Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kyoungho Suk | -- | 2802 | 2023-06-10 10:22:11 | | | |
| 2 | Rita Xu | -59 word(s) | 2743 | 2023-06-12 05:05:51 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Afridi, R.; Suk, K. Microglial Responses to Stress-Induced Depression. Encyclopedia. Available online: https://encyclopedia.pub/entry/45410 (accessed on 08 February 2026).
Afridi R, Suk K. Microglial Responses to Stress-Induced Depression. Encyclopedia. Available at: https://encyclopedia.pub/entry/45410. Accessed February 08, 2026.
Afridi, Ruqayya, Kyoungho Suk. "Microglial Responses to Stress-Induced Depression" Encyclopedia, https://encyclopedia.pub/entry/45410 (accessed February 08, 2026).
Afridi, R., & Suk, K. (2023, June 10). Microglial Responses to Stress-Induced Depression. In Encyclopedia. https://encyclopedia.pub/entry/45410
Afridi, Ruqayya and Kyoungho Suk. "Microglial Responses to Stress-Induced Depression." Encyclopedia. Web. 10 June, 2023.
Copy Citation
Growing evidence suggests that microglial inflammatory activation is crucial in psychiatric disorders, including major depressive disorder (MDD). Chronic exposure to stressful stimuli, a significant risk factor for MDD, has been associated with the activation of peripheral and central immune cells, leading to inflammation. The aim is to discuss microglial responses to stress-induced depression comprehensively. Animal models used in preclinical studies of depression often utilize stressors to induce pathology similar to depression. These studies offer compelling evidence of microglial inflammatory activation, resulting in neuropathology and depressive-like behavior.
chronic stress
depression
microglia
neuroinflammation
1. Introduction
Major depressive disorder (MDD) is one of the most heterogeneous neuropsychiatric disorders that affects approximately 280 million people worldwide [1]. The disease is characterized by several core behavioral symptoms, including anhedonia, low self-esteem, sleep disturbances, and suicidal ideation [2]. Structural and functional abnormalities have been identified in the depressed brain, including decreased volumes of the prefrontal cortex (PFC) and hippocampus [3]. Various mechanisms have been proposed for decades to regulate depressive behaviors, most of which focused on restoring neuronal health and activity [4][5][6]. The monoamine hypothesis garnered the most attention; therefore, most antidepressant drugs aimed to replenish monoamine levels in the brain. However, the limited effectiveness of classical antidepressants in patients suggests the involvement of more intricate and multifaceted pathologies in depression.
Chronic stress is a major risk factor for MDD and has been associated with increased hypothalamic-pituitary-adrenal (HPA) axis activity and inflammatory activation of immune cells [7]. Increased levels of proinflammatory cytokines in the serum of depressed patients have inspired neuroscientists to investigate the possible role of neuroinflammation in depressive behavior [8]. This ushered in a new era in the neuropsychiatric field, leading to identifying brain immune cells, particularly microglia, as one of the prominent regulators of inflammation in the depressed brain. Microglia are the second major type of glial cells and primary immune cells that guard the brain parenchyma; these highly receptive cells respond to any changes in the brain microenvironment and adopt various structural and functional phenotypes in a context-dependent manner. In addition to their immune functions, microglia also regulate neuronal functions. For example, microglia aid in forming neural circuits in the developing brain through synaptic pruning and stripping, secretion of neurotrophic factors, and phagocytosis of dying neurons [9]. Microglial pruning of synaptic elements has also been implicated in cognition [10]. In the adult brain, microglia regulate dendritic spine formation via BDNF signaling, which plays a role in learning and memory [10]. Other brain cells, such as neurons and astrocytes, engage with microglia to maintain brain homeostasis [11].
Brain samples of patients with MDD have shown that chronic exposure to stressful stimuli affects microglial activation states [12]. Significant inflammatory activation of microglia was found in stress-responsive brain regions of MDD patients, including the PFC, nucleus accumbens, amygdala, and hippocampus [13][14][15]. Similar findings were also found in animal models of chronic stress-induced depression, where microglial inflammatory activation was positively correlated with depressive-like behaviors [16]. Proinflammatory cytokines released by inflammatory microglia can bind to their cognate neuronal receptors in stress neurocircuitry and regulate behavior [17][18][19]. Moreover, these cytokines can decrease the levels of neurotrophic factors, negatively impacting neuronal health. What exactly triggers microglial activation in stress-related depression remains unclear, though various pathways likely drive microglial immune activation, which has been reviewed previously [20][21][22].
2. Possible Triggers of Microglial Inflammatory Activation in Stress-Induced Depression
Tissue damage or infection can induce microglial inflammatory activation by releasing proinflammatory mediators [23]. In contrast, the exact triggers of microglial inflammatory activation under sterile inflammatory conditions such as chronic psychosocial stress are unknown. Chronic psychosocial stress causes cellular and structural changes in the brain, resulting in altered neurocircuitry and depressive behavior [24][25][26]. Researchers discuss inflammatory factors that trigger the activation of microglial cells in response to psychosocial stress in animal models (Figure 1). Animal models used in preclinical studies of depression often use stressors to induce depression-like pathology [27]. While some of these models may not accurately reflect the actual pathophysiology of human depression, they consistently exhibit features such as hyperactive HPA axis, impaired neuroplasticity and neurogenesis, and altered neurotransmitters that can be related to human depression [28][29][30][31]. These models have greatly contributed to the understanding of depression, particularly in revealing the role of neuroinflammation in its pathophysiology [27].
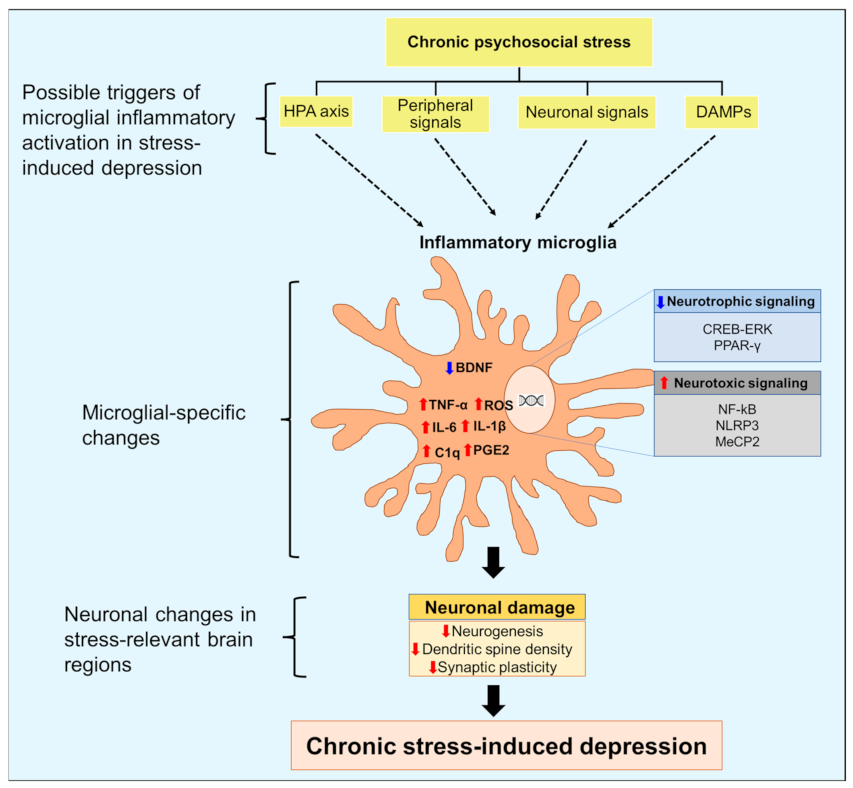
Figure 1. Triggers and role of microglial inflammatory activation in the pathogenesis of depression. Chronic psychosocial stress can increase hyperactivity of the hypothalamic-pituitary-adrenal axis, activation of peripheral immune cells, and release of damage-associated molecular patterns (DAMPs). Stress can also disturb communication between microglia and neurons that regulate microglial immune responses. Inflammatory signaling in microglia increases the expression of proinflammatory cytokines and the generation of reactive oxygen species (ROS). Inflammatory microglia also show decreased neurotrophic signaling, which hampers the release of brain-derived neurotrophic factor (BDNF) from microglia. These changes culminate in neuronal damage, including decreased neurogenesis, dendritic spine density, and impaired synaptic plasticity, leading to depression. ↓, decrease; ↑, increase; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; IL, interleukin; C1q, complement component 1q; TNF, tumor necrosis factor; MeCP2, methyl-CpG binding protein 2; CREB, cAMP response element binding protein; ERK, extracellular signal-regulated kinase; PPAR-γ, peroxisome proliferator-activated receptor gamma, NLRP3, nucleotide-binding domain, leucine-rich repeat, pyrin domain-containing protein 3.
2.1. Hyperactivity of HPA Axis
Neuroendocrine responses to psychosocial stressors are an important compensatory mechanism [32]. The classical “fight-or-flight ” response leads to hyperactivity of the HPA axis, increasing circulating glucocorticoids and catecholamines that return to baseline levels after the threat wanes [33]. In chronic psychosocial stress, however, persistently increased HPA activity exerts deleterious effects on the brain [34]. Chronic stress can lead to maladaptive changes in the HPA axis, which can contribute to the development of depression [33]. In vivo models have shown that the upregulation of HPA activity varies according to the type of stressor, which also reflects variable levels of glucocorticoids in a subset of patients with MDD [34].
The brain responds to stress by identifying potential threats and triggering corresponding physiological and behavioral reactions that can either be beneficial or harmful [32]. The brain is the main target of glucocorticoid actions, which are elevated after exposure to stressful stimuli [32]. All major cell types of the brain, including neurons, astrocytes, and microglia, express glucocorticoid receptors [35][36]. These receptors are expressed in distant limbic–midbrain and cortical brain regions, including the hippocampus, amygdala, and prefrontal anterior cingulate cortex, suggesting the role of glucocorticoids in stress-related mood disorders [37]. Importantly, distinct hippocampus regions show varied sensitivities to glucocorticoid activity [31]. The hippocampus plays diverse roles in memory and behavior due to functional segregation along its longitudinal axis. The dorsal hippocampus primarily contributes to spatial learning and memory, whereas the ventral hippocampus mainly regulates anxiety, which is influenced by stress. Due to its direct connection to the hypothalamus, the ventral hippocampus is more prone to the deleterious effects of glucocorticoids compared to the dorsal hippocampus [38].
Given the impact of the HPA axis on stress neurocircuitry, the increased HPA axis may drive the phenotypic transition of microglia in chronic stress-induced depression [30]. Indeed, recent literature has reported that increased HPA axis activity drives the primed state of microglia and induces the inflammatory phenotype in stress-sensitive brain regions [30][39][40]. Increased serum glucocorticoid levels were found in both preclinical and clinical studies of MDD [41]. Glucocorticoids also increased NLRP3 inflammasome signaling in the hippocampal region of mice subjected to chronic restraint stress [39]. Increased levels of high-mobility group box 1 (HMGB1) in limbic regions of the rat brain were reported in a model of inescapable tail shock, where subsequent administration of an antagonist blocked glucocorticoid signaling and attenuated the increase of HMGB1 levels [40]. In addition, increased inflammatory signaling in microglia was observed in a mouse model of corticosterone-induced depression [42][43]. Increased levels of proinflammatory cytokines in microglia were accompanied by depressive-like behavior in mice injected with corticosterone [44].
2.2. Peripheral Signals: Brain-Immune Axis
The brain is a distinct structure separated from the rest of the body by the blood-brain barrier (BBB). BBB acts as a selective barrier, regulating peripheral access to the brain parenchyma. The loss of BBB integrity has been documented in MDD pathophysiology, but the role of peripheral signals in microglial activation in vivo is debatable [45]. Immune dysfunction has been documented in patients with MDD as well as in preclinical models of depression [46][47][48]. Peripheral immune cells are the major sources of circulating proinflammatory cytokines that can induce inflammatory activation of microglia [49]. In addition, peripheral immune cells infiltrate the brain parenchyma in various animal models of depression [50]. Whether these peripheral immune cells trigger microglial activation in depression is unclear.
In a CSDS model, susceptible mice exhibited decreased expression of claudin-5, a tight junction protein in the BBB, which allows peripheral immune cells and proinflammatory cytokines to enter the brain [29]. Moreover, transcriptomic analysis of endothelial cells from susceptible mice revealed increased expression of genes associated with the proinflammatory tumor necrosis factor-α (TNF-α) and the NF-κB pathway [29]. The study also found decreased claudin-5 expression in post-mortem samples of patients with MDD [29]. Thus, a compromised BBB can allow proinflammatory signals from the periphery to act on microglia in a depressed brain.
Increased trafficking of monocytes to the perivascular space and parenchyma was also observed in the repeated social defeat model [50]. Using chimeric mice expressing the green fluorescent protein in lysozyme M (LysM)-positive myeloid cells, the study found an increased infiltration of monocytes in various brain regions of defeated mice. Interestingly, significant increases in IL-1β, chemokine (C-C motif) ligand 2 (CCL2), and microglial activation were also found in brain regions in which peripheral macrophages infiltrated [50]. Finally, the study found that crosstalk between chemokine receptor-2 (CCR2) and fractalkine receptor (CX3CR1) recruits macrophages to the brain parenchyma under stressful conditions [50].
Contrary to these findings, another group found that peripheral immune cells do not play a role in microglial inflammatory activation in acute and chronic social defeat stress models [51]. Here, chronic social defeat increased phagocytic microglial cells in the brain without recruiting peripheral immune signals, indicating that microglia are solely responsible for generating inflammation in the brain during chronic stress. Moreover, peripheral signals can attenuate pathways involved in monoamine synthesis. A recent study showed that lipopolysaccharide-binding protein (LBP) expression increased both peripherally and centrally in mice following exposure to stressful stimuli [52]. LBP expression also increased in microglial cells and inhibited enzymes involved in synthesizing monoamine neurotransmitters. These findings suggest a bidirectional communication between neuroendocrine stimuli and the immune system in the pathology of depression.
2.3. Neuronal Signals Shape Microglial Responses
Microglia and neurons work together by secreting diverse molecules to regulate brain homeostasis. Particularly, neuronal-derived soluble factors, including colony-stimulating factor 1 (CSF1), CX3CL1, and transforming growth factor-β (TGFβ), play crucial roles in regulating microglial immune functions [53][54]. Dysregulation of neuronal activity and neuronal atrophy following stress alters neuronal-derived factors that maintain microglial activity, leading to increased inflammatory signaling in microglial cells [55]. Mice exposed to the chronic unpredictable stress model displayed increased expression of CSF1 in the PFC as well as CSF1 receptor (CSF1R) in microglial cells in the same region [53]. Augmented CSF1 signaling in microglia increased phagocytosis of neuronal elements, which reduced dendritic spine density. Interestingly, the knockdown of neuronal CSF1 decreased microglial phagocytosis and attenuated behavioral deficits in stressed mice. Impaired CX3CL1-CX3CR1 signaling between neurons and microglia has also been shown in an animal model of chronic stress, causing inflammatory activation of microglial cells [56]. Microglial deletion of CX3CR1 prevented mice from developing depression-like behavior after stress exposure. Ultimately, CX3CR1 deficiency attenuated chronic stress-induced proinflammatory gene expression in microglia and prevented neuronal dysfunction [57].
2.4. Role of Damage-Associated Molecular Patterns (DAMPs)
Studies have reported that damage-associated molecular patterns (DAMPs), including heat shock proteins, HMGB1, and S100 proteins, can initiate sterile neuroinflammatory processes in animal models of chronic psychosocial stress [58]. Microglia can recognize these DAMPs and transmit signals to intracellular NLRP3 inflammasomes through TLRs and RAGE [47][59]. These immune receptors have been shown to promote microglial inflammatory signaling in an animal model of chronic stress and depression.
In addition to increased mRNA levels of HMGB1 in hippocampal microglia, higher expression of RAGE and activation of NLRP3 inflammasomes were found in the CUMS model of depression [59]. The increased HMGB1-RAGE signaling in hippocampal microglia coincided with depressive-like behavior in mice exposed to chronic unpredictable stress. Increased expression of S100a8 and S100a9 was also found in the PFC of susceptible mice subjected to repeated social defeat stress [47]. Microglia-specific reduction of TLR2/4 expression by using a viral strategy, however, prevented mice from developing depressive-like behavior after repeated social defeat stress [47]. Increased HMGB1 expression was also observed in the hippocampal region in rat brains following inescapable tail shock. Increased HMGB1 expression positively correlated with heightened NLRP3 inflammasome signaling [60].
Chronic stress can trigger not only previously well-recognized DAMPs but also extracellular nucleosomes and histones. Increased histones and nucleosomes were found in the cerebrospinal fluid of the CUMS mice, which positively correlated with IL-1β levels in PFC [61]. Higher levels of nucleosomes promoted microglial inflammatory signaling in a C-type lectin receptor 2D (Clec2d)-dependent manner, increasing oxidative stress and IL-1β secretion [61]. Knockdown of Clec2d in PFC reduced microglial inflammatory activation and depressive-like behavior in CUMS mice.
3. Microglia as a Potential Therapeutic Target for Treatment of Stress-Induced Depression
Inflammatory activation of microglia in various limbic brain regions is a hallmark of chronic psychosocial stress not only in rodents but also in humans [62][63]. Patients with MDD exhibit increased proinflammatory cytokines in cerebrospinal fluid, decreased neurogenesis, and impaired synaptic plasticity [62][63]. Inflammatory activation of microglia is strongly linked to neuronal deficits in MDD pathology; therefore, microglial inflammation is a potential therapeutic target for treating depression. Various strategies have been used effectively in in vivo models of depression to mitigate inflammatory activation of microglia (Table 1).
Table 1. Targeting microglial inflammatory activation in animal models of depression.
| Putative Microglial Targets | Targeting Strategies | Animal Models | Brain Regions | Outcomes | References |
|---|---|---|---|---|---|
| ↓ NLRP3 signaling | MCC950 | CUMS (Mice) | PFC | ↓ Depressive-like behavior ↓ Neuroinflammatory markers ↓ IL-1β |
[64] |
| ↓ NLRP3 signaling | Astragalin | CUMS (Mice) | Hippocampus | ↓ Depressive-like behavior ↓ Neuroinflammatory markers ↓ IL-1β |
[65] |
| ↓ p38 MAPK signaling ↓ NF-κB signaling ↓ HMGB1/RAGE/TLR4 signaling |
Roflupram | CUMS (Mice) | Hippocampus PFC | ↓ depressive-like behavior ↓ proinflammatory cytokines |
[66] |
| ↑ BDNF signaling | Viral-mediated overexpression of IL-4 | CMS (Mice) | Hippocampus | ↑ Neurogenesis ↓ Depressive-like behavior ↓ Proinflammatory cytokines ↑ Arg-1 positive microglia |
[67] |
| ↑ BDNF by increasing Nrf2 signaling ↓ MeCP2 expression |
Sulforaphane | CSDS (Mice) | PFC | ↑ Resilience to stress ↑ Synaptic plasticity ↓ Proinflammatory cytokines |
[68] |
| ↑ ERK-NRBP1-CREB signaling ↑ microglial BDNF |
(R)-Ketamine | CSDS (Mice) | PFC | ↑ Dendritic spine density long-lasting antidepressant action |
[69] |
| ↓ NLRP3 signaling ↑ Autophagy |
Ketamine | CRS (rats) | PFC Hippocampus | ↑ Synaptic plasticity ↓ Depressive-like behavior |
[70] |
| ↓ CSF1 receptor expression ↓ CD11b ↓ (CR3)-C3 phagocytic pathway |
Diazepam | CUS (Mice) | PFC | ↑ Dendritic spine density long-lasting antidepressant action |
[71] |
| ↓ ERK 1/2 signaling ↓ Phagocytic microglia |
Minocycline | CMS (Mice) | Hippocampus | ↑ Neurogenesis ↓ Depressive-like behavior |
[72] |
| ↓ HMGB1 release | CUMS (Mice) | ↑ Cognitive performance ↓ Depressive-like behavior |
[73] | ||
| ↓ Phagocytic microglia | CSDS (Mice) | ↓ Proinflammatory cytokines ↓ Synaptic loss ↓ Behavioral despair |
[74] | ||
| ↓ Phagocytic and inflammatory microglia | CUMS (Mice) | PFC Hippocampus | ↑ Kynurenic acid ↓ Behavioral despair |
[75] | |
| ↑ LXR- β signaling ↓ NF-κB signaling ↓ NLRP3 signaling ↓ IL-1β ↓ Phagocytic microglia |
TO90137 | CUMS Corticosterone-induced depression | Basolateral amygdala | ↓ Neuroinflammation ↓ Depressive-like behavior |
[76] |
| ↑ LXR- β signaling ↓ NF-κB signaling |
GW3965 | CUMS (Mice) | Hippocampus | ↓ Inflammatory markers ↓ Synaptic impairment |
[77] |
| ↑ PPAR-γ signaling ↑ Neuroprotective microglia |
Asperosaponin VI | CMS (Mice) | Hippocampus | ↑ Microglial-neuronal interactions ↓ Synaptic deficits |
[78] |
| Not discussed | murine recombinant IL-10 | Learned helplessness (mice) | Hippocampus | ↑ Dendritic spine density ↑ Cognitive performance |
[79] |
| Not discussed | Dimethyl fumarate | CUMS (mice) | Hippocampus | ↓ Neuroinflammatory markers ↓ Cognitive impairment |
[80] |
↓, decrease; ↑, increase; CUMS, chronic unpredictable mild stress; CSDS, chronic social defeat stress; CMS, chronic mild stress; CRS, chronic restraint stress; CUS; chronic unpredictable stress; PFC, prefrontal cortex; NLRP3, nucleotide-binding domain, leucine-rich repeat, pyrin domain-containing protein 3; MAPK, mitogen-activated protein kinases, NF-κB, nuclear factor kappa-light-chain-enhanced of activated B cells, IL-1β, interleukin-1β; HMGB1, high mobility group box 1; RAGE, receptors for advanced glycation end products; TLR, toll-like receptors; BDNF, brain-derived neurotrophic factor; PPAR-γ, peroxisome proliferator-activated receptor gamma, MeCP2, methyl-CpG binding protein 2; CREB, cAMP response element binding protein; ERK, extracellular signal-regulated kinase; LXR, Liver X receptors; CSF1, colony-stimulating factor 1.
4. Conclusions and Future Perspectives
Microglial inflammatory activation plays a key role in MDD pathology. Several preclinical and clinical studies of MDD have reported increased neuroinflammatory signaling in various brain regions that regulate mood and behavior. However, the exact triggers of microglial inflammatory signaling in MDD pathology remain unclear. Recent studies suggest the possible role of elevated glucocorticoids in inducing the inflammatory phenotype of microglia, but the underlying mechanisms are unknown. The brain-immune axis is another factor that can induce microglial inflammatory activation during psychosocial stress.
Despite these ambiguities, inflammatory activation of microglia and neuronal deficits are consistently observed in animal models of stress-induced depressive-like behavior. Increased proinflammatory signaling in microglia hampers neuronal health through phagocytosis of synaptic elements, altered synaptic plasticity, and decreased neurogenesis. Therefore, reducing microglial inflammatory activation is a potential strategy for treating MDD. Further research is needed to elucidate the molecular mechanisms involved in microglia activation and their impact on neurons in stress-induced depression. Future studies should better identify specific therapeutic targets to treat MDD.
References
- Global Health Data Exchange (GHDx); Institute of Health Metrics and Evaluation: Seattle, WA, USA, 2023.
- Alexopoulos, G.S. Mechanisms and treatment of late-life depression. Transl. Psychiatry 2019, 9, 188.
- Koolschijn, P.C.; van Haren, N.E.; Lensvelt-Mulders, G.J.; Hulshoff Pol, H.E.; Kahn, R.S. Brain volume abnormalities in major depressive disorder: A meta-analysis of magnetic resonance imaging studies. Hum. Brain Mapp. 2009, 30, 3719–3735.
- Kornhuber, J.; Gulbins, E. New Molecular Targets for Antidepressant Drugs. Pharmaceuticals 2021, 14, 894.
- Malki, K.; Keers, R.; Tosto, M.G.; Lourdusamy, A.; Carboni, L.; Domenici, E.; Uher, R.; McGuffin, P.; Schalkwyk, L.C. The endogenous and reactive depression subtypes revisited: Integrative animal and human studies implicate multiple distinct molecular mechanisms underlying major depressive disorder. BMC Med. 2014, 12, 73.
- Wang, B.; Shi, H.; Ren, L.; Miao, Z.; Wan, B.; Yang, H.; Fan, X.; Gustafsson, J.A.; Sun, M.; Xu, X. Ahi1 regulates serotonin production by the GR/ERbeta/TPH2 pathway involving sexual differences in depressive behaviors. Cell Commun. Signal. 2022, 20, 74.
- Wang, Y.; Liu, L.; Gu, J.H.; Wang, C.N.; Guan, W.; Liu, Y.; Tang, W.Q.; Ji, C.H.; Chen, Y.M.; Huang, J.; et al. Salt-inducible kinase 1-CREB-regulated transcription coactivator 1 signalling in the paraventricular nucleus of the hypothalamus plays a role in depression by regulating the hypothalamic-pituitary-adrenal axis. Mol. Psychiatry 2022, 28, 76–82.
- Sukhram, S.D.; Yilmaz, G.; Gu, J. Antidepressant Effect of Ketamine on Inflammation-Mediated Cytokine Dysregulation in Adults with Treatment-Resistant Depression: Rapid Systematic Review. Oxid Med. Cell. Longev. 2022, 2022, 1061274.
- Schlegelmilch, T.; Henke, K.; Peri, F. Microglia in the developing brain: From immunity to behaviour. Curr. Opin. Neurobiol. 2011, 21, 5–10.
- Cornell, J.; Salinas, S.; Huang, H.Y.; Zhou, M. Microglia regulation of synaptic plasticity and learning and memory. Neural Regen. Res. 2022, 17, 705–716.
- Wohleb, E.S. Neuron-Microglia Interactions in Mental Health Disorders: “For Better, and For Worse”. Front. Immunol. 2016, 7, 544.
- Gu, S.; Li, Y.; Jiang, Y.; Huang, J.H.; Wang, F. Glymphatic Dysfunction Induced Oxidative Stress and Neuro-Inflammation in Major Depression Disorders. Antioxidants 2022, 11, 2296.
- Miller, A.H.; Maletic, V.; Raison, C.L. Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biol. Psychiatry 2009, 65, 732–741.
- Schnieder, T.P.; Trencevska, I.; Rosoklija, G.; Stankov, A.; Mann, J.J.; Smiley, J.; Dwork, A.J. Microglia of prefrontal white matter in suicide. J. Neuropathol. Exp. Neurol. 2014, 73, 880–890.
- Torres-Platas, S.G.; Cruceanu, C.; Chen, G.G.; Turecki, G.; Mechawar, N. Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain Behav. Immun. 2014, 42, 50–59.
- Tynan, R.J.; Naicker, S.; Hinwood, M.; Nalivaiko, E.; Buller, K.M.; Pow, D.V.; Day, T.A.; Walker, F.R. Chronic stress alters the density and morphology of microglia in a subset of stress-responsive brain regions. Brain Behav. Immun. 2010, 24, 1058–1068.
- DiSabato, D.J.; Nemeth, D.P.; Liu, X.; Witcher, K.G.; O’Neil, S.M.; Oliver, B.; Bray, C.E.; Sheridan, J.F.; Godbout, J.P.; Quan, N. Interleukin-1 receptor on hippocampal neurons drives social withdrawal and cognitive deficits after chronic social stress. Mol. Psychiatry 2021, 26, 4770–4782.
- Kaur, C.; Sivakumar, V.; Zou, Z.; Ling, E.A. Microglia-derived proinflammatory cytokines tumor necrosis factor-alpha and interleukin-1beta induce Purkinje neuronal apoptosis via their receptors in hypoxic neonatal rat brain. Brain Struct. Funct. 2014, 219, 151–170.
- Stellwagen, D.; Beattie, E.C.; Seo, J.Y.; Malenka, R.C. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. J. Neurosci. 2005, 25, 3219–3228.
- Wang, H.; He, Y.; Sun, Z.; Ren, S.; Liu, M.; Wang, G.; Yang, J. Microglia in depression: An overview of microglia in the pathogenesis and treatment of depression. J. Neuroinflamm. 2022, 19, 132.
- Woodburn, S.C.; Bollinger, J.L.; Wohleb, E.S. The semantics of microglia activation: Neuroinflammation, homeostasis, and stress. J. Neuroinflamm. 2021, 18, 258.
- Troubat, R.; Barone, P.; Leman, S.; Desmidt, T.; Cressant, A.; Atanasova, B.; Brizard, B.; El Hage, W.; Surget, A.; Belzung, C.; et al. Neuroinflammation and depression: A review. Eur. J. Neurosci. 2021, 53, 151–171.
- Block, M.L.; Hong, J.S. Microglia and inflammation-mediated neurodegeneration: Multiple triggers with a common mechanism. Prog. Neurobiol. 2005, 76, 77–98.
- Wang, Y.L.; Han, Q.Q.; Gong, W.Q.; Pan, D.H.; Wang, L.Z.; Hu, W.; Yang, M.; Li, B.; Yu, J.; Liu, Q. Microglial activation mediates chronic mild stress-induced depressive- and anxiety-like behavior in adult rats. J. Neuroinflamm. 2018, 15, 21.
- Duman, R.S.; Aghajanian, G.K. Synaptic dysfunction in depression: Potential therapeutic targets. Science 2012, 338, 68–72.
- Wang, J.; Chen, H.S.; Li, H.H.; Wang, H.J.; Zou, R.S.; Lu, X.J.; Wang, J.; Nie, B.B.; Wu, J.F.; Li, S.; et al. Microglia-dependent excessive synaptic pruning leads to cortical underconnectivity and behavioral abnormality following chronic social defeat stress in mice. Brain Behav. Immun. 2023, 109, 23–36.
- Afridi, R.; Suk, K. Neuroinflammatory Basis of Depression: Learning From Experimental Models. Front. Cell. Neurosci. 2021, 15, 691067.
- Steiner, J.; Bielau, H.; Brisch, R.; Danos, P.; Ullrich, O.; Mawrin, C.; Bernstein, H.G.; Bogerts, B. Immunological aspects in the neurobiology of suicide: Elevated microglial density in schizophrenia and depression is associated with suicide. J. Psychiatr. Res. 2008, 42, 151–157.
- Dudek, K.A.; Dion-Albert, L.; Lebel, M.; LeClair, K.; Labrecque, S.; Tuck, E.; Ferrer Perez, C.; Golden, S.A.; Tamminga, C.; Turecki, G.; et al. Molecular adaptations of the blood-brain barrier promote stress resilience vs. depression. Proc. Natl. Acad. Sci. USA 2020, 117, 3326–3336.
- Frank, M.G.; Thompson, B.M.; Watkins, L.R.; Maier, S.F. Glucocorticoids mediate stress-induced priming of microglial proinflammatory responses. Brain Behav. Immun. 2012, 26, 337–345.
- Sahay, A.; Hen, R. Adult hippocampal neurogenesis in depression. Nat. Neurosci. 2007, 10, 1110–1115.
- McEwen, B.S. The neurobiology of stress: From serendipity to clinical relevance. Brain Res. 2000, 886, 172–189.
- McEwen, B.S. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol. Rev. 2007, 87, 873–904.
- Varghese, F.P.; Brown, E.S. The Hypothalamic-Pituitary-Adrenal Axis in Major Depressive Disorder: A Brief Primer for Primary Care Physicians. Prim. Care Companion J. Clin. Psychiatry 2001, 3, 151–155.
- Meijer, O.C.; Buurstede, J.C.; Schaaf, M.J.M. Corticosteroid Receptors in the Brain: Transcriptional Mechanisms for Specificity and Context-Dependent Effects. Cell. Mol. Neurobiol. 2019, 39, 539–549.
- Niraula, A.; Wang, Y.; Godbout, J.P.; Sheridan, J.F. Corticosterone Production during Repeated Social Defeat Causes Monocyte Mobilization from the Bone Marrow, Glucocorticoid Resistance, and Neurovascular Adhesion Molecule Expression. J. Neurosci. 2018, 38, 2328–2340.
- Wang, Q.; Van Heerikhuize, J.; Aronica, E.; Kawata, M.; Seress, L.; Joels, M.; Swaab, D.F.; Lucassen, P.J. Glucocorticoid receptor protein expression in human hippocampus; stability with age. Neurobiol. Aging 2013, 34, 1662–1673.
- Levone, B.R.; Codagnone, M.G.; Moloney, G.M.; Nolan, Y.M.; Cryan, J.F.; OF, O.L. Adult-born neurons from the dorsal, intermediate, and ventral regions of the longitudinal axis of the hippocampus exhibit differential sensitivity to glucocorticoids. Mol. Psychiatry 2021, 26, 3240–3252.
- Feng, X.; Zhao, Y.; Yang, T.; Song, M.; Wang, C.; Yao, Y.; Fan, H. Glucocorticoid-Driven NLRP3 Inflammasome Activation in Hippocampal Microglia Mediates Chronic Stress-Induced Depressive-Like Behaviors. Front. Mol. Neurosci. 2019, 12, 210.
- Frank, M.G.; Annis, J.L.; Watkins, L.R.; Maier, S.F. Glucocorticoids mediate stress induction of the alarmin HMGB1 and reduction of the microglia checkpoint receptor CD200R1 in limbic brain structures. Brain Behav. Immun. 2019, 80, 678–687.
- Horowitz, M.A.; Cattaneo, A.; Cattane, N.; Lopizzo, N.; Tojo, L.; Bakunina, N.; Musaelyan, K.; Borsini, A.; Zunszain, P.A.; Pariante, C.M. Glucocorticoids prime the inflammatory response of human hippocampal cells through up-regulation of inflammatory pathways. Brain Behav. Immun. 2020, 87, 777–794.
- Bai, G.; Qiao, Y.; Lo, P.C.; Song, L.; Yang, Y.; Duan, L.; Wei, S.; Li, M.; Huang, S.; Zhang, B.; et al. Anti-depressive effects of Jiao-Tai-Wan on CORT-induced depression in mice by inhibiting inflammation and microglia activation. J. Ethnopharmacol. 2022, 283, 114717.
- Horchar, M.J.; Wohleb, E.S. Glucocorticoid receptor antagonism prevents microglia-mediated neuronal remodeling and behavioral despair following chronic unpredictable stress. Brain Behav. Immun. 2019, 81, 329–340.
- Mao, Z.F.; Ouyang, S.H.; Zhang, Q.Y.; Wu, Y.P.; Wang, G.E.; Tu, L.F.; Luo, Z.; Li, W.X.; Kurihara, H.; Li, Y.F.; et al. New insights into the effects of caffeine on adult hippocampal neurogenesis in stressed mice: Inhibition of CORT-induced microglia activation. FASEB J. 2020, 34, 10998–11014.
- Najjar, S.; Pearlman, D.M.; Devinsky, O.; Najjar, A.; Zagzag, D. Neurovascular unit dysfunction with blood-brain barrier hyperpermeability contributes to major depressive disorder: A review of clinical and experimental evidence. J. Neuroinflamm. 2013, 10, 142.
- Lehmann, M.L.; Weigel, T.K.; Poffenberger, C.N.; Herkenham, M. The Behavioral Sequelae of Social Defeat Require Microglia and Are Driven by Oxidative Stress in Mice. J. Neurosci. 2019, 39, 5594–5605.
- Nie, X.; Kitaoka, S.; Tanaka, K.; Segi-Nishida, E.; Imoto, Y.; Ogawa, A.; Nakano, F.; Tomohiro, A.; Nakayama, K.; Taniguchi, M.; et al. The Innate Immune Receptors TLR2/4 Mediate Repeated Social Defeat Stress-Induced Social Avoidance through Prefrontal Microglial Activation. Neuron 2018, 99, 464–479 e467.
- Savitz, J.; Drevets, W.C. Bipolar and major depressive disorder: Neuroimaging the developmental-degenerative divide. Neurosci. Biobehav. Rev. 2009, 33, 699–771.
- Afridi, R.; Seol, S.; Kang, H.J.; Suk, K. Brain-immune interactions in neuropsychiatric disorders: Lessons from transcriptome studies for molecular targeting. Biochem. Pharmacol. 2021, 188, 114532.
- Wohleb, E.S.; Powell, N.D.; Godbout, J.P.; Sheridan, J.F. Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J. Neurosci. 2013, 33, 13820–13833.
- Lehmann, M.L.; Cooper, H.A.; Maric, D.; Herkenham, M. Social defeat induces depressive-like states and microglial activation without involvement of peripheral macrophages. J. Neuroinflamm. 2016, 13, 224.
- Fang, M.; Li, Y.; Liao, Z.; Wang, G.; Cao, Q.; Li, Y.; Duan, Y.; Han, Y.; Deng, X.; Wu, F.; et al. Lipopolysaccharide-binding protein expression is increased by stress and inhibits monoamine synthesis to promote depressive symptoms. Immunity 2023, 56, 620–634.e11.
- Wohleb, E.S.; Terwilliger, R.; Duman, C.H.; Duman, R.S. Stress-Induced Neuronal Colony Stimulating Factor 1 Provokes Microglia-Mediated Neuronal Remodeling and Depressive-like Behavior. Biol. Psychiatry 2018, 83, 38–49.
- Butovsky, O.; Jedrychowski, M.P.; Moore, C.S.; Cialic, R.; Lanser, A.J.; Gabriely, G.; Koeglsperger, T.; Dake, B.; Wu, P.M.; Doykan, C.E.; et al. Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nat. Neurosci. 2014, 17, 131–143.
- Milior, G.; Lecours, C.; Samson, L.; Bisht, K.; Poggini, S.; Pagani, F.; Deflorio, C.; Lauro, C.; Alboni, S.; Limatola, C.; et al. Fractalkine receptor deficiency impairs microglial and neuronal responsiveness to chronic stress. Brain Behav. Immun. 2016, 55, 114–125.
- Rimmerman, N.; Schottlender, N.; Reshef, R.; Dan-Goor, N.; Yirmiya, R. The hippocampal transcriptomic signature of stress resilience in mice with microglial fractalkine receptor (CX3CR1) deficiency. Brain Behav. Immun. 2017, 61, 184–196.
- Liu, Y.; Zhang, T.; Meng, D.; Sun, L.; Yang, G.; He, Y.; Zhang, C. Involvement of CX3CL1/CX3CR1 in depression and cognitive impairment induced by chronic unpredictable stress and relevant underlying mechanism. Behav. Brain Res. 2020, 381, 112371.
- Fleshner, M.; Frank, M.; Maier, S.F. Danger Signals and Inflammasomes: Stress-Evoked Sterile Inflammation in Mood Disorders. Neuropsychopharmacology 2017, 42, 36–45.
- Franklin, T.C.; Wohleb, E.S.; Zhang, Y.; Fogaca, M.; Hare, B.; Duman, R.S. Persistent Increase in Microglial RAGE Contributes to Chronic Stress-Induced Priming of Depressive-like Behavior. Biol. Psychiatry 2018, 83, 50–60.
- Weber, M.D.; Frank, M.G.; Tracey, K.J.; Watkins, L.R.; Maier, S.F. Stress induces the danger-associated molecular pattern HMGB-1 in the hippocampus of male Sprague Dawley rats: A priming stimulus of microglia and the NLRP3 inflammasome. J. Neurosci. 2015, 35, 316–324.
- Wu, H.; Bao, H.; Liu, C.; Zhang, Q.; Huang, A.; Quan, M.; Li, C.; Xiong, Y.; Chen, G.; Hou, L. Extracellular Nucleosomes Accelerate Microglial Inflammation via C-Type Lectin Receptor 2D and Toll-Like Receptor 9 in mPFC of Mice With Chronic Stress. Front. Immunol. 2022, 13, 854202.
- Campbell, S.; Marriott, M.; Nahmias, C.; MacQueen, G.M. Lower hippocampal volume in patients suffering from depression: A meta-analysis. Am. J. Psychiatry 2004, 161, 598–607.
- Lucassen, P.J.; Stumpel, M.W.; Wang, Q.; Aronica, E. Decreased numbers of progenitor cells but no response to antidepressant drugs in the hippocampus of elderly depressed patients. Neuropharmacology 2010, 58, 940–949.
- Liu, Q.; Zhang, M.M.; Guo, M.X.; Zhang, Q.P.; Li, N.Z.; Cheng, J.; Wang, S.L.; Xu, G.H.; Li, C.F.; Zhu, J.X.; et al. Inhibition of Microglial NLRP3 with MCC950 Attenuates Microglial Morphology and NLRP3/Caspase-1/IL-1beta Signaling in Stress-induced Mice. J. Neuroimmune Pharmacol. 2022, 17, 503–514.
- Tong, Y.; Fu, H.; Xia, C.; Song, W.; Li, Y.; Zhao, J.; Zhang, X.; Gao, X.; Yong, J.; Liu, Q.; et al. Astragalin Exerted Antidepressant-like Action through SIRT1 Signaling Modulated NLRP3 Inflammasome Deactivation. ACS Chem. Neurosci. 2020, 11, 1495–1503.
- Xie, J.; Bi, B.; Qin, Y.; Dong, W.; Zhong, J.; Li, M.; Cheng, Y.; Xu, J.; Wang, H. Inhibition of phosphodiesterase-4 suppresses HMGB1/RAGE signaling pathway and NLRP3 inflammasome activation in mice exposed to chronic unpredictable mild stress. Brain Behav. Immun. 2021, 92, 67–77.
- Zhang, J.; Rong, P.; Zhang, L.; He, H.; Zhou, T.; Fan, Y.; Mo, L.; Zhao, Q.; Han, Y.; Li, S.; et al. IL4-driven microglia modulate stress resilience through BDNF-dependent neurogenesis. Sci. Adv. 2021, 7, eabb9888.
- Tang, R.; Cao, Q.Q.; Hu, S.W.; He, L.J.; Du, P.F.; Chen, G.; Fu, R.; Xiao, F.; Sun, Y.R.; Zhang, J.C.; et al. Sulforaphane activates anti-inflammatory microglia, modulating stress resilience associated with BDNF transcription. Acta Pharmacol. Sin. 2022, 43, 829–839.
- Yao, W.; Cao, Q.; Luo, S.; He, L.; Yang, C.; Chen, J.; Qi, Q.; Hashimoto, K.; Zhang, J.C. Microglial ERK-NRBP1-CREB-BDNF signaling in sustained antidepressant actions of (R)-ketamine. Mol. Psychiatry 2022, 27, 1618–1629.
- Lyu, D.; Wang, F.; Zhang, M.; Yang, W.; Huang, H.; Huang, Q.; Wu, C.; Qian, N.; Wang, M.; Zhang, H.; et al. Ketamine induces rapid antidepressant effects via the autophagy-NLRP3 inflammasome pathway. Psychopharmacology 2022, 239, 3201–3212.
- Bollinger, J.L.; Horchar, M.J.; Wohleb, E.S. Diazepam limits microglia-mediated neuronal remodeling in the prefrontal cortex and associated behavioral consequences following chronic unpredictable stress. Neuropsychopharmacology 2020, 45, 1766–1776.
- Bassett, B.; Subramaniyam, S.; Fan, Y.; Varney, S.; Pan, H.; Carneiro, A.M.D.; Chung, C.Y. Minocycline alleviates depression-like symptoms by rescuing decrease in neurogenesis in dorsal hippocampus via blocking microglia activation/phagocytosis. Brain Behav. Immun. 2021, 91, 519–530.
- Wang, B.; Huang, X.; Pan, X.; Zhang, T.; Hou, C.; Su, W.J.; Liu, L.L.; Li, J.M.; Wang, Y.X. Minocycline prevents the depressive-like behavior through inhibiting the release of HMGB1 from microglia and neurons. Brain Behav. Immun. 2020, 88, 132–143.
- Han, Q.Q.; Shen, S.Y.; Chen, X.R.; Pilot, A.; Liang, L.F.; Zhang, J.R.; Li, W.H.; Fu, Y.; Le, J.M.; Chen, P.Q.; et al. Minocycline alleviates abnormal microglial phagocytosis of synapses in a mouse model of depression. Neuropharmacology 2022, 220, 109249.
- Cheng, D.; Qin, Z.S.; Zheng, Y.; Xie, J.Y.; Liang, S.S.; Zhang, J.L.; Feng, Y.B.; Zhang, Z.J. Minocycline, a classic antibiotic, exerts psychotropic effects by normalizing microglial neuroinflammation-evoked tryptophan-kynurenine pathway dysregulation in chronically stressed male mice. Brain Behav. Immun. 2023, 107, 305–318.
- Li, C.; Wu, H.; Sen Ta Na, H.; Wang, L.; Zhong, C.; Deng, B.; Liu, C.; Bao, H.; Sang, H.; Hou, L. Neuronal-microglial liver X receptor beta activating decrease neuroinflammation and chronic stress-induced depression-related behavior in mice. Brain Res. 2022, 1797, 148112.
- Xu, X.; Xiao, X.; Yan, Y.; Zhang, T. Activation of liver X receptors prevents emotional and cognitive dysfunction by suppressing microglial M1-polarization and restoring synaptic plasticity in the hippocampus of mice. Brain Behav. Immun. 2021, 94, 111–124.
- Jiang, X.; Yi, S.; Liu, Q.; Su, D.; Li, L.; Xiao, C.; Zhang, J. Asperosaponin VI ameliorates the CMS-induced depressive-like behaviors by inducing a neuroprotective microglial phenotype in hippocampus via PPAR-gamma pathway. J. Neuroinflamm. 2022, 19, 115.
- Worthen, R.J.; Garzon Zighelboim, S.S.; Torres Jaramillo, C.S.; Beurel, E. Anti-inflammatory IL-10 administration rescues depression-associated learning and memory deficits in mice. J. Neuroinflamm. 2020, 17, 246.
- de Souza, A.G.; Lopes, I.S.; Filho, A.; Cavalcante, T.M.B.; Oliveira, J.V.S.; de Carvalho, M.A.J.; de Lima, K.A.; Juca, P.M.; Mendonca, S.S.; Mottin, M.; et al. Neuroprotective effects of dimethyl fumarate against depression-like behaviors via astrocytes and microglia modulation in mice: Possible involvement of the HCAR2/Nrf2 signaling pathway. Naunyn Schmiedebergs Arch. Pharmacol. 2022, 395, 1029–1045.
More
Information
Subjects:
Pathology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
857
Revisions:
2 times
(View History)
Update Date:
12 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




