Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yulia Lukina | -- | 8216 | 2023-06-10 19:18:31 | | | |
| 2 | Dean Liu | Meta information modification | 8216 | 2023-06-12 04:07:23 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Lukina, Y.; Safronova, T.; Smolentsev, D.; Toshev, O. Calcium Phosphate Cements as Carriers of Functional Substances. Encyclopedia. Available online: https://encyclopedia.pub/entry/45418 (accessed on 07 February 2026).
Lukina Y, Safronova T, Smolentsev D, Toshev O. Calcium Phosphate Cements as Carriers of Functional Substances. Encyclopedia. Available at: https://encyclopedia.pub/entry/45418. Accessed February 07, 2026.
Lukina, Yulia, Tatiana Safronova, Dmitriiy Smolentsev, Otabek Toshev. "Calcium Phosphate Cements as Carriers of Functional Substances" Encyclopedia, https://encyclopedia.pub/entry/45418 (accessed February 07, 2026).
Lukina, Y., Safronova, T., Smolentsev, D., & Toshev, O. (2023, June 10). Calcium Phosphate Cements as Carriers of Functional Substances. In Encyclopedia. https://encyclopedia.pub/entry/45418
Lukina, Yulia, et al. "Calcium Phosphate Cements as Carriers of Functional Substances." Encyclopedia. Web. 10 June, 2023.
Copy Citation
Interest in calcium phosphate cements as materials for the restoration and treatment of bone tissue defects is still high. Despite commercialization and use in the clinic, the calcium phosphate cements have great potential for development. Existing approaches to the production of calcium phosphate cements as drugs are analyzed.
calcium phosphate cement
hydroxyapatite
brushite
functionalization
bone tissue
1. Introduction
Bone tissue is a part of the human musculoskeletal system, which participates in the transfer of force from one part of the body to another under controlled tension, and protects and fixes internal organs. In addition to performing a mechanical function, bone tissue performs a biological function, as it participates in metabolism [1][2][3][4]. Bone tissue is a reservoir of calcium and phosphate ions in the form of hydroxyapatite, so it plays an important role maintaining the proper calcium levels, along with in other organs [5][6].
Bone tissue’s ability to regenerate effectively, maintain mineralization and repair itself after damage depends on its ability to dynamically remodel. However, the regenerative process is limited by the ability to self-repair: osteogenic insufficiency occurs if the critical size of the defect is exceeded, and the defect is filled with fibrous connective tissue.
There are many different clinical circumstances under which a significant part of bone or a whole bone is lost. Bone defects can be caused by various reasons. They can be associated with various pathogenic conditions and clinical outcomes, including injuries (fractures), infections (osteomyelitis), tumors, osteoporosis, and many other bone diseases [7].
According to statistics, 20 million orthopedic surgeries in the world per year are performed, 70% of which require the use of bone implant material for filling and repairing bone defects [8].
Various osteoplastic materials can be used to fill in bone defects caused by various diseases, or for the purpose of their prevention. They are able to deliver functional substances locally, fill in defects and serve as a material for bone tissue reconstruction.
Calcium phosphate cements are similar in composition to the mineral component of bone tissue. They have a high specific surface area and are used in medicine as osteoplastic materials. Blocks and granules made of pre-hardened cement are a promising type of skeleton for the restoration of bone defects. They have an increased rate of resorption compared to matrices obtained by high-temperature processing. Functional substances can be volumetrically incorporated into them at the stage of mixing the components. The kinetics of the release of functional substances may vary. The release of Ca2+ ions during resorption can affect the differentiation of osteogenic cells and the level of inflammatory cytokines.
The functionalization of calcium phosphate cements is of great clinical interest for the treatment or prevention of various diseases of bone tissue. Prolonged elution is the main requirement for carrier materials of pharmaceutical substances. Another important requirement is the absence of a mutual negative influence of the functional substance and the matrix on each other’s properties. The release of functional substances from calcium phosphate cements depends on many parameters of the matrix, specific interactions between the functional substance and the matrix, as well as environmental factors.
A significant advantage of calcium phosphate cements is the wide range of changes in the properties of matrices that can affect the release of functional substances and the process of bone tissue restoration.
2. Causes of Bone Tissue Damage and Ways of Treatment
2.1. Injury
Bone injuries caused by trauma can occur in patients of all ages. This can be the result of traffic accidents, falls, and many other reasons. Bone fractures are one of the most common types of injuries.
Injuries caused by trauma can be divided into long bones and spine, or maxillofacial and craniofacial, depending on their localization. The most common places of bone fractures are the femur, shoulder (mainly humerus), hip (femoral neck), wrist (radius/ulna), tibia (distal third), ankle, vertebra, and maxillofacial and craniofacial areas (jaw bone, cranial vault) [9].
The mechanism of bone repair is a multi-stage organized restorative procedure, involving a number of vital progenitor cells along with inflammatory, endothelial and hematopoietic cells [10][11]. Cortical tissue, periosteum, bone marrow and external soft tissues contribute to the healing process. It depends on many parameters present in the damaged tissue, such as growth factors, hormones and nutrients, pH, oxygen saturation, and the performed mechanical stabilization of the fracture [12].
The bone restoration procedure consists of several overlapping phases [8][10][13], which can be combined into three main phases: inflammation, bone formation, and bone modeling (Figure 1).
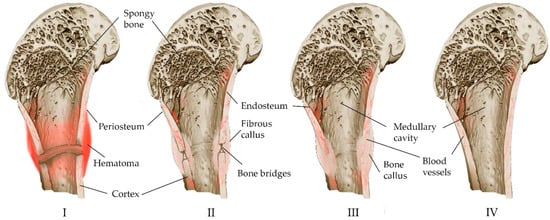
Figure 1. Schematic representation of the stages of fracture self-healing: (I) The acute stage of the fracture, a hematoma forms around the damaged area of the bone, the bone tissue of the ends of the fragments partially dies (colored dark), local enzymatic activity increases. (II) Development of connective tissue corn. Accompanied by inflammation: cells participating in the inflammatory response appear at the site of the fracture; osteoclasts and osteoblasts are active, non-viable tissue is processed; with the formation of a “cloud” of new tissue at the site of the fracture, structured bone bridges appear inside the corn connecting the fragments. (III) Consolidation: the newly formed tissue acquires the correct bone structure, and trabeculae appear. (IV) Remodeling: the newly formed bone acquires its final structure.
Inflammation begins immediately after a bone fracture and lasts for several days. In the fracture area, blood vessels are damaged, bleeding occurs, hematoma forms caused by osteocyte necrosis due to hypoxia, and thrombosis ensues with the formation of fibrin mesh along the fracture line [8][9][10][14][15][16]. The fibrin mesh isolates the fracture site and serves as a framework for the infiltration of inflammatory cells and macrophages, neutrophils and mast cells that release cytokines and growth factors. Macrophages that have migrated to the site of inflammation remove the temporary fibrin matrix, necrotic cells and bone fragments [9]. As a result, the hematoma and acute inflammatory reactions disappear after a week.
The release of cytokines and growth factors together with pro-inflammatory stimuli leads to a high production of prostaglandins [12]. Newly formed capillaries from the periosteum, and fibroblasts and bone formations of an invasive hematoma appear. Fibroblasts secrete a large number of collagen fibers, and after differentiation, the hematoma forms a fibrous callus, which is characterized by neovascularization, migration of mesenchymal cells, and fibroblast ingrowth [8][15]. Neovasculogenesis in combination with the further production of growth factor and prostaglandins contributes to the differentiation of mesenchymal stem cells towards chondrogenic or osteogenic, and the initial formation of bone tissue [8][17].
The process of the transformation of fibrous callus into bone callus occurs at 2–3 weeks, mainly due to the differentiation of progenitor cells into osteogenic cells (chondrocytes and osteoblasts), secreting matrix osteoids, which gradually replace the fibrous callus due to the deposition of calcium salts (mineralization) [8][16]. It is believed that the relative distance of cells to blood vessels is one of the factors in the differentiation of progenitor cells into osteoblasts, along with the release profiles of cytokines and growth factors. The distance must be kept to a minimum because osteoblasts depend on oxidative metabolism, and require a constant and substantial supply of oxygen and nutrients. Therefore, they accumulate in the immediate vicinity of the newly formed blood vessels. The adapted metabolism of chondrocytes is designed to survive and function in a poorly vascularized environment, so they mature farther from the blood vessels [9].
Chondrocytes proliferate, forming a cartilaginous callus, and then turn into a bone callus via the internal osteogenesis of cartilage [8]. Gradually, the soft callus is replaced by a hard callus at 3–4 months [16], which is visible on radiographs [10].
Bone remodeling, the final stage of healing, lasts for more than several months. In the process of regeneration, the bone regenerates and returns to its original shape [10][12], and the callus is rebuilt according to the needs of biomechanics. Osteoclasts absorb excess callus and recanalize the bone marrow cavity. Insufficient bone callus is replenished due to membrane osteogenesis [8].
Trabecular bone repair occurs without significant external callus formation. After the inflammatory stage, intramembranous ossification predominates in bone formation. This is explained by a significant angiogenic response [16].
The healing of bone fractures is a complex regenerative process. Pathological conditions (the presence of cracks, impaired blood flow, concomitant infection and extensive damage to soft tissues), insufficient mechanical stability and metabolic disorders (diabetes, age-related osteoporosis, genetic factors) are inhibitory factors [17][18]. Up to 10% of people experience delayed healing or bone non-fusion. Biomechanical stability is a critical factor in the healing process of fractures. Internal or external fixation is intended to improve stability and promote healing [19]. The stability of the fracture is of paramount importance to the prevention and treatment of fracture-associated infection [16][20]. Antibiotic-loaded spacers are used in the staged reconstruction of bone non-fusion and bone defects [21].
Poor bone quality in patients with osteoporosis and diabetes can lead to a more complex fracture structure and problems with fixation [7]. The shell of soft tissues can be disrupted due to chronic vascular disease, the prolonged use of steroids, and a decrease in skin turgor. This leads to open injuries, even with low-energy fractures [22].
The use of exogenous bioactive factors at the site of a defect to accelerate bone repair has been under extensive investigation in the field of bone regeneration. Differences in structure determine their different physiological roles and their special role in ensuring the growth of new bones [11][23][24][25]. Vascular endothelial growth factor (VEGF), insulin-like growth factors (IGFs), fibroblast growth factors (FGFs), bone morphogenetic proteins (BMPs), platelet-derived growth factor (PDGF), and transforming growth factors (TGFs) are the most widely studied in areas of bone regeneration at present. Effective results of regeneration in bone defects depend on the growth factor delivery system.
The concept of using bioactive agents such as tetracycline (antibiotic) and flurbiprofen (non-steroidal anti-inflammatory drug—NSAID) and their chemically modified analogues for early bone formation has recently been proposed in bone regeneration studies [23]. They are effective antiresorptive agents, interfere with intracellular calcium concentration and act as strong inhibitors of osteoclasts. However, there are conflicting data on the effectiveness of the use of NSAIDs. They lead to a delay in healing and a decrease in the content of minerals and matrix of the callus, inhibit remodeling, and cause non-fusion, according to the results of some studies [17][26], and have shown almost no effect on fracture healing in other studies [27]. NSAIDs are useful in everyday clinical practice for pain relief due to their pronounced analgesic activity and anti-inflammatory effects, but conflicting preclinical results, as well as the lack of well-randomized clinical data, suggest that more research is needed [17].
Bisphosphonates and anabolic agents inhibit bone resorption and can be used to accelerate bone tissue repair [28]. Despite the possible stoppage of remodeling and an increase in bone fragility [29], the negative consequences do not outweigh their beneficial effect at the moment; for example, in the prevention of additional fractures in patients with osteoporosis, who are often diagnosed after primary fractures [17].
2.2. Osteomyelitis
Osteomyelitis is an inflammatory bone disease caused by an infectious microorganism, often accompanied by bone destruction. It is most often caused by the local spread of infection after injuries, orthopedic operations or joint replacement. The disease may be limited to a specific area of bone or several areas, such as the bone marrow, cortical, periosteum and surrounding soft tissues [18][30][31]. Different types of osteomyelitis require different medical and surgical therapeutic strategies. The most common osteomyelitis is secondary to the site of infection after injury, surgery, or the installation of an articular prosthesis. Osteomyelitis secondary to vascular insufficiency (diabetic foot) or hematogenic origin is less common.
The main causative agents of bone infections are Gram-positive cocci, including Staphylococcus aureus, coagulase-negative staphylococci (Staphylococcus epidermidis), enterococci (Enterococcus faecalis) and streptococci, and Gram-negative anaerobic bacteria (Escherichia coli, Pseudomonas aeruginosa) [32][33][34][35]. S. aureus has a high level of antibiotic resistance, which has long been recognized. Many other microorganisms, including S. epidermidis [36] and a number of staphylococci, have demonstrated increasing antibiotic resistance in recent years [37]. It has been reported that up to 40% of S. epidermidis strains [38] and 32% of S. aureus strains [39] isolated from orthopedic postoperative and implant-related infections are resistant to gentamicin.
Methicillin-resistant S. aureus (MRSA) is considered particularly virulent due to the production and release of a number of extracellular and cell-associated factors [32][40][41]: bacterial adhesins (microbial surface components that recognize and specifically interact with a single host protein component such as fibrinogen, fibronectin, collagen, and others), toxins, capsular polysaccharides (avoiding host defenses), exotoxins, and various hydrolases (invasion or penetration into tissues by a specific attack on host cells or degradation of extracellular matrix components). S. aureus internalized by cultured osteoblasts can survive inside cells [41][42]. As soon as S. aureus enters the cell, the activity and viability of osteoblasts decrease, and the expression of an apoptosis-inducing ligand associated with tumor necrosis factor is induced [32]. Infected osteoblasts secrete cytokines, chemokines, and growth factors. They are involved in the immune response and promote bone destruction, bacterial adhesion, and biofilm formation [32][43]. A biofilm is a microbial community and consists of cells that adhere to a substrate, an interface, or to each other. They are embedded in an extracellular polymer matrix and exhibit an altered phenotype in terms of growth, gene expression, and protein production [41][44]. Biofilm complicates treatment by acting as an impenetrable barrier that prevents the penetration of antibiotics and immune cells.
The strategy for the treatment of osteomyelitis is based on the use of antibiotics systemically and/or locally, alone or in combination with debridement. Many factors, including patient-specific factors, microorganism type and antibiotic susceptibility, location, spread, implant loosening, and most importantly, the type of infection determined by the time of onset (acute or chronic), affect the overall treatment algorithm [45][46].
In periprosthetic infections, methicillin-resistant S. aureus (MRSA) predominantly causes early infections, whereas methicillin-sensitive S. aureus (MSSA) causes delayed and late infections [33].
Acute purulent inflammation is characteristic of acute osteomyelitis (Figure 2). Various inflammatory factors and leukocytes contribute to tissue necrosis and destruction of bone trabeculae and bone matrix. Vascular channels are compressed and obliterated by the inflammatory process. The ischemia resulting from the process also contributes to bone necrosis. Segments of bone that are deprived of blood supply may separate as sequesters and continue to contain bacteria despite antibiotic treatment [41]. However, antibiotic therapy alone is often sufficient to treat acute osteomyelitis [18]. The high activity of osteoclasts causes bone loss and localized osteoporosis. Meanwhile, the bones converge, in some cases excessively, causing the periosteum to converge and new bone to form [41].
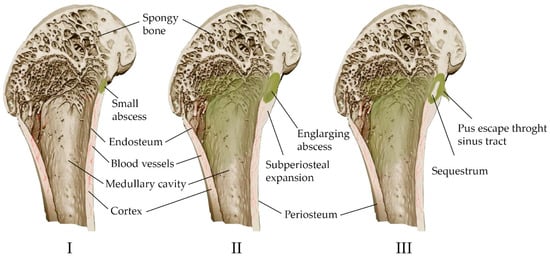
Figure 2. Scheme of the development of hematogenous osteomyelitis in the bone. (I) Formation of a primary bone abscess (necrosis and purulent fusion of the bone marrow and adjacent bone, limited by the walls of healthy tissue). (II) Subperiosteal abscess (pus through the haversian canals of the bone spreads under the periosteum, exfoliating it from the bone). (III) Fistula formation (pus breaks out through soft tissues); the formation of sequesters is possible—areas of the bone completely devoid of vascular nutrition and lying freely.
Chronic osteomyelitis is still difficult to treat, and has significant morbidity and a high risk of recurrence. It is associated with avascular bone necrosis and sequestration. The infection usually does not begin to regress until the site of persistent contamination has been surgically removed. Antibiotic therapy alone is usually not enough to treat chronic osteomyelitis, although antibiotics relieve many symptoms [18][47]. Rare complications of chronic osteomyelitis include squamous cell carcinoma at the site of tissue drainage and amyloidosis [48].
Systemic antibacterial therapy becomes ineffective in the presence of a vascular area or poorly vascularized scar tissue in osteomyelitis, since antibiotics cannot reach infected tissue [41][45].
In such cases, there is a significant need to develop matrices with antibacterial properties.
Thus, the specific etiology of bone defects caused by inflammation imposes many requirements on the design of medical materials, including the ability to cope with both the inhibition of inflammatory responses and the stimulation of bone tissue regeneration [18][45]. Several principles for topical antibiotic treatment are particularly important: maintaining a concentration above the minimum inhibitory concentration (MIC) of antibiotics that affects bacteria for at least three to six weeks, and adequate tissue penetration so as not to cause local and systemic toxicity. High concentrations of antibiotics (up to 1000-fold increase) are needed to destroy the introduced microorganisms in the case of a formed biofilm.
One of the main advantages of the biodegradable system is the absence of secondary surgical procedures to remove foreign material after the release of antibiotics, such as PMMA cement, has ceased. Additional possibilities for using resorbable systems include changing the release of antibiotics, and the possibility of targeted adjustment of the wound environment by the products of material degradation [49]. Resorption must be complete to leave no substrate for bacterial colonization and to promote the integration of the host tissue. The kinetics of elution of an antibiotic from a material are closely related to its characteristics, provided by composition, surface area, porosity, and other factors (Hanssen, 2005).
The most commonly used antibiotics are gentamicin, rifampicin, vancomycin and tobramycin [46].
It is necessary to take into account the local concentration of antibiotics when they are applied topically, since high concentrations of antibacterial drugs have a cytotoxic effect on cell viability, cause osteogenic differentiation, and reduce the expression levels of genes and the proteins of collagen [50].
2.3. Tumor
The main concept of orthopedic oncology is the prevention of amputation. It is performed in 10–20% of patients with malignant bone tumors [51]. The tumor is characterized by the replacement of healthy bone tissue with tumor tissue, with the inclusion of the medullary canal and the soft tissues surrounding the bone in the pathological process (Figure 3). Many tumors originate in the metaphyseal–diaphyseal regions of long bones and can be segmentally resected while preserving the joint [52]. Approaches have been developed for the treatment of complications associated with malignant neoplasms. These include either anticancer intervention, such as chemotherapy, hormone therapy, radiation therapy, and resection, or bone-supportive care, such as calcium, analgesics, and vitamin D supplements [53].
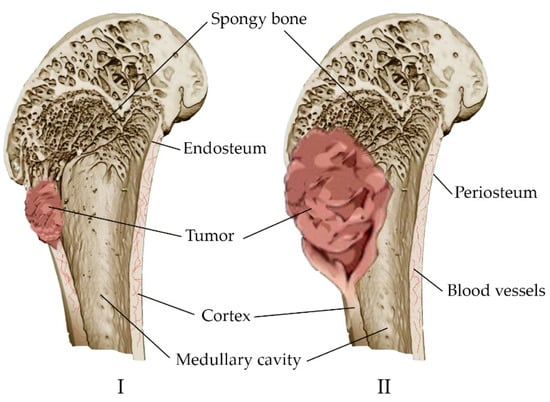
Figure 3. Development of a bone tumor: (I) Destruction of a bone site by a tumor; (II) replacement of healthy bone tissue with tumor tissue, inclusion of the bone marrow canal and soft tissues in the pathological process.
Metastatic bone lesions are common in patients with other types of cancer (lung, breast, prostate, and colorectal cancer). Most severe bone metastases are also treated with reconstructive surgery. This is followed in some cases by postoperative radiation or chemotherapy. Patients after body reconstruction still show a risk of developing severe complications such as tumor recurrence [54].
Depending on the type of cancer, metastatic lesions can be characterized as osteolytic, osteoblastic or mixed lesions (containing both elements), in which the regulation of the normal process of bone remodeling is disrupted [55]. Osteolytic lesions are characterized by the leaching of the mineral part of the bone, its thinning, and fractures. Osteoblastic metastases, on the contrary, are characterized by compaction of the mineral part of the bone, since the cells of different tumors can both directly destroy bone tissue and stimulate cells that renew it. Osteolysis is also observed in prostate cancer, despite the tendency to osteoblasting in metastases. However, there is a shift in the balance towards the formation of a new matrix and its mineralization. The increase in bone volume is due to the replacement of existing trabecular tissue with abnormally woven bone, which creates a general appearance of sclerosis [56].
Osteolytic metastases can cause severe pain, pathological fractures, life-threatening hypercalcemia due to elevated blood calcium levels, spinal cord compression, and other nerve compression syndromes [57]. Patients with osteoblastic metastases feel pain in bones and pathological fractures due to poor bone quality produced by osteoblasts [55].
Drugs that block bone resorption may reduce bone pain and the risk of pathological fractures in metastatic lesions. One of the effective groups of drugs used in osteolytic bone disease and osteoblastic bone disease associated with prostate cancer metastasis is bisphosphonates [53][55][56][58][59]. Bisphosphonates have the ability to inhibit resorption processes in the bone by reducing the activity of osteoclasts. Bisphosphonates inhibit the release of growth factors, inhibiting bone resorption, and thus block the feedback from tumor cells. This helps to reduce the activity of tumor cell proliferation. Bisphosphonates can induce apoptosis in malignant cells similar to the process observed in osteoclasts, and reduce the adhesion of tumor cells to bone, reducing the risk of new metastatic lesions.
If there is a high probability of a pathological fracture of metastatic bone tissue, prophylactic fixation is recommended [60]. This involves the resection of the tumor and reconstruction of the damaged bone [61]. Plates, intramedullary rods and screws are used as fixators in the reconstruction of a damaged bone. With lesions of more than 50% of the bone diameter or joint lesions, prostheses are installed [62][63]. The purpose of reconstruction is palliative. It reduces pain and restores the function of the affected bone throughout the life of the patient. Among the complications of reconstructive treatment of metastatic lesions of the bone tissue are infection (0–11.7%), aseptic loosening (0–12.5%), mechanical damage (0–14.7%), and tumor recurrence (3.1–14.7%), despite the fact that chemotherapy is prescribed after reconstruction [54][64][65][66][67].
The combination of high doses of methotrexate, cisplatin, ifosfamide, and doxorubicin results in increased survival, but the use of anticancer drugs is limited by serious side effects. Poor bone blood supply, drug resistance, and nonspecific absorption require the use of highly toxic doses of anticancer drugs [58]. Therefore, there is a need to develop locally delivered carrier materials with reduced side effects and/or without effects for the treatment and prevention of growth-related bone cancers.
Surgical resection together with radiation/chemotherapy is a clinically accepted treatment regimen. For biomaterial therapy, surgical intervention is necessary to remove tissue and to provide space for the formation and integration of new bone. Biomaterials are used as bone substitutes after tumor surgery. Adjuvant treatments, such as systemic radiation therapy and chemotherapy, are used to prevent relapse.
The localization of radio/chemotherapy is a more highly effective method of the treatment and prevention of relapse after tissue reconstruction, since simultaneous processes of tumor inhibition and bone regeneration are possible with the mobilization of drugs via bone replacement material [68].
Many surgeons use cement reconstruction techniques to minimize postoperative complications. Bone cement made of polymethylmethacrylate is used as a carrier of chemotherapeutic drugs in an attempt to reduce tumor recurrence, and serves as an addition to bone reconstruction [54].
At the same time, inorganic calcium phosphate cements attract the attention of researchers because their biological properties can counteract the toxic nature of the inclusion of anticancer drugs, and promote osteogenesis. The presence of calcium phosphate cement can help to avoid the spread of tumor cells, and prevent the development of new lesions in the surrounding tissues during resection inside the focus [69]. They can also be used as carriers of antitumor drugs or radioactive substances, and administered to patients to achieve antitumor effects [70][71]. These cement modifications for the delayed release of antitumor drugs, magnetic tumor targeting, or radiological modifications are designed to simplify the treatment process and reduce systemic side effects and pain in patients [72].
2.4. Osteoporosis
An imbalance in bone metabolism between bone resorption mediated by osteoclasts and bone formation mediated by osteoblasts leads to the occurrence of metabolic diseases in bones, including osteoporosis and osteomalacia (mineralization deficiency caused by a a lack of calcium or phosphorus, or insufficient activity of osteoblasts) [73][74]. Osteoporosis is a leading public health problem and one of the most common chronic diseases. It affects more than 200 million people worldwide [75]. A systemic metabolic disorder in the bones leads to a gradual loss of bone mass and damage to microarchitectonics, along with the weakening of bone strength (Figure 4). This leads to low-energy fractures [18][76]. A fracture can occur anywhere in the skeleton, although fractures of the wrist, hip and spine are the most common [73]. Both men and women lose bone mass as they age, with women gradually losing 50% of trabecular and 30% of cortical bone over a lifetime, while men lose two-thirds of this amount [77]. The prevention of osteoporosis can be achieved via a balanced diet containing calcium, phosphorus and vitamin D. These improve bone reabsorption and repair, except in cases of hereditary bone diseases [73][78].
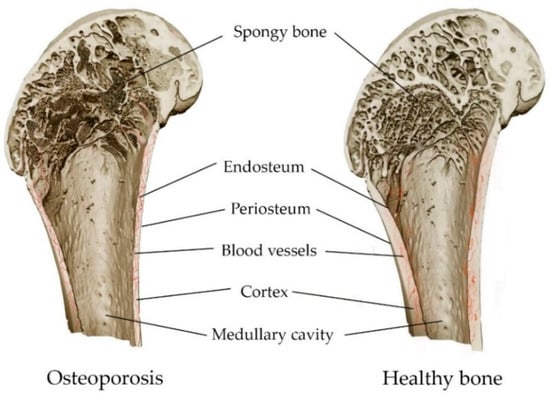
Figure 4. Comparison of osteoporosis and healthy bone: decrease in bone density, and thinning of bone structures, as a result of osteoporosis.
The pathogenesis of osteoporosis is mainly associated with bone homeostasis, with the balance of bone remodeling between formation and resorption by specific cells, including osteoblasts, osteocytes and osteoclasts. Mesenchymal stem cells of the bone marrow are multipotent cells with the ability to differentiate into lines of osteoblasts, chondrocytes and adipocytes [79]. Osteoporosis is an increase in the adipose tissue of the bone marrow due to a shift in differentiation into adipocytes rather than osteoblasts. In addition, activation of the main signaling pathways of bone metabolism can both promote the differentiation of pre-osteoclasts into osteoclasts, and prevent it by suppressing the RANKL membrane protein [18].
A common and effective strategy for the treatment of osteoporosis is antiresorptive therapy, which targets osteoclasts and reduces the rate of bone resorption [18][58][76][80]. The therapeutic efficacy of bisphosphonates and monoclonal antibodies has been confirmed by the successful use of pharmaceutical preparations alendronate, risedronate, zoledronate, raloxifene ibandronate, teriparatide, abaloparatide and denosumab [58][73][76][81]. Bisphosphonates can minimize the chances of vertebral fractures by 50–60% and hip fractures by 50% [82]. Anabolic agents (romosozumab, teriparatide, and abaloparatide) are currently approved for the treatment of osteoporosis because the drugs may promote bone regeneration and reduce bone fractures [73][81].
In addition, osteoblasts are able to respond to various modalities of the extracellular signal, including the concentration of extracellular free ionized calcium Ca2+, regardless of systemic factors [83].
Local prolonged drug delivery systems in effective therapeutic doses to the site of bone disease can contribute to the effective treatment of various metabolic diseases of the bone tissue, with less adverse effects [73][84][85]. Minimal invasiveness can be considered as a concept to reduce the risk and number of complications [18].
Thus, calcium phosphate cements can be used as carriers of antitumor, antibacterial, radioactive, anabolic, antiresorptive, anti-inflammatory and osteoinductive drugs, providing the output of functional substances at the implantation site (Figure 5).
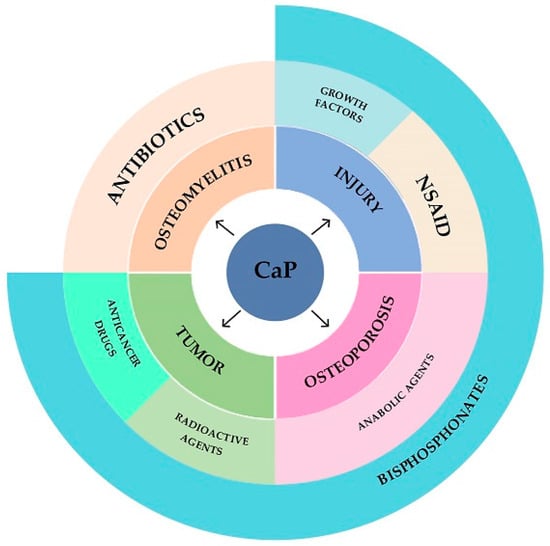
Figure 5. Diagram of applicability of calcium phosphate cements.
2.5. Calcium Phosphate Cements for Bone Treatment
Bone tissue is able to spontaneously heal fractures or defects, but regeneration is limited to small areas of the defect. The critical bone tissue defect size is commonly taken to mean the smallest bone defect in a particular bone of a particular living organism that does not spontaneously heal up, or shows less than 10% bone regeneration over its life [86]. Bone grafts are needed to facilitate the repair process if the size of the defect is too large for the bone’s natural ability to heal. Calcium phosphate cements have a similar bone mineral chemistry, and can adapt perfectly to the shape of the defect, making them a convenient option for use as a synthetic bone graft.
Bone is the main storage site for calcium and other ions in the body. Bone fractures, especially during surgical treatment, are associated with changes in microelement homeostasis [87]. Calcium introduction is effective in reducing the risk of postoperative hypocalcemia [88][89][90]. Together with other ions such as magnesium, they have a positive effect on the healing of bone fractures [91]. Ca2+ is an important homing signal; it brings together various cell types required to initiate bone remodeling. A high concentration of Ca2+ can induce osteoblast proliferation and chemotaxis by binding to an extracellular calcium-sensitive G-protein-coupled receptor [92][93].
Cytokines C-reactive protein (CRP), interleukins IL-1β, IL-6 and tumor necrosis factor alpha TNF-α play a pro-inflammatory role, and are important mediators of the immune response and inflammatory response. The disordered expression of IL-1β, IL-6 and TNF-α is considered an effective biomarker of inflammation associated with the development and process of fractures [94]. An increase in the content of Ca and Mg, and a decrease in inflammatory cytokines, are observed after short-term treatment with trace elements. Their addition can be an effective way to combat inflammation, as it contributes to the restoration of bone fractures [89]. The disordered expression of IL-1β, IL-6 and TNF-α is considered an effective biomarker of inflammation associated with the development and process of fractures [94]. There is an increase in the content of Ca and Mg, and a decrease in inflammatory cytokines, after short-term treatment with trace elements. The addition of trace elements can be an effective way to fighting inflammation, and contribute to the reconstruction of bone fractures [89].
Calcium phosphate cements can be used to strengthen vertebral bodies affected by osteoporosis [95] in cranio-maxillofacial surgery [96][97], as well as for treating fractures and revisions, mainly to fill defects resulting from the surgical removal of cysts and tumors, trauma and osteolytic defects, or in the surgical treatment of infections [98].
Resorbable calcium phosphate cements prepared by low-temperature technology containing drugs or other biologically active substances and cells can potentially act as multifunctional carriers and drug delivery systems [99][100]. Figure 6 shows the process of bone defect replacement during the implantation of calcium phosphate material in the form of a block, granules or cement paste.
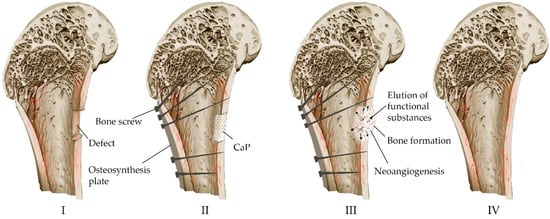
Figure 6. Scheme of replacement of bone defects with the help of calcium phosphate material: (I) Formation of a defect along the boundaries of viable tissues (resection of compromised areas as a result of high-energy trauma, inflamed areas in osteomyelitis, tumor lesions, etc.). (II) Installation of metal structures for mechanical strength for the period of healing and implantation of osteoplastic material to close the defect. (III) Reconstruction of osteoplastic material in the defect, resorption, biodegradation and formation of bone. (IV) Bone remodeling.
Currently, calcium phosphate materials are used in the field of traumatology and orthopedics in the form of powders, granules, blocks, cements and coatings on metal implants [101][102][103][104][105][106]. Due to the high strength of ionic bonds, calcium phosphate materials are brittle and cannot carry heavy loads. They are used as osteoplastic materials in small defects or operated with the additional use of structures on which the load is shifted.
Calcium phosphate cements are osteoconductive and bioactive, and they integrate into bone tissues without forming a connective tissue capsule [107]. The cements are resorbable, moldable and easy to handle. They can be injected into bone cavities under conditions of limited surgical access, and completely fill cracks (defects) in situ in the operating room, thus minimizing surgical intervention. They provide good fixation and close contact between the bone and the material [108]. Cements harden, acquiring their own mechanical strength [105].
3. Concepts for the Production of Calcium Phosphate Cements
Calcium phosphate blocks and granules obtained as a result of the hardening of calcium phosphate cement are a promising type of scaffold for restoring bone defects due to their similarity in composition with the mineral component of bone tissue, high specific surface area, and increased resorption rate compared to matrices obtained by high-temperature processing. In addition, the volumetric incorporation of functional substances at the stage of mixing the components can be used to change the release kinetics, in contrast to the surface impregnation of high-temperature calcium phosphate materials [101][109][110][111][112]. Matrixes can be formed by casting cement paste into molds, by interacting pre-pressed initial components in an aqueous medium via setting and hardening, and by 3D printing; they can serve as a substrate in tissue engineering and are an excellent platform for incorporating functional substances [101][113][114][115].
Cements are usually obtained from powders of one or more calcium phosphates and an aqueous solution. When mixing powders of calcium phosphates and an aqueous solution, a paste is obtained. It hardens within minutes. Another form of supply of calcium phosphate cement is pre-mixed cement components in the form of a cement paste. There are three main approaches used to prepare premixed cements: (1) one-component cement in the form of calcium phosphate paste mixed with a non-aqueous liquid (hardening when mixed with biological fluids); (2) two- or more-component cement in the form of pastes (hardening when mixed); (3) one-component cement in the form of a paste of calcium phosphate in an aqueous liquid, followed by freezing (hardening during thawing). This form of delivery is presented in the commercial products of VitalOs from CalciphOs/Produits Dentaires SA and VELOX from InnoTERE GmbH [116].
The constant solidification volume of cements and low heat release (minor exothermicity at low heat release rate) [72][109][117] are properties that that enable their use.
An increase in mechanical strength is facilitated by the isolation of particles of non-equiaxed morphology (lamellar, needle-shaped), which provide mechanical engagement. The cements fuse well with the bone, gradually dissolving and being replaced by new bone tissue.
Powders and blocks of cements can be sterilized by γ-irradiation without the loss of biocompatibility and bioactivity [118]. Steam sterilization, ethanol sterilization, and ethylene oxide sterilization with complete degassing after sterilization are also mentioned [112][115][119]. Gamma radiation for powder components and filtration for liquid components are used in the sterilization of cement formulations [120].
Calcium phosphate cements can be divided into two categories according to the final product, despite the large number of possible preparation methods: (1) based on hydroxyapatite; (2) based on dicalcium phosphate dihydrate CaHPO4·2H2O (DCPD) (or brushite) [105][109][121][122].
A significant part of the commercial cement materials used in medicine for the treatment of bone tissue defects contain minerals belonging to the CaO–P2O5–H2O system [123]. The production of calcium phosphate cements is usually realized according to two scenarios, with two chemical processes: acid–base interaction and hydrolysis.
As a result of the acid–base reaction, cements based on hydroxyapatite Ca10(PO4)6(OH)2 (HA) (Ca/P = 1.67)/calcium-deficient hydroxyapatite Ca10−x(HPO4)y(PO4)1−y)6(OH)2 (CDHA) (Ca/P = 1.5) and brushite CaHPO4·2H2O (DCPD) (Ca/P = 1)/monetite (dicalcium phosphate anhydrite) CaHPO4 (DCPA) (Ca/P = 1) are obtained by reacting tetracalcium phosphate Ca4(PO4)2O (TTCP) (Ca/P = 2) or β-tricalcium phosphate β-Ca3(PO4)2 (β-TCP) (Ca/P = 1.5) with DCPD (Ca/P = 1)/DCPA or monocalcium phosphate monohydrate Ca(H2PO4)2·H2O (MCPM) (Ca/P = 0.5), without the formation of acidic or basic co-products. Monetite is formed under conditions of water deficiency and low pH [104][124].
Only one precursor is involved in the hydrolysis reaction. When mixed with the liquid phase, it becomes hydrated. The reaction proceeds with the dissolution of PO43− and Ca2+ ions. On the surface of the α-tricalcium phosphate α-Ca3(PO4)2 (α-TCP) or amorphous calcium phosphate Ca3(PO4)2 (ACP), particles of precipitated crystals of calcium-deficient hydroxyapatite form during the hydrolysis process.
HA and brushite cements differ significantly in setting time, mechanical strength, resorption rate in the body, and pH values. In this regard, approaches to improving the properties of cements differ.
HA cements are long-hardening; the setting time is no earlier than 30 min. A decrease in cohesion upon contact with blood and partial mixing with it may occur during prolonged hardening, and this will lead to a loss of quality or migration from the implantation site [125][126][127].
Miyamoto and colleagues stated that the cement sets consistently despite partial decomposition of the cement paste on contact with blood during setting. However, fast-setting cements may be less sensitive to contact with blood [128].
Particle size and the degree of crystallinity strongly affect the degree of reactivity of cements and, as a result, the rate and integral completeness of the reaction [129][130]. ACP is the most reactive because it has the least stable crystalline phase. It is followed by α-TCP and finally β-TCP [131]. A decrease in the particle size leads to an increase in the surface area, and an increase in the reactivity and the reaction rate [101][132][133].
The introduction of a seed of crystallization increases the rate of hydration and hardening [133][134][135]. The seed plays the role of a substrate that can be used for heterogeneous nucleation. The nucleation barrier does not exist if the substrate is identical to the nascent crystal.
HA cements are relatively insoluble in aqueous solutions at neutral pH. Solubility increases at acidic pH values, as their own pH values correspond to physiological values.
Brushite cements are fast-setting, with setting times less than 1 min, as well as being soluble, highly bioresorbable and biocompatible, and relatively acidic (pH around 4).
The rate of resorption of cements in the body can be regulated by various technological parameters: L/P ratio, porosity, phase composition (ion substitutions, introduction of additional components of cement powder), crystallinity, as well as the presence and quantity of additives.
The introduction of additives into the system or due to ionic substitutions can increase the setting time of cement. For example, in the production of brushite cement, the setting time and compressive strength were increased by replacing calcium in TCP with magnesium by up to 10%. Moreover, the initial setting time increased to 33 min with magnesium content [136]. Changing the particle size of the initial components affects the setting time and strength of the cement.
In the literature, the compressive strength of HA cements is 20–83 MPa, while that of brushite cements −1–24 MPa, and tensile strength of brushite cements −0.7–4.5 MPa, and that of HA cements is up to 15 MPa. Such data were obtained by measuring the strength under different conditions. Cement samples dried in air or at a slight increase in temperature have the maximum strength values.
Among the ways to increase the strength of cements are the following: reducing the liquid/powder ratio (L/P or LPR) when receiving cement paste, introducing additives, changing the particle size [137][138], and introducing fillers (fibers, granules) inert with respect to the cement stone. As fillers, granules or fibers of the inorganic compounds β-TCP, HA, gypsum, bioglass, carbon, silica, wollastonite and zirconium dioxide can be used. They are biocompatible, non-resorbable or poorly resorbable compounds [139][140][141], with the exception of resorbable β-TCP and some bioglasses.
Fibers and granules made from resorbable polymers such as polylactic acid (PLA) [142], polylactide-co-glycolide (PLGA) [143], polyvinyl alcohol (PVA) [144], gelatin or chitosan [145][146] increase initial strength and create porosity over time. In this regard, the characteristics of the cement stone change; this affects the behavior of the material in vivo. Such fillers can be attributed to additives as modifiers of cements.
Calcium phosphate cements have a porosity of 30–60% by volume, typically depending on the composition of the cement. The porosity is mostly open. Pore sizes up to 1 micron do not provide bone tissue ingrowth, and resorption occurs from the surface of the material. The porosity of calcium phosphate cements is due to excess mixing water. As the amount of water decreases, the porosity decreases, and hence the mechanical properties improve. This leads to a decrease in the resorption rate and the deterioration of rheological properties [109].
The absence of macroporosity is attributed to the disadvantages of calcium phosphate cements, in particular HA, while the presence of microporosity is an advantage. This is due to the fact that microporosity creates a surface microrelief for cell retention, and improves osteogenesis [146][147][148][149][150]. In addition, microporosity is a positive factor in calcium phosphate cements–functional substance delivery systems. However, microporosity reduction due to prepressing is used to obtain cement scaffolds with increased strength [151], and in combination with removable fillers to form macroporosity, it reduces strength to a lesser extent [110][152]. Another approach to reducing porosity is to reduce the particle size of the original components [153].
Ionic substitutions can also affect porosity. For example, when using silicon beta-tricalcium phosphate (Si-β-TCP) in the preparation of brushite cement, the pore size decreases in direct proportion to the amount of Si from micro- to nano-size, and the surface area increases [154].
Macroporosity provides blood with access to contact surfaces, and allows angiogenesis [146][155]. The absence of macroporosity is solved by introducing soluble additives and removing them before or after implantation (in vivo).
4. Calcium Phosphate Cements as Carriers of Functional Substances
The incorporation of active molecules into calcium phosphate cement can be achieved by dissolving it in the liquid phase, by mixing with the powder phase, or by simultaneously mixing with the powder and liquid phases [238], including with granules of resorbable fillers. The surface adsorption of drugs to the cement surface by incubating the scaffold in a drug solution is another possible approach. Surface impregnation for cements is rarely used. The kinetic release of drugs depends on the functionalization, microstructure and resorbability of the CPC matrix [116,129]. The gradual release of drugs is ensured by a homogeneous distribution in the cement stone volume, which can be effective in the treatment of various bone diseases such as tumors, osteoporosis or osteomyelitis [115]. Cells, injectable calcium phosphate cements, and functional agents, used alone or in combination, can promote tissue regeneration in a minimally invasive manner to restore function, reduce risk, reduce complications, and reduce treatment costs [34].
The functionalization of calcium phosphate cements is of great clinical interest for the treatment or prevention of various bone diseases. The main requirement of this for all carrier materials is prolonged elution. The release of functional substances depends on many factors:
- -
-
Resorption rate (depends on crystallinity, porosity, phase composition, presence of additives, surface roughness, L/P ratio, molding method, curing conditions, geometric shape and matrix size);
- -
-
Size and size distribution of pores (depends on the phase composition, presence and concentration of additives and their nature, L/P ratio, molding method, hardening conditions);
- -
-
pH of the cement stone (depends on the phase composition, solubility);
- -
-
Solubility of a functional substance (depending on the type, chemical nature);
- -
-
The possibility of interaction between the functional substance and the matrix (depends on the chemical formula);
- -
-
The size of the molecule of the functional substance;
- -
-
Quantity and uniformity of distribution of the immobilized functional substance;
- -
-
Method of immobilization of the functional substance;
- -
-
Type of supply of calcium phosphate cement (paste or cement stone).
The release of functional substances also depends on environmental factors. There are specific interactions between the ions formed during the dissolution of the drug and the ions of saline buffer solutions. This may play an important role in determining release mechanisms and in shaping the release profile [239]. Saline buffer solutions such as PBS or SBF promote the precipitation of HA and its deposition on the surface of the cement. It acts as a barrier to the diffusion of drugs from the volume, thereby reducing the release rate [137].
Environmental conditions are different in experiments in vivo and in vitro. This affects the release. The amount of vancomycin released in vivo has been reported to be half that of vancomycin released in vitro [240]. It is necessary to take into account that high concentrations of the antibiotic can adversely affect osteogenesis. It was reported that high concentrations of gentamicin sulfate inhibited the production of alkaline phosphatase by cells [241].
The kinetics of release of functional substances from calcium phosphate cements are controlled by diffusion, since matrices are resorbed more slowly compared to the release of functional substances. The mechanism of material resorption can be connected to the diffusion mechanism in the case of a more highly resorbable brushite cement [242,243].
The release curves of functional substances from calcium phosphate matrices most often show bimodal release. In this case, a typical initial release occurs within the first 24 h, followed by a sustained slow release [152]. This corresponds to the Higuchi model. It describes the release of functional substances from matrix systems as a diffusion process, based on Fick’s law and depending on the square root of time. The burst of release in the initial period of time reflects the slight diffusion of functional substances from the surface layer. After this period of time, diffusion from the inner surface of the matrix is somewhat difficult; this slows down the release of functional substances and thereby prolongs the release.
Multi-stage release profiles potentially offer much greater advantages over monotonic drug elution kinetics. The rapid initial release is able to effectively stop the pathological process, while its longer second release phase will gradually support the healing process [245].
The rate of release of functional substances depends on the morphology of the cement stone particles, as it leads to a different degree of adsorption of the substance on the inner surface. Desorption of functional substances from the surface of calcium phosphates with needle morphology of crystals is higher compared to desorption with lamellar morphology [246].
The surface charge of molecules of functional substances increases adsorption with the surface of calcium phosphate cement due to electrostatic attraction. Positively charged functional substances bind to the surface of calcium phosphate, since there are many negatively charged phosphate and hydroxide ions on the surface. Negatively charged functional substances bind to the surface of calcium phosphate with a large amount of calcium ions [247], such as cefaclor and ciprofloxacin, which have carboxyl groups [248]. The functional groups of BMP-2 (hydroxyl, amine and carboxyl) have a high affinity for calcium phosphates.
However, the presence of cephalexin has been reported to inhibit the growth of hydroxyapatite crystals. This is due to the ability of carboxylic acid molecules to be adsorbed on the surfaces of the initial components and nascent crystals, and to inhibit the growth of the target phase [249].
The values of the surface zeta potential are negative for calcium phosphates, but they differ depending on the arrangement of atoms on two types of crystal planes along the (a) axis and along the (c) axis [247]. Plane (a) is rich in positively charged calcium ions, and plane (c) is rich in negatively charged phosphate and hydroxide ions [250]. The different habitus of calcium phosphate crystals determines the different surface zeta potential due to the different arrangement of atoms. Negatively charged functional substances are easily adsorbed on needle-shaped crystals with developed (a) planes. Positively charged functional substances are adsorbed on lamellar crystals with developed (c) planes [246]. Neutral functional substances are adsorbed to a lesser extent and more easily desorbed from the surface of calcium phosphates, for example, metronidazole [248] or di(ethylenediamineplatinum)medronate [246]. Irregular crystals have intermediate zeta potentials and differently oriented planes [247].
Low crystallinity and high specific surface area allow the mobilization of more functional substances [251].
An increase in open porosity leads to an increase in surface area and the faster elution of functional substances from the cement matrix, since the drug solution is released through the phenomenon of capillary flow [252]. The volumetric flow rate of the eluted substance is proportional to the radius of the capillary. The influence of the pore size is more intense when it is similar to the size of the molecules of functional substances. Molecules of functional substances can freely diffuse from much larger pores. Release is controlled by diffusion to a greater extent than by structural factors [253]. This aspect is extremely important in the incorporation of giant molecules of some protein growth factors, such as the bone morphogenetic protein (BMP).
The release rate of functional substances from injection formulations is higher due to the setting period and the initial hardening time until the structure of the cement stone is formed. The kinetics depend on setting time, cohesion, microenvironment and hardening conditions [254,255]. Thus, drug release rates from brushite cement after 3 min and 1 h of curing showed explosive release during the first 8 h and slower release over 4 days. At the same time, the initial substances were found in the composition after 3 min. Most of the reagents turned into brushite after 1 h. The morphology of brushite crystals changed slightly from 1 to 15 h, which is confirmed by the values of the total porosity and tortuosity coefficient [255].
The functional substance should not impair the physical properties of the cements, and the cement should not change the active principle of the functional substance in delivery systems [238]. Brushite cements have a high ion concentration and an acid setting reaction—properties that can reduce or inhibit the effects of certain drugs [238].
In particular, the antibiotic groups of tetracyclines tend to chelate Ca2+ ions. This affects the primary formation of the nuclei of crystallization in brushite cements, prevents the deposition of minerals, and inhibits mineralization. Consequently, the incorporation of tetracyclines increases the setting time [256,257].
On the other hand, functional substances loaded in the form of salts into cement stone can affect the structure of the cement stone. For example, the addition of lidocaine hydrochloride increases the size of needle and plate CDHA crystals in TCP-based cement stone [239]. Increasing the size of the crystals ensures a good interaction between the preparation and the surface of the cement crystals. Large needles and plates provide greater adsorption, greater chemical binding and greater dissolution of lidocaine hydrochloride from the surface of cement crystals. This increases the release rate without changing the phase composition of the cement [239,258].
The excipients in the composition of pharmaceuticals ensure their shape, consistency, strength and degradation properties, and can affect the properties of cement stone. For example, sodium stearate inhibits the hydration of α-TCP and slows down the setting reaction. This contributes to an increase in the release rate of the functional substance at the first stage [137].
One of the options for changing the kinetics of the output of a functional agent is its encapsulation in a biodegradable polymer. As a result of encapsulation, the kinetics of elution are limited by diffusion from the polymer. Reduced initial explosive elution of the drug and more prolonged elution at a later date were observed when the substance was incorporated into PGLA microspheres, compared with direct incorporation [259].
The kinetics of elution of functional substances can be determined using the following methods: ultraviolet–visible spectroscopy (UV-Vis), high-performance liquid chromatography (HPLC) and fluorescent polarization immunoassay (FPIA). The electrochemical impedance spectroscopy (EIS) of Pasqual Group positions is a method for determining the amount of a drug without aliquot selection, as required by traditional methods [239].
5. Conclusions
The functionalization of calcium phosphate cement is one of the most important directions for solving the problems of the treatment and restoration of bone tissue.
Calcium phosphate cements have a chemical composition similar to the inorganic component of bone tissue. They are biocompatible, resorbable, injectable and self-hardening. They can take the form of a bone defect and fit tightly to the bone bed. The Ca2+ ion is an important homing signal; it brings together various cell types necessary to initiate bone remodeling. Ca2+ ions affect protein growth factors, and additionally stimulate the expression of osteogenic marker genes.
At present, the most common approach to using calcium phosphate cements as scaffolds is the bulk incorporation of anti-inflammatory, antitumor, antiresorptive, and osteogenic functional substances.
References
- Doblaré, M.; Garcıa, J.M.; Gómez, M.J. Modelling bone tissue fracture and healing: A review. Eng. Fract. Mech. 2004, 71, 1809–1840.
- Pajarinen, J.; Lin, T.; Gibon, E.; Kohno, Y.; Maruyama, M.; Nathan, K.; Lu, L.; Yao, Z.; Goodman, S.B. Mesenchymal stem cell-macrophage crosstalk and bone healing. Biomaterials 2019, 196, 80–89.
- Zhang, X.; Hassan, M.G.; Scheller, E.L. Neural regulation of bone marrow adipose tissue. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101522.
- Li, Y.; Meng, Y.; Yu, X. The unique metabolic characteristics of bone marrow adipose tissue. Front. Endocrinol. 2019, 10, 69.
- Jouret, F.; Wu, J.; Hull, M.; Rajendran, V.; Mayr, B.; Schöfl, C.; Geibel, J.; Caplan, M.J. Activation of the Ca2+-sensing receptor induces deposition of tight junction components to the epithelial cell plasma membrane. J. Cell Sci. 2013, 126, 5132–5142.
- Kitay, A.M.; Geibel, J.P. Stomach and bone. In Understanding the Gut-Bone Signaling Axis: Mechanisms and Therapeutic Implications; Springer: Berlin/Heidelberg, Germany, 2017; pp. 97–131.
- Rammelt, S. Management of ankle fractures in the elderly. EFORT Open Rev. 2016, 1, 239–246.
- Qiu, Z.-Y.; Cui, Y.; Wang, X.-M. Natural Bone Tissue and Its Biomimetic. In Mineralized Collagen Bone Graft Substitutes; Woodhead Publishing: Sawston, UK, 2019; pp. 1–22.
- Ho-Shui-Ling, A.; Bolander, J.; Rustom, L.E.; Johnson, A.W.; Luyten, F.P.; Picart, C. Bone regeneration strategies: Engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials 2018, 180, 143–162.
- Ansari, M. Bone tissue regeneration: Biology, strategies and interface studies. Prog. Biomater. 2019, 8, 223–237.
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2014, 11, 45–54.
- Dimitriou, R.; Tsiridis, E.; Giannoudis, P.V. Current concepts of molecular aspects of bone healing. Injury 2005, 36, 1392–1404.
- Schindeler, A.; McDonald, M.M.; Bokko, P.; Little, D.G. Bone remodeling during fracture repair: The cellular picture. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2008; Volume 19, pp. 459–466.
- Sikavitsas, V.I.; Temenoff, J.S.; Mikos, A.G. Biomaterials and bone mechanotransduction. Biomaterials 2001, 22, 2581–2593.
- Radi, Z.A.; Khan, N.K. Effects of cyclooxygenase inhibition on bone, tendon, and ligament healing. J. Inflamm. Res. 2005, 54, 358–366.
- Foster, A.L.; Moriarty, T.F.; Zalavras, C.; Morgenstern, M.; Jaiprakash, A.; Crawford, R.; Burch, M.-A.; Boot, W.; Tetsworth, K.; Miclau, T.; et al. The influence of biomechanical stability on bone healing and fracture-related infection: The legacy of Stephan Perren. Injury 2021, 52, 43–52.
- Pountos, I.; Georgouli, T.; Blokhuis, T.J.; Pape, H.C.; Giannoudis, P.V. Pharmacological agents and impairment of fracture healing: What is the evidence? Injury 2008, 39, 384–394.
- Zhou, J.; Zhang, Z.; Joseph, J.; Zhang, X.; Ferdows, B.E.; Patel, D.N.; Chen, W.; Banfi, G.; Molinaro, R.; Cosco, D.; et al. Biomaterials and nanomedicine for bone regeneration: Progress and future prospects. Exploration 2021, 1, 20210011.
- Perren, S.M. Evolution of the internal fixation of long bone fractures. The scientific basis of biological internal fixation: Choosing a new balance between stability and biology. J. Bone Jt. Surg. Br. 2002, 84, 1093–1110.
- Metsemakers, W.J.; Kuehl, R.; Moriarty, T.F.; Richards, R.G.; Verhofstad, M.H.J.; Borens, O.; Kates, S.; Morgenstern, M. Infection after fracture fixation: Current surgical and microbiological concepts. Injury 2018, 49, 511–522.
- Masquelet, A.C.; Fitoussi, F.; Begue, T.; Muller, G.P. Reconstruction of the long bones by the induced membrane and spongy autograft. Ann. Chir. Plast. Esthet. 2000, 45, 346–353.
- Court-Brown, C.M.; Biant, L.C.; Clement, N.D.; Bugler, K.E.; Duckworth, A.D.; McQueen, M.M. Open fractures in the elderly. The importance of skin ageing. Injury 2015, 46, 189–194.
- Toosi, S.; Behravan, J. Osteogenesis and bone remodeling: A focus on growth factors and bioactive peptides. Biofactors 2019, 46, 326–340.
- Siddiqui, J.A.; Partridge, N.C. Physiological bone remodeling: Systemic regulation and growth factor involvement. Physiology 2016, 31, 233–245.
- Yun, Y.R.; Jang, J.H.; Jeon, E.; Kang, W.; Lee, S.; Won, J.E.; Kim, H.W.; Wall, I. Administration of growth factors for bone regeneration. Regen. Med. 2012, 7, 369–385.
- Goodman, S.; Ma, T.; Trindade, M.; Ikenoue, T.; Matsuura, I.; Wong, N.; Fox, N.; Genovese, M.; Regula, D.; Smith, R.L. COX-2 selective NSAID decreases bone ingrowth in vivo. J. Orthop. Res. 2002, 20, 1164–1169.
- Reikeraas, O.; Engebretsen, L. Effects of ketoralac tromethamine and indomethacin on primary and secondary bone healing. Arch. Orthop. Traum. Surg. 1998, 118, 50–52.
- Amanat, N.; Brown, R.; Bilston, L.E.; Little, D.G. A single systemic dose of pamidronate improves bone mineral content and accelerates restoration of strength in a rat model of fracture repair. J. Orthop. Res. 2005, 235, 1029–1034.
- Mashiba, T.; Hirano, T.; Turner, C.H.; Forwood, M.R.; Johnston, C.C.; Burr, D.B. Suppressed bone turnover by bisphosphonates increases microdamage accumulation and reduces some biomechanical properties in dog rib. J. Bone Miner. Res. 2000, 15, 613–620.
- Zhong, C.; Wu, Y.; Lin, H.; Liu, R. Advances in the antimicrobial treatment of osteomyelitis. Compos. B Eng. 2022, 249, 110428.
- Kavanagh, N.; Ryan, E.J.; Widaa, A.; Sexton, G.; Fennell, J.; O’Rourke, S.; Cahill, K.C.; Kearney, C.J.; O’Brien, F.J.; Kerrigan, S.W. Staphylococcal osteomyelitis: Disease progression, treatment challenges, and future directions. Clin. Microbiol. Rev. 2018, 31, e00084-17.
- Chen, Z.Y.; Gao, S.; Zhang, Y.W.; Zhou, R.B.; Zhou, F. Antibacterial biomaterials in bone tissue engineering. J. Mater. Chem. B 2021, 9, 2594–2612.
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409.
- Kernéis, S.; Plainvert, C.; Barnier, J.P.; Tazi, A.; Dmytruk, N.; Gislain, B.; Loubinoux, J.; Sayed, F.E.; Cattoir, V.; Desplaces, N.; et al. Clinical and microbiological features associated with group B Streptococcus bone and joint infections, France 2004–2014. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1679–1684.
- Costa-Pinto, A.R.; Lemos, A.L.; Tavaria, F.K.; Pintado, M. Chitosan and hydroxyapatite based biomaterials to circumvent periprosthetic joint infections. Materials 2021, 14, 804.
- Cherifi, S.; Byl, B.; Deplano, A.; Nonhoff, C.; Denis, O.; Hallin, M. Comparative epidemiology of Staphylococcus epidermidis isolates from patients with catheter-related bacteremia and from healthy volunteers. J. Clin. Microbiol. 2013, 51, 1541–1547.
- Li, B.; Webster, T.J. Bacteria antibiotic resistance: New challenges and opportunities for implant—Associated orthopedic infections. J. Orthop. Res. 2018, 36, 22–32.
- Campoccia, D.; Montanaro, L.; Von Eiff, C.; Pirini, V.; Ravaioli, S.; Becker, K.; Arciola, C.R. Cluster analysis of ribotyping profiles of Staphylococcus epidermidis isolates recovered from foreign body—Associated orthopedic infections. J. Biomed. Mater. Res. A 2009, 88, 664–672.
- Campoccia, D.; Baldassarri, L.; Pirini, V.; Ravaioli, S.; Montanaro, L.; Arciola, C.R. Molecular epidemiology of Staphylococcus aureus from implant orthopaedic infections: Ribotypes, agr polymorphism, leukocidal toxins and antibiotic resistance. Biomaterials 2008, 29, 4108–4116.
- Sarkissian, E.J.; Gans, I.; Gunderson, M.A.; Myers, S.H.; Spiegel, D.A.; Flynn, J.M. Community-acquired Methicillin-resistant Staphylococcus aureus Musculoskeletal Infections. J. Pediatr. Orthop. 2016, 36, 323–327.
- Lew, D.P.; Waldvogel, F.A. Osteomyelitis. Lancet 2004, 364, 369–379.
- Hudson, M.C.; Ramp, W.K.; Nicholson, N.C.; Williams, A.S.; Nousiainen, M.T. Internalization of Staphylococcus aureus by cultured osteoblasts. Microb. Pathog. 1995, 19, 409–419.
- Redlich, K.; Smolen, J.S. Inflammatory bone loss: Pathogenesis and therapeutic intervention. Nat. Rev. Drug Discov. 2012, 11, 234–250.
- Stewart, P.S. Mechanisms of antibiotic resistance in bacterial biofilms. Int. J. Med. Microbiol. 2002, 292, 107–113.
- Ene, R.; Nica, M.; Ene, D.; Cursaru, A.; Cirstoiu, C. Review of calcium-sulphate-based ceramics and synthetic bone substitutes used for antibiotic delivery in PJI and osteomyelitis treatment. EFORT Open Rev. 2021, 6, 297.
- Van de Belt, H.; Neut, D.; Schenk, W.; van Horn, J.R.; van der Mei, H.C.; Busscher, H.J. Infection of orthopedic implants and the use of antibiotic-loaded bone cements: A review. Acta Orthop. Scand. 2001, 72, 557–571.
- Calhoun, J.H.; Manring, M.M. Adult Osteomyelitis. Infect. Dis. Clin. N. Am. 2005, 19, 765–786.
- Gruber, H.E. Bone and the immune system. Proc. Soc. Exp. Biol. Med. 1991, 197, 219–225.
- Hanssen, A.D. Local antibiotic delivery vehicles in the treatment of musculoskeletal infection. Clin. Orthop. Relat. Res. 2005, 437, 91–96.
- Wiesli, M.G.; Kaiser, J.P.; Gautier, E.; Wick, P.; Maniura-Weber, K.; Rottmar, M.; Wahl, P. Influence of ceftriaxone on human bone cell viability and in vitro mineralization potential is concentration-and time-dependent. Bone Jt. Res. 2021, 10, 218–225.
- Fuchs, B.; Ossendorf, C.; Leerapun, T.; Sim, F.H. Intercalary segmental reconstruction after bone tumor resection. Eur. J. Surg. Oncol. 2008, 34, 1271–1276.
- Zekry, K.M.; Yamamoto, N.; Hayashi, K.; Takeuchi, A.; Alkhooly, A.Z.A.; Abd-Elfattah, A.S.; Elsaid, A.N.S.; Ahmed, A.R.; Tsuchiya, H. Reconstruction of intercalary bone defect after resection of malignant bone tumor. J. Orthop. Surg. 2019, 27, 2309499019832970.
- Kanakis, I.; Kousidou, O.C.; Karamanos, N.K. In vitro and in vivo antiresorptive effects of bisphosphonates in metastatic bone disease. In Vivo 2005, 19, 311–318.
- Phull, S.S.; Yazdi, A.R.; Ghert, M.; Towler, M.R. Bone cement as a local chemotherapeutic drug delivery carrier in orthopedic oncology: A review. J. Bone Oncol. 2021, 26, 100345.
- Roodman, G.D. Mechanisms of Bone Metastasis. N. Engl. J. Med. 2004, 350, 1655–1664.
- Oades, G.M.; Coxon, J.; Colston, K.W. The potential role of bisphosphonates in prostate cancer. Prostate Cancer Prostatic Dis. 2002, 5, 264–272.
- Zagzag, J.; Hu, M.I.; Fisher, S.B.; Perrier, N.D. Hypercalcemia and cancer: Differential diagnosis and treatment. CA Cancer J. Clin. 2018, 68, 377–386.
- Mbese, Z.; Aderibigbe, B.A. Bisphosphonate-Based conjugates and derivatives as potential therapeutic agents in osteoporosis, bone cancer and metastatic bone cancer. Int. J. Mol. Sci. 2021, 22, 6869.
- Berenson, J.R.; Rosen, L.S.; Howell, A.; Porter, L.; Coleman, R.E.; Morley, W.; Dreicer, R.; Kuross, S.A.; Lipton, A.; Seaman, J.J. Zoledronic acid reduces skeletal-related events in patients with osteolytic metastases: A double-blind, randomized dose–response study. Cancer 2001, 91, 1191–1200.
- Mirels, H. Metastatic Disease in Long Bones a Proposed Scoring System for Diagnosing Impending Pathologic Fractures. Clin. Orthop. Relat. Res. 1989, 249, 256–264.
- Chen, T.H.; Chen, W.M.; Huang, C.K. Reconstruction after intercalary resection of malignant bone tumours: Comparison between segmental allograft and extracorporeally-irradiated autograft. J. Bone Jt. Surg. Br. Vol. 2005, 87, 704–709.
- Harvey, N.; Ahlmann, E.R.; Allison, D.C.; Wang, L.; Menendez, L.R. Endoprostheses last longer than intramedullary devices in proximal femur metastases. Clin. Orthop. Relat. Res. 2012, 470, 684–691.
- Zhao, J.; Yu, X.C.; Xu, M.; Zheng, K.; Hu, Y.C.; Wang, F.; Lun, D.X. Intercalary prosthetic reconstruction for pathologic diaphyseal humeral fractures due to metastatic tumors: Outcomes and improvements. J. Shoulder Elb. Surg. 2018, 27, 2013–2020.
- Henrichs, M.P.; Krebs, J.; Gosheger, G.; Streitbuerger, A.; Nottrott, M.; Sauer, T.; Hoell, S.; Singh, G.; Hardes, J. Modular tumor endoprostheses in surgical palliation of long-bone metastases: A reduction in tumor burden and a durable reconstruction. World J. Surg. Oncol. 2014, 12, 330.
- Bernthal, N.M.; Schwartz, A.J.; Oakes, D.A.; Kabo, J.M.; Eckardt, J.J. How long do endoprosthetic reconstructions for proximal femoral tumors last? Clin. Orthop. Relat. Res. 2010, 468, 2867–2874.
- Bickels, J.; Wittig, J.C.; Kollender, Y.; Henshaw, R.M.; Kellar-Graney, K.L.; Meller, I.; Malawer, M.M. Distal femur resection with endoprosthetic reconstruction: A long-term followup study. Clin. Orthop. Relat. Res. 2002, 400, 225–235.
- Wafa, H.; Reddy, K.; Grimer, R.; Abudu, A.; Jeys, L.; Carter, S.; Tillman, R. Does total humeral endoprosthetic replacement provide reliable reconstruction with preservation of a useful extremity? Clin. Orthop. Relat. Res. 2015, 473, 917–925.
- Tan, B.; Tang, Q.; Zhong, Y.; Wei, Y.; He, L.; Wu, Y.; Wu, J.; Liao, J. Biomaterial-based strategies for maxillofacial tumour therapy and bone defect regeneration. Int. J. Oral Sci. 2021, 13, 9.
- Tanzawa, Y.; Tsuchiya, H.; Shirai, T.; Nishida, H.; Hayashi, K.; Takeuchi, A.; Tomita, K.; Kawahara, M. Potentiation of the antitumor effect of calcium phosphate cement containing anticancer drug and caffeine on rat osteosarcoma. J. Orthop. Sci. 2011, 16, 77–84.
- Donanzam, B.A.; Campos, T.P.R.; Dalmázio, I.; Valente, E.S. Synthesis and Characterization of Calcium Phosphate Loaded with Ho-166 and Sm-153: A Novel Biomaterial for Treatment of Spine Metastases. J. Mater. Sci. Mater. Med. 2013, 24, 2873–2880.
- Dolci, L.S.; Panzavolta, S.; Torricelli, P.; Albertini, B.; Sicuro, L.; Fini, M.; Bigi, A.; Passerini, N. Modulation of Alendronate release from a calcium phosphate bone cement: An in vitro osteoblast-osteoclast co-culture study. Int. J. Pharm. 2019, 554, 245–255.
- Wang, X.H.; Jia, S.J.; Hao, D.J. Advances in the modification of injectable calcium-phosphate-based bone cements for clinical application. Chin. Med. J. 2020, 133, 2610–2612.
- Natesan, V.; Kim, S.J. Metabolic bone diseases and new drug developments. Biomol. Ther. 2022, 30, 309.
- Anderson, H.C.; Harmey, D.; Camacho, N.P.; Garimella, R.; Sipe, J.B.; Tague, S.; Bi, X.; Johnson, K.; Terkeltaub, R.; Millán, J.L. Sustained osteomalacia of long bones despite major improvement in other hypophosphatasia-related mineral deficits in tissue nonspecific alkaline phosphatase/nucleotide pyrophosphatase phosphodiesterase 1 double-deficient mice. Am. J. Pathol. 2005, 166, 1711–1720.
- Albahri, A.S.; Alwan, J.K.; Taha, Z.K.; Ismail, S.F.; Hamid, R.A.; Zaidan, A.A.; Albahri, O.S.; Zaidan, B.B.; Alamoodi, A.H.; Alsalem, M.A. IoT-based telemedicine for disease prevention and health promotion: State-of-the-art. J. Netw. Comput. Appl. 2021, 173, 102873.
- Anastasilakis, A.D.; Polyzos, S.A.; Makras, P. Therapy of endocrine disease: Denosumab vs bisphosphonates for the treatment of postmenopausal osteoporosis. Eur. J. Endocrinol. 2018, 179, R31–R45.
- Drake, M.T.; Clarke, B.L.; Lewiecki, E.M. The pathophysiology and treatment of osteoporosis. Clin. Ther. 2015, 37, 1837–1850.
- Chinoy, A.; Mughal, M.Z.; Padidela, R. Metabolic bone disease of prematurity: Causes, recognition, prevention, treatment and long-term consequences. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F560–F566.
- Fan, Y.; Hanai, J.I.; Le, P.T.; Bi, R.; Maridas, D.; DeMambro, V.; Figueroa, C.A.; Kir, S.; Zhou, X.; Mannstadt, M.; et al. Parathyroid hormone directs bone marrow mesenchymal cell fate. Cell Metab. 2017, 25, 661–672.
- Fleisch, H. Bisphosphonates in osteoporosis. Eur. Spine J. 2003, 12, S142–S146.
- Fabre, S.; Funck-Brentano, T.; Cohen-Solal, M. Anti-sclerostin antibodies in osteoporosis and other bone diseases. J. Clin. Med. 2020, 9, 3439.
- Carbone, L.D.; Gonzalez, B.; Miskevics, S.; Ray, C.; Etingen, B.; Guihan, M.; Craven, B.C.; George, V.; Weaver, F.M. Association of bisphosphonate therapy with incident of lower extremity fractures in persons with spinal cord injuries or disorders. Arch. Phys. Med. Rehabil. 2020, 101, 633–641.
- Dvorak, M.M.; Riccardi, D. Ca2+ as an extracellular signal in bone. Cell Calcium 2004, 35, 249–255.
- Wassif, R.K.; Elkayal, M.; Shamma, R.N.; Elkheshen, S.A. Recent advances in the local antibiotics delivery systems for management of osteomyelitis. Drug Deliv. 2021, 28, 2392–2414.
- Nandi, S.K.; Bandyopadhyay, S.; Das, P.; Samanta, I.; Mukherjee, P.; Roy, S.; Kundu, B. Understanding osteomyelitis and its treatment through local drug delivery system. Biotechnol. Adv. 2016, 34, 1305–1317.
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247.
- Galusha, A.L.; Howard, L.J.; Kruger, P.C.; Marks, T.; Parsons, P.J. Bone mineral composition among long-term parenteral nutrition patients: Postmortem assessment of calcium, phosphorus, magnesium, and select trace elements. J. Parenter. Enteral. Nutr. 2020, 45, 175–182.
- Xing, T.; Hu, Y.; Wang, B.; Zhu, J. Role of oral calcium supplementation alone or with vitamin D in preventing post-thyroidectomy hypocalcaemia: A meta-analysis. Medicine 2019, 98, e14455.
- Song, Y.; Xu, L.; Jin, X.; Chen, D.; Jin, X.; Xu, G. Effect of calcium and magnesium on inflammatory cytokines in accidentally multiple fracture adults: A short-term follow-up. Medicine 2022, 101, e28538.
- Chapuy, M.C.; Arlot, M.E.; Duboeuf, F.; Brun, J.; Crouzet, B.; Arnaud, S.; Delmas, P.D.; Meunier, P.J. Vitamin D3 and calcium to prevent hip fractures in elderly women. N. Engl. J. Med. 1992, 327, 1637–1642.
- Notomi, T.; Kuno, M.; Hiyama, A.; Nozaki, T.; Ohura, K.; Ezura, Y.; Noda, M. Role of lysosomal channel protein TPC2 in osteoclast differentiation and bone remodeling under normal and low-magnesium conditions. J. Biol. Chem. 2017, 292, 20998–21010.
- Pi, M.; Faber, P.; Ekema, G.; Jackson, P.D.; Ting, A.; Wang, N.; Fontilla-Poole, M.; Mays, R.W.; Brunden, K.R.; Harrington, J.J.; et al. Identification of a novel extracellular cation-sensing G-protein-coupled receptor. J. Biol. Chem. 2005, 280, 40201–40209.
- Li, W.; Chen, W.; Lin, Y. The efficacy of parathyroid hormone analogues in combination with bisphosphonates for the treatment of osteoporosis: A meta-analysis of randomized controlled trials. Medicine 2015, 94, e1156.
- Che, J.; Yang, J.; Zhao, B.; Zhang, G.; Wang, L.; Peng, S.; Shang, P. The effect of abnormal iron metabolism on osteoporosis. Biol. Trace Elem. Res. 2020, 195, 353–365.
- Krenzlin, H.; Foelger, A.; Mailänder, V.; Blase, C.; Brockmann, M.; Düber, C.; Ringel, C.; Keric, N. Novel biodegradable composite of calcium phosphate cement and the collagen I mimetic P-15 for pedicle screw augmentation in osteoporotic bone. Biomedicines 2021, 9, 1392.
- Lin, Y.; Huang, S.; Zou, R.; Gao, X.; Ruan, J.; Weir, M.D.; Reynolds, M.A.; Qin, W.; Chang, X.; Fu, H.; et al. Calcium phosphate cement scaffold with stem cell co-culture and prevascularization for dental and craniofacial bone tissue engineering. Dent. Mater. 2019, 35, 1031–1041.
- Van Oirschot, B.; Mikos, A.G.; Liu, Q.; van den Beucken, J.J.; Jansen, J.A. Fast Degradable Calcium Phosphate Cement for Maxillofacial Bone Regeneration. Tissue Eng. Part A 2023, 29, 161–171.
- Lewandrowski, K.-U.; DGresser, J.; Wise, D.L.; Trantolo, D.J. Bioresorbable bone graft substitutes of different osteoconductivities: A histologic evaluation of osteointegration of poly(propylene glycol-co-fumaric acid)-based cement implants in rats. Biomaterials 2000, 21, 757–764.
- Pupilli, F.; Ruffini, A.; Dapporto, M.; Tavoni, M.; Tampieri, A.; Sprio, S. Design Strategies and Biomimetic Approaches for Calcium Phosphate Scaffolds in Bone Tissue Regeneration. Biomimetics 2022, 7, 112.
- Tavoni, M.; Dapporto, M.; Tampieri, A.; Sprio, S. Bioactive calcium phosphate-based composites for bone regeneration. J. Compos. Sci. 2021, 5, 227.
- Lukina, Y.; Panov, Y.; Panova, L.; Senyagin, A.; Bionyshev-Abramov, L.; Serejnikova, N.; Kireynov, A.; Sivkov, S.; Gavryushenko, N.; Smolentsev, D.; et al. Chemically Bound Resorbable Ceramics as an Antibiotic Delivery System in the Treatment of Purulent–Septic Inflammation of Bone Tissue. Ceramics 2022, 5, 26.
- Skriabin, A.S.; Shakurov, A.V.; Vesnin, V.R.; Lukina, Y.S.; Tsygankov, P.A.; Bionyshev-Abramov, L.L.; Serejnikova, N.B.; Vorob’ev, E.V. Titanium Membranes with Hydroxyapatite/Titania Bioactive Ceramic Coatings: Characterization and In Vivo Biocompatibility Testing. ACS Omega 2022, 7, 47880–47891.
- Drevet, R.; Benhayoune, H. Electrodeposition of Calcium Phosphate Coatings on Metallic Substrates for Bone Implant Applications: A Review. Coatings 2022, 12, 539.
- Lukina, Y.; Kotov, S.; Bionyshev-Abramov, L.; Serejnikova, N.; Chelmodeev, R.; Fadeev, R.; Toshev, O.; Tavtorkin, A.; Ryndyk, M.; Smolentsev, D.; et al. Low-Temperature Magnesium Calcium Phosphate Ceramics with Adjustable Resorption Rate. Ceramics 2023, 6, 11.
- Lodoso-Torrecilla, I.; van den Beucken, J.J.; Jansen, J.A. Calcium phosphate cements: Optimization toward biodegradability. Acta Biomater. 2021, 119, 1–12.
- Safronova, T.V.; Selezneva, I.I.; Tikhonova, S.A.; Kiselev, A.S.; Davydova, G.A.; Shatalova, T.B.; Larionov, D.S.; Rau, J.V. Biocompatibility of biphasic α, β-tricalcium phosphate ceramics in vitro. Bioact. Mater. 2020, 5, 423–427.
- Yuan, H.; Li, Y.; de Bruijn, J.; de Groot, K.; Zhang, X. Tissue responses of calcium phosphate cement: A study in dogs. Biomaterials 2000, 21, 1283–1290.
- Hurle, K.; Oliveira, J.M.; Reis, R.L.; Pina, S.; Goetz-Neunhoeffer, F. Ion-doped brushite cements for bone regeneration. Acta Biomater. 2021, 123, 51–71.
- Bohner, M. Physical and chemical aspects of calcium phosphates used in spinal surgery. Eur. Spine J. 2001, 10 (Suppl. S2), S114–S121.
- Guo, H.; Su, J.; Wei, J.; Kong, H.; Liu, C. Biocompatibility and osteogenicity of degradable Ca-deficient hydroxyapatite scaffolds from calcium phosphate cement for bone tissue engineering. Acta Biomater. 2009, 5, 268–278.
- Barba, A.; Maazouz, Y.; Diez-Escudero, A.; Rappe, K.; Espanol, M.; Montufar, E.B.; Öhman-Mägi, C.; Persson, C.; Fontecha, P.; Manzanares, M.-C.; et al. Osteogenesis by foamed and 3D-printed nanostructured calcium phosphate scaffolds: Effect of pore architecture. Acta Biomater. 2018, 79, 135–147.
- Takeyama, H.; Maruta, M.; Sato, T.; Kajimoto, N.; Fujii, E.; Matsuura, T.; Tsuru, K. Fabrication of bioresorbable hydroxyapatite bone grafts through the setting reaction of calcium phosphate cement. Dent. Mater. J. 2022, 41, 882–888.
- Wu, Y.; Woodbine, L.; Carr, A.M.; Pillai, A.R.; Nokhodchi, A.; Maniruzzaman, M. 3D printed calcium phosphate cement (CPC) scaffolds for anti-cancer drug delivery. Pharmaceutics 2020, 12, 1077.
- Trombetta, R.P.; Ninomiya, M.J.; El-Atawneh, I.M.; Knapp, E.K.; Bentley, K.L.D.M.; Dunman, P.M.; Schwarz, E.M.; Kates, S.L.; Awad, H. Calcium Phosphate Spacers for the Local Delivery of Sitafloxacin and Rifampin to Treat Orthopedic Infections: Efficacy and Proof of Concept in a Mouse Model of Single-Stage Revision of Device-Associated Osteomyelitis. Pharmaceutics 2019, 11, 94.
- Bagnol, R.; Sprecher, C.; Peroglio, M.; Chevalier, J.; Mahou, R.; Büchler, P.; Richards, G.; Eglin, D. Coaxial micro-extrusion of a calcium phosphate ink with aqueous solvents improves printing stability, structure fidelity and mechanical properties. Acta Biomater. 2021, 125, 322–332.
- Irbe, Z.; Loca, D. Soluble phosphate salts as setting aids for premixed calcium phosphate bone cement pastes. Ceram. Int. 2021, 47, 24012–24019.
- Criado, A.; Yokhana, S.; Rahman, T.; McCarty, S.; Andrecovich, C.; Ren, W.; Yassir, W.K. Biomechanical strength comparison of pedicle screw augmentation using poly-dicalcium phosphate dihydrate (P-DCPD) and polymethylmethacrylate (PMMA) cements. Spine Deform. 2020, 8, 165–170.
- Kucko, N.W.; Li, W.; García Martinez, M.A.; Rehman, I.U.; Ulset, A.S.T.; Christensen, B.E.; Leewenburgh, S.C.G.; Herber, R.P. Sterilization effects on the handling and degradation properties of calcium phosphate cements containing poly (D, L–lactic–co–glycolic acid) porogens and carboxymethyl cellulose. J. Biomed. Mater. Res. 2019, 107, 2216–2228.
- Cichoń, E.; Czechowska, J.P.; Krok-Borkowicz, M.; Allinson, S.L.; Stępień, K.; Smith, A.; Douglas, T.E.L.; Zima, A. Biosurfactants as foaming agents in calcium phosphate bone cements. Biomater. Adv. 2023, 145, 213273.
- De Lacerda Schickert, S.; Jansen, J.A.; Bronkhorst, E.M.; van den Beucken, J.J.; Leeuwenburgh, S.C. C. Stabilizing dental implants with a fiber-reinforced calcium phosphate cement: An in vitro and in vivo study. Acta Biomater. 2020, 110, 280–288.
- Fosca, M.; Rau, J.V.; Uskoković, V. Factors influencing the drug release from calcium phosphate cements. Bioact. Mater. 2022, 7, 341–363.
- Schröter, L.; Kaiser, F.; Stein, S.; Gbureck, U.; Ignatius, A. Biological and mechanical performance and degradation characteristics of calcium phosphate cements in large animals and humans. Acta Biomater. 2020, 117, 1–20.
- Safronova, T.V. Inorganic Materials for Regenerative Medicine. Inorg. Mater. 2021, 57, 443–474.
- Aberg, J.; Brisby, H.; Henriksson, H.; Lindahl, A.; Thomsen, P.; Engqvist, H. Premixed acidic calcium phosphate cement: Characterization of strength and microstructure. J. Biomed. Mater. Res. 2010, 93, 436–441.
- Bohner, M.; Doebelin, N.; Baroud, G. Theoretical and experimental approach to test the cohesion of calcium phosphate pastes. Eur. Cell. Mater. 2006, 12, 26–35.
- Martin, R.I.; Brown, P.W. Formation of hydroxyapatite in serum. J. Mater. Sci. Mater. Med. 1994, 5, 96–102.
- Eanes, E.; Hailer, A. Anionic Effects on the Size and Shape of Apatite Crystals Grown from Physiological Solutions. Calcif. Tissue Int. 2000, 66, 449–455.
- Miyamoto, Y.; Ishikawa, R.; Fukao, H.; Sawada, M.; Nagayama, M.; Kon, M.; Asaoka, K. In vivo setting behavior of fast-setting calcium phosphate cement. Biomaterials 1995, 16, 855–860.
- Moreno, D.; Vargas, F.; Ruiz, J.; López, M.E. Solid-state synthesis of alpha tricalcium phosphate for cements used in biomedical applications. Bol. Soc. Esp. Ceram. Vidr. 2020, 59, 193–200.
- Hurle, K.; Neubauer, J.; Bohner, M.; Doebelin, N.; Goetz-Neunhoeffer, F. Effect of amorphous phases during the hydraulic conversion of α-TCP into calcium-deficient hydroxyapatite. Acta Biomater. 2014, 10, 3931–3941.
- Brunner, T.J.; Grass, R.N.; Bohner, M.; Stark, W.J. Effect of particle size, crystal phase and crystallinity on the reactivity of tricalcium phosphate cements for bone reconstruction. J. Mater. Chem. 2007, 17, 4072–4078.
- Bohner, M.; Brunner, T.J.; Stark, W.J. Controlling the reactivity of calcium phosphate cements. J. Mater. Chem. 2008, 18, 5669–5675.
- Liu, C.; Shao, H.; Chen, W.; Zheng, H. Effect of granularity of raw materials on the hydration and hardening process of calcium phosphate cement. Biomaterials 2003, 24, 4103–4113.
- Bermudez, O.; Boltong, M.G.; Driessens, F.C.M.; Planell, J.A. Development of an octocalcium phosphate cement. Mater. Sci. Mater. Med. 1994, 5, 144–146.
- Brown, P.W.; Hocker, N.; Hoyle, S. Variations in Solution Chemistry During the Low-Temperature Formation of Hydroxyapatite. J. Am. Ceram. Soc. 1991, 74, 1848–1854.
- Saleh, A.T.; Ling, L.S.; Hussain, R. Injectable magnesium-doped brushite cement for controlled drug release application. J. Mater. Sci. 2016, 51, 7427–7439.
- Hofmann, M.P.; Mohammed, A.R.; Perrie, Y.; Gbureck, U.; Barralet, J.E. High-strength resorbable brushite bone cement with controlled drug-releasing capabilities. Acta Biomater. 2009, 5, 43–49.
- Mirtchi, A.; Lemaitre, J.; Munting, E. Calcium phosphate cements: Action of setting regulators in the properties of the β-tricalcium phosphate—Monocalcium phosphate cements. Biomaterials 1989, 10, 475–480.
- Xu, H.H.K.; Eichmiller, F.C.; Giuseppetty, A.A. Reinforcement of a self-setting calcium phosphate cement with different fibers. J. Biomed. Mater. Res. 2000, 52, 107–114.
- Nezafati, N.; Moztarzadeh, F.; Hesaraki, S.; Mozafari, M. Synergistically reinforcement of a self-setting calcium phosphate cement with bioactive glass fibers. Ceram. Int. 2011, 37, 927–934.
- White, A.A.; Best, S.M.; Kinloch, I.A. Hydroxyapatite–carbon nanotube composites for biomedical applications: A review International. J. Appl. Ceram. Technol. 2007, 4, 1–13.
- Zuo, Y.; Yang, F.; Wolke, J.G.; Li, Y.; Jansen, J.A. Incorporation of biodegradable electrospun fibers into calcium phosphate cement for bone regeneration. Acta Biomater. 2010, 6, 1238–1247.
- Maenz, S.; Kunisch, E.; Mühlstädt, M.; Böhm, A.; Kopsch, V.; Bossert, J.; Kinne, R.W.; Jandt, K.D. Enhanced mechanical properties of a novel, injectable, fiber-reinforced brushite cement. J. Mech. Behav. Biomed. Mater. 2014, 39, 328–338.
- Kucko, N.W.; de Lacerda Schickert, S.; Sobral Marques, T.; Herber, R.P.; Van den Beuken, J.J.; Zuo, Y.; Leeuwenburgh, S.C. Tough and osteocompatible calcium phosphate cements reinforced with poly (vinyl alcohol) fibers. ACS Biomater. Sci. Eng. 2019, 5, 2491–2505.
- Pan, Z.; Jiang, P.; Fan, Q.; Ma, B.; Cai, H. Mechanical and biocompatible influences of chitosan fiber and gelatin on calcium phosphate cement. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 82, 246–252.
- Yin, Y.; Ye, F.; Yao, K.; Cui, J.; Song, X. Gelatin manipulation of latent macropores formulation in brushite cement. J. Mater. Sci. Mater. Med. 2003, 14, 255–261.
- Lodoso-Torrecilla, I.; Stumpel, F.; Jansen, J.A.; van den Beucken, J.J. Early-stage macroporosity enhancement in calcium phosphate cements by inclusion of poly (N-vinylpyrrolidone) particles as a porogen. Mater. Today Commun. 2020, 23, 100901.
- Li, X.; Wang, Y.; Chen, F.; Chen, X.; Xiao, Y.; Zhang, X. Design of macropore structure and micro-nano topography to promote the early neovascularization and osteoinductivity of biphasic calcium phosphate bioceramics. Mater. Des. 2022, 216, 110581.
- Zhang, J.; Luo, X.; Barbieri, D.; Barradas, A.M.C.; de Bruijn, J.D.; van Blitterswijk, C.A.; Yuan, H. The Size of Surface Microstructures as an Osteogenic Factor in Calcium Phosphate—Ceramics. Acta Biomater. 2014, 10, 3254–3263.
- Chan, O.; Coathup, M.J.; Nesbitt, A.; Ho, C.Y.; Hing, K.A.; Buckland, T.; Campion, C.; Blunn, G.W. The effects of microporosity on osteoinduction of calcium phosphate bone graft substitute biomaterials. Acta Biomater. 2012, 8, 2788–2794.
- Barralet, J.E.; Grover, L.M.; Gbureck, U. Ionic modification of calcium phosphate cement viscosity. Part II: Hypodermic injection and strength improvement of brushite cement. Biomaterials 2004, 25, 2197–2203.
- Lukina, Y.S.; Panova, L.V.; Panov, Y.M.; Krut’ko, D.P.; Gavryushenko, N.S.; Lemenovskii, D.A. Application of SC-CO2 in the Technology of the Preparation of Calcium Phosphate Matrices for the Treatment of Septic Purulent Inflammations of Bone Tissues. Russ. J. Phys. Chem. B 2022, 16, 1221–1230.
- Espanol, M.; Perez, R.A.; Montufar, E.B.; Marichal, C.; Sacco, A.; Ginebra, M.P. Intrinsic porosity of calcium phosphate cements and its significance for drug delivery and tissue engineering applications. Acta Biomater. 2009, 5, 2752–2762.
- Lucas-Aparicio, J.; Manchón, Á.; Rueda, C.; Pintado, C.; Torres, J.; Alkhraisat, M.H.; López-Cabarcos, E. Silicon-calcium phosphate ceramics and silicon-calcium phosphate cements: Substrates to customize the release of antibiotics according to the idiosyncrasies of the patient. Mater. Sci. Eng. C 2020, 106, 110173.
- Takagi, S.; Chow, L. Formation of macropores in calcium phosphate cement implants. Mater. Sci. Mater. Med. 2001, 12, 135–139.
- Ginebra, M.P.; Canal, C.; Espanol, M.; Pastorino, D.; Montufar, E.B. Calcium phosphate cements as drug delivery materials. Adv. Drug Deliv. Rev. 2012, 64, 1090–1110.
- Pasqual, J.A.R.; Pereira, B.L.R.; Colpo, J.C.; Egea, J.R.J.; Santos, L.A.L.; Sousa, V.C. Monitoring of the interaction of calcium phosphate cement and lidocaine hydrochloride by electrochemical impedance spectroscopy during the drug release process. J. Appl. Electrochem. 2021, 51, 463–471.
- Uchida, K.; Sugo, K.; Nakajima, T.; Nakawaki, M.; Takano, S.; Nagura, N.; Takaso, M.; Urabe, K. In Vivo Release of Vancomycin from Calcium Phosphate Cement. BioMed Res. Int. 2018, 2018, 4560647.
- Liu, S.-M.; Chen, W.-C.; Ko, C.-L.; Chang, H.-T.; Chen, Y.-S.; Haung, S.-M.; Chang, K.-C.; Chen, J.-C. In Vitro Evaluation of Calcium Phosphate Bone Cement Composite Hydrogel Beads of Cross-Linked Gelatin-Alginate with Gentamicin-Impregnated Porous Scaffold. Pharmaceuticals 2021, 14, 1000.
- Haghbin-Nazarpak, M.; Moztarzadeh, F.; Solati-Hashjin, M.; Mirhabibi, A.R.; Tahriri, M. Preparation, characterization and gentamicin sulfate release investigation of biphasic injectable calcium phosphate bone cement. Ceram. Silikáty 2010, 54, 334–340.
- Khashaba, R.M.; Lockwood, P.E.; Lewis, J.B.; Messer, R.L.; Chutkan, N.B.; Borke, J.L. Cytotoxicity, calcium release, and pH changes generated by novel calcium phosphate cement formulations. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 93, 297–303.
- Lu, Z.; Tsai, M.; Lu, D.; Wang, J.; Wientjes, M.G.; Au, J.L.S. Tumor-penetrating microparticles for intraperitoneal therapy of ovarian cancer. J. Pharmacol. Exp. Ther. 2008, 327, 673–682.
- Palazzo, B.; Iafisco, M.; Laforgia, M.; Margiotta, N.; Natile, G.; Bianchi, C.L.; Walsh, B.; Mann, S.; Roveri, N. Biomimetic Hydroxyapatite–Drug Nanocrystals as Potential Bone Substitutes with Antitumor Drug Delivery Properties. Adv. Funct. Mater. 2007, 17, 2180–2188.
- Zhuang, Z.; Aizawa, M. Protein adsorption on single-crystal hydroxyapatite particles with preferred orientation to a(b)- and c-axes. J. Mater. Sci. Mater. Med. 2013, 24, 1211–1216.
- Akashi, A.; Matsuya, Y.; Unemori, M.; Akamine, A. Release profile of antimicrobial agents from α-tricalcium phosphate cement. Biomaterials 2001, 22, 2713–2717.
- Hesaraki, S.; Zamanian, A.; Moztarzadeh, F. The influence of the acidic component of the gas-foaming porogen used in preparing an injectable porous calcium phosphate cement on its properties: Acetic acid versus citric acid. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 86, 208–216.
- Peroos, S.; Du, Z.; de Leeuw, N.H. A computer modelling study of the uptake, structure and distribution of carbonate defects in hydroxy-apatite. Biomaterials 2006, 27, 2150–2161.
- Matsumoto, T.; Okazaki, M.; Inoue, M.; Yamaguchi, S.; Kusunose, T.; Toyonaga, T.; Hamada, Y.; Takahashi, J. Hydroxyapatite particles as a controlled release carrier of protein. Biomaterials 2004, 25, 3807–3812.
- Stähli, C.; Bohner, M.; Bashoor-Zadeh, M.; Doebelin, N.; Baroud, G. Aqueous impregnation of porous β-tricalcium phosphate scaffolds. Acta Biomater. 2010, 6, 2760–2772.
- Uskokovíc, V. Entering the Era of Nanoscience: Time to Be So Small. J. Biomed. Nanotechnol. 2013, 9, 1441–1470.
- Su, W.-Y.; Chen, Y.-C.; Lin, F.-H. A New Type of Biphasic Calcium Phosphate Cement as a Gentamicin Carrier for Osteomyelitis. Evid. Based Complement. Altern. Med. 2013, 2013, 801374.
- Mestres, G.; Kugiejko, K.; Pastorino, D.; Unosson, J.; Öhman, C.; Ott, M.K.; Ginebra M-PPersson, C. Changes in the drug release pattern of fresh and set simvastatin-loaded brushite cement. Mater. Sci. Eng. C 2016, 58, 88–96.
- Ratier, A.; Freche, M.; Lacout, J.L.; Rodriguez, F. Behaviour of an injectable calcium phosphate cement with added tetracycline. Int. J. Pharm. 2004, 274, 261–268.
- Alkhraisat, M.H.; Rueda, C.; Cabrejos-Azama, J.; Lucas-Aparicio, J.; Mariño, F.T.; García-Denche, J.T.; Blanco Jerez, L.; Gbureck, U.; Cabarcos, E.L. Loading and release of doxycycline hyclate from strontium-substituted calcium phosphate cement. Acta Biomater. 2010, 6, 1522–1528.
- Chen, A.; Holt-Hindle, P. Platinum-based nanostructured materials: Synthesis, properties, and applications. Chem. Rev. 2010, 110, 3767–3804.
- Schnieders, J.; Gbureck, U.; Vorndran, E.; Schossig, M.; Kissel, T. The effect of porosity on drug release kinetics from vancomycin microsphere/calcium phosphate cement composites. J. Biomed. Mater. Res. Part A 2011, 99, 391–398.
More
Information
Subjects:
Materials Science, Biomaterials
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
879
Revisions:
2 times
(View History)
Update Date:
12 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




