
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Muhammad Ali | -- | 3281 | 2023-06-08 12:10:46 | | | |
| 2 | Lindsay Dong | + 2 word(s) | 3283 | 2023-06-09 03:06:47 | | |
Video Upload Options
Polyphenols have gained widespread attention as they are effective in the prevention and management of various diseases, including cancer diseases (CD) and rheumatoid arthritis (RA). They are natural organic substances present in fruits, vegetables, and spices. Polyphenols interact with various kinds of receptors and membranes. They modulate different signal cascades and interact with the enzymes responsible for CD and RA. These interactions involve cellular machinery, from cell membranes to major nuclear components, and provide information on their beneficial effects on health.
1. Introduction
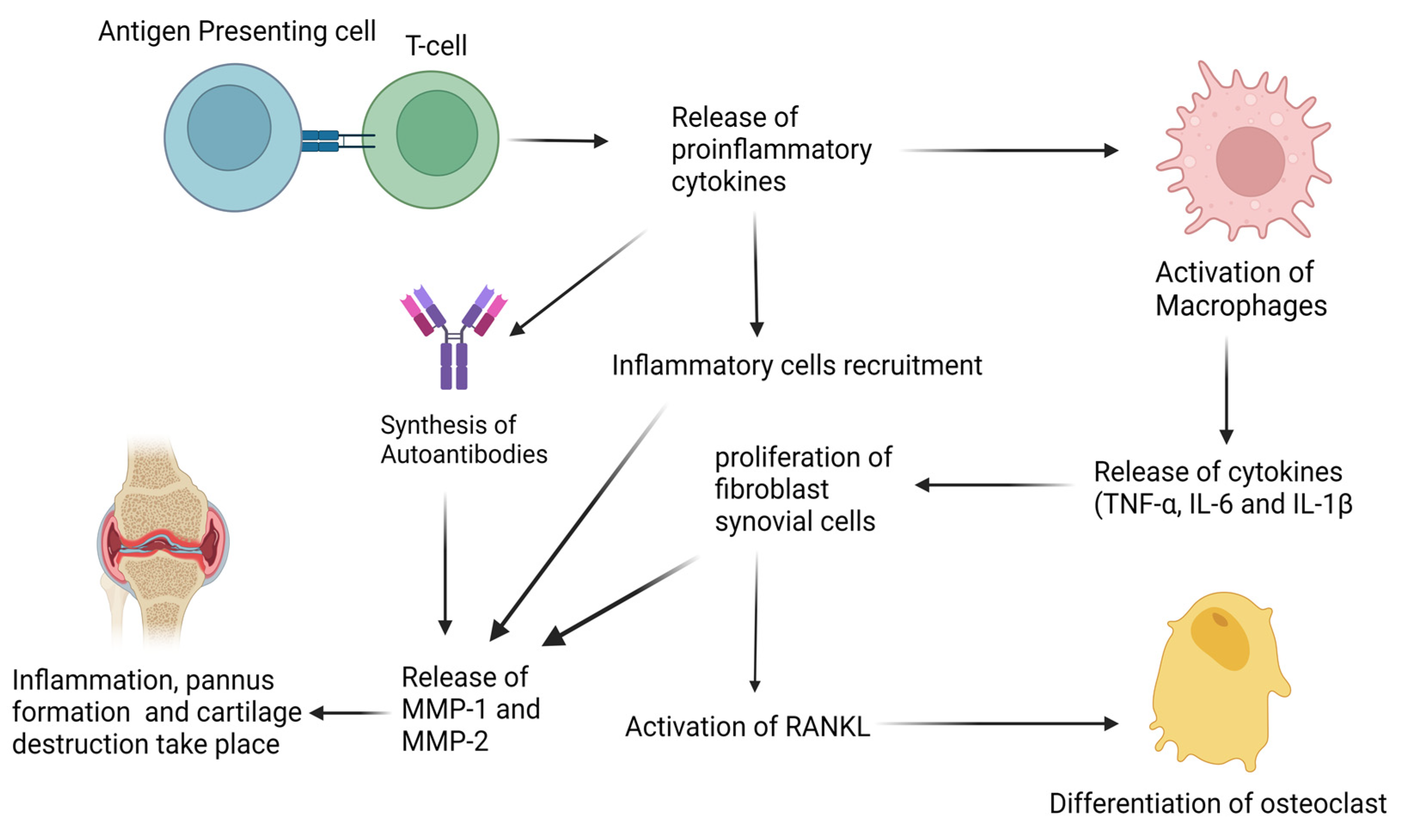
2. Biological Basis of Rheumatoid Arthritis
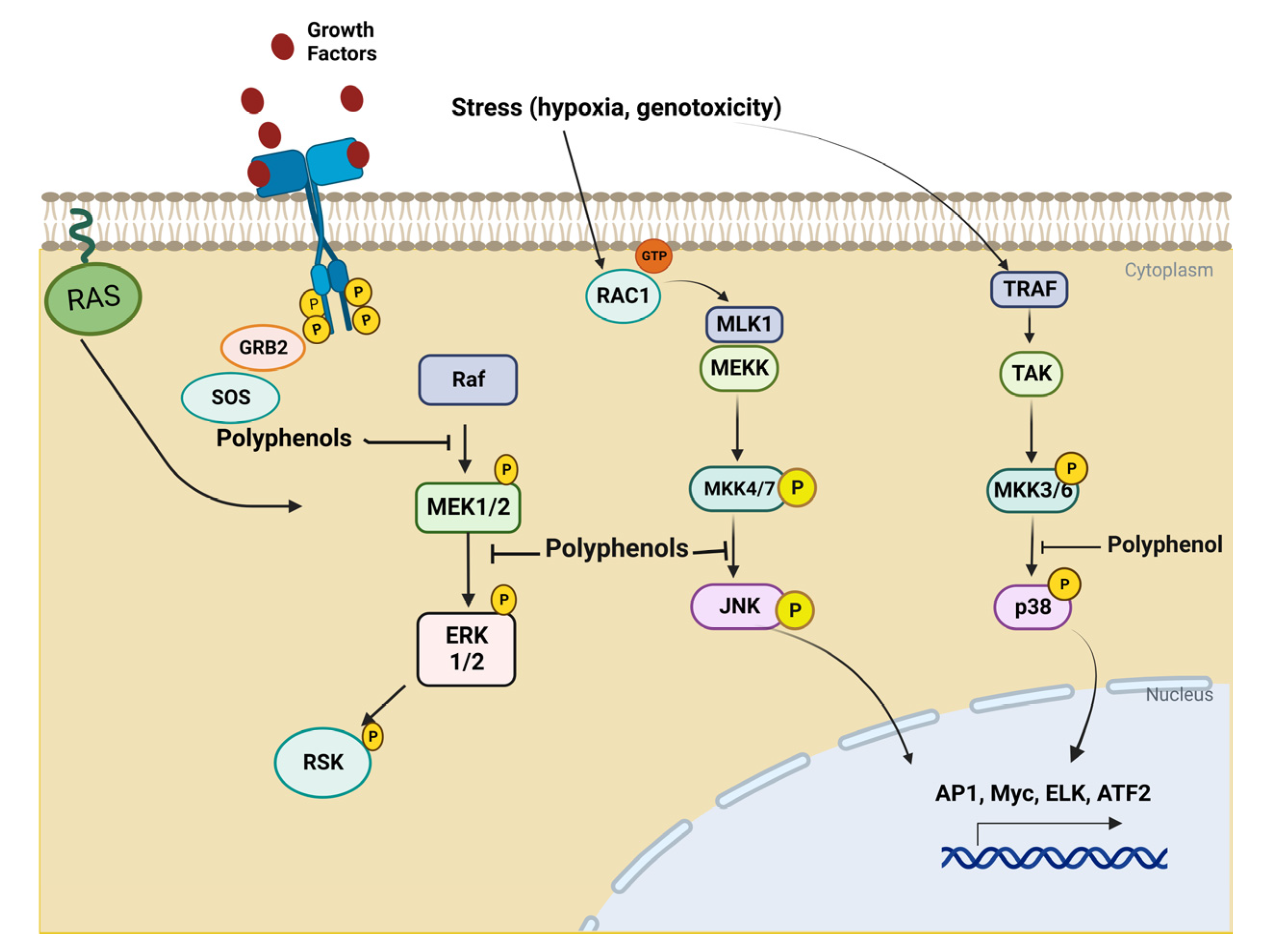
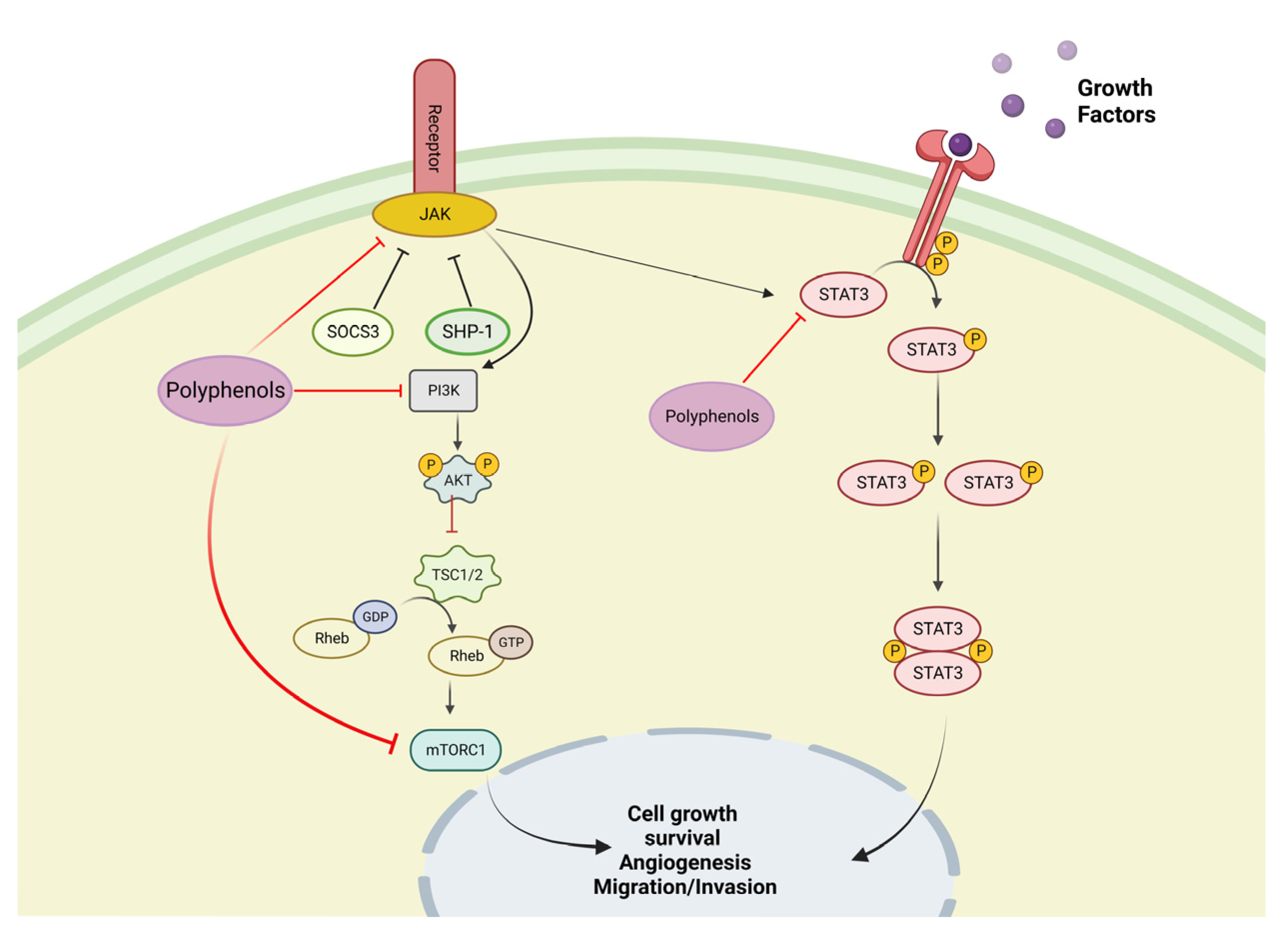
3. Biological Basis of Cancer Disease
4. In Vitro Test with Polyphenols
4.1. In Vitro Test with Olive Oil Polyphenols
4.1.1. In Vitro Test with Tyrosol on Rheumatoid Arthritis Cellular Models
4.1.2. In Vitro Test with Oleocanthal on Rheumatoid Arthritis Cellular Models
4.1.3. In Vitro Test with Oleuropein on Rheumatoid Arthritis Cellular Models
4.1.4. In Vitro Test with Hydroxytyrosol on Cancer Cell Lines
4.1.5. In Vitro Test with Luteolin on Cancer Cell Lines
4.2. In Vitro Test with Spice Polyphenols
4.2.1. In Vitro Test with Curcumin Polyphenols on Rheumatoid Arthritis Cellular Models
4.2.2. In Vitro Test with Curcumin Polyphenols on Cancer Cell Lines
4.2.3. In Vitro Test with Ginger Polyphenols on Cancer Cell Lines
4.2.4. In Vitro Test with Stilbenes on Rheumatoid Arthritis Cellular Models
4.3. In Vitro Test with Grapes Polyphenols
Studies by Jang et al. examined the anti-tumor activity of resveratrol on androgen-sensitive human prostate adenocarcinoma cells (LNCaP), a human prostate cancer cell line. Prostate cancer is the second-most common cause of cancer-related mortality. Studies have shown that prostate cancer is affected by the action of dihydrotestosterone on androgen receptors. C-X-C chemokine receptor type 4 (CXCR4) is a receptor that is highly expressed in prostate cancer cells. Dihydrotestosterone proliferates LNCaP prostate cancer cells. The results showed that resveratrol and its combination with AMD3100 (CXCR4 inhibitor) reduced the cell viability promoted by dihydrotestosterone [57]. Studies conducted by Aires et al. concluded that 3-o-sulfate-Resveratrol, a metabolite of resveratrol, inhibits human colon cancer cell lines due to S-phase stem cell accumulation, the apoptosis process, and DNA damage to the colon [58].
References
- Akanda, M.R.; Uddin, M.N.; Kim, I.-S.; Ahn, D.; Tae, H.-J.; Park, B.-Y. The Biological and Pharmacological Roles of Polyphenol Flavonoid Tilianin. Eur. J. Pharm. 2019, 842, 291–297.
- Avtanski, D.; Poretsky, L. Phyto-Polyphenols as Potential Inhibitors of Breast Cancer Metastasis. Mol. Med. 2018, 24, 29.
- Moalin, M.; van Strijdonck, G.P.F.; Beckers, M.; Hagemen, G.J.; Borm, P.J.; Bast, A.; Haenen, G.R.M.M. A Planar Conformation and the Hydroxyl Groups in the B and C Rings Play a Pivotal Role in the Antioxidant Capacity of Quercetin and Quercetin Derivatives. Molecules 2011, 16, 9636–9650.
- Mateen, S.; Moin, S.; Zafar, A.; Khan, A.Q. Redox Signaling in Rheumatoid Arthritis and the Preventive Role of Polyphenols. Clin. Chim. Acta 2016, 463, 4–10.
- Li, S.; Hu, T.; Yuan, T.; Cheng, D.; Yang, Q. Nucleoside Diphosphate Kinase B Promotes Osteosarcoma Proliferation through C-Myc. Cancer Biol. Ther. 2018, 19, 565–572.
- Behl, T.; Sharma, A.; Sharma, L.; Sehgal, A.; Singh, S.; Sharma, N.; Zengin, G.; Bungau, S.; Toma, M.M.; Gitea, D.; et al. Current Perspective on the Natural Compounds and Drug Delivery Techniques in Glioblastoma Multiforme. Cancers 2021, 13, 2765.
- Hazafa, A.; Rehman, K.U.; Jahan, N.; Jabeen, Z. The Role of Polyphenol (Flavonoids) Compounds in the Treatment of Cancer Cells. Nutr. Cancer 2020, 72, 386–397.
- Silva, S.; Sepodes, B.; Rocha, J.; Direito, R.; Fernandes, A.; Brites, D.; Freitas, M.; Fernandes, E.; Bronze, M.R.; Figueira, M.E. Protective Effects of Hydroxytyrosol-Supplemented Refined Olive Oil in Animal Models of Acute Inflammation and Rheumatoid Arthritis. J. Nutr. Biochem. 2015, 26, 360–368.
- Kurowska, W.; Kuca-Warnawin, E.H.; Radzikowska, A.; Maśliński, W. The Role of Anti-Citrullinated Protein Antibodies (ACPA) in the Pathogenesis of Rheumatoid Arthritis. Cent. Eur. J. Immunol. 2017, 42, 390–398.
- Bizzaro, N.; Bartoloni, E.; Morozzi, G.; Manganelli, S.; Riccieri, V.; Sabatini, P.; Filippini, M.; Tampoia, M.; Afeltra, A.; Sebastiani, G.; et al. Anti-Cyclic Citrullinated Peptide Antibody Titer Predicts Time to Rheumatoid Arthritis Onset in Patients with Undifferentiated Arthritis: Results from a 2-Year Prospective Study. Arthritis Res. Ther. 2013, 15, R16.
- Klareskog, L.; Stolt, P.; Lundberg, K.; Källberg, H.; Bengtsson, C.; Grunewald, J.; Rönnelid, J.; Erlandsson Harris, H.; Ulfgren, A.-K.; Rantapää-Dahlqvist, S.; et al. A New Model for an Etiology of Rheumatoid Arthritis: Smoking May Trigger HLA–DR (Shared Epitope)–Restricted Immune Reactions to Autoantigens Modified by Citrullination. Arthritis Rheum. 2006, 54, 38–46.
- Konig, M.F.; Abusleme, L.; Reinholdt, J.; Palmer, R.J.; Teles, R.P.; Sampson, K.; Rosen, A.; Nigrovic, P.A.; Sokolove, J.; Giles, J.T.; et al. Aggregatibacter Actinomycetemcomitans–Induced Hypercitrullination Links Periodontal Infection to Autoimmunity in Rheumatoid Arthritis. Sci. Transl. Med. 2016, 8, 369.
- Firestein, G.S.; McInnes, I.B. Immunopathogenesis of Rheumatoid Arthritis. Immunity 2017, 46, 183–196.
- Xu, Y.; Wu, Q. Prevalence Trend and Disparities in Rheumatoid Arthritis among US Adults, 2005–2018. J. Clin. Med. 2021, 10, 3289.
- Tian, J.; Chen, J.; Gao, J.; Li, L.; Xie, X. Resveratrol Inhibits TNF-α-Induced IL-1β, MMP-3 Production in Human Rheumatoid Arthritis Fibroblast-like Synoviocytes via Modulation of PI3kinase/Akt Pathway. Rheumatol. Int. 2013, 33, 1829–1835.
- Rocha, J.; Sepodes, B.; Eduardo-Figueira, M. Phenolic Compounds Impact on Rheumatoid Arthritis, Inflammatory Bowel Disease and Microbiota Modulation. Pharmaceutics 2021, 13, 145.
- Marino, A.; Paterniti, I.; Cordaro, M.; Morabito, R.; Campolo, M.; Navarra, M.; Esposito, E.; Cuzzocrea, S. Role of Natural Antioxidants and Potential Use of Bergamot in Treating Rheumatoid Arthritis. PharmaNutrition 2015, 3, 53–59.
- Shrivastava, A.K.; Singh, H.V.; Raizada, A.; Singh, S.K.; Pandey, A.; Singh, N.; Yadav, D.S.; Sharma, H. Inflammatory Markers in Patients with Rheumatoid Arthritis. Allergol. Immunopathol. 2015, 43, 81–87.
- Zengin, O.; Onder, M.E.; Kalem, A.; Bilici, M.; Türkbeyler, I.H.; Ozturk, Z.A.; Kisacik, B.; Onat, A.M. New Inflammatory Markers in Early Rheumatoid Arthritis. Z Rheumatol. 2018, 77, 144–150.
- Vetal, S.; Bodhankar, S.L.; Mohan, V.; Thakurdesai, P.A. Anti-Inflammatory and Anti-Arthritic Activity of Type-A Procyanidine Polyphenols from Bark of Cinnamomum Zeylanicum in Rats. Food Sci. Hum. Wellness 2013, 2, 59–67.
- Ramiro, S.; Sepriano, A.; Chatzidionysiou, K.; Nam, J.L.; Smolen, J.S.; van der Heijde, D.; Dougados, M.; van Vollenhoven, R.; Bijlsma, J.W.; Burmester, G.R.; et al. Safety of Synthetic and Biological DMARDs: A Systematic Literature Review Informing the 2016 Update of the EULAR Recommendations for Management of Rheumatoid Arthritis. Ann. Rheum. Dis. 2017, 76, 1101.
- Ganesan, M.; Eikenberry, A.; Poluektova, L.Y.; Kharbanda, K.K.; Osna, N.A. Role of Alcohol in Pathogenesis of Hepatitis B Virus Infection. World J. Gastroenterol. 2020, 26, 883–903.
- Debras, C.; Chazelas, E.; Srour, B.; Kesse-Guyot, E.; Julia, C.; Zelek, L.; Agaësse, C.; Druesne-Pecollo, N.; Galan, P.; Hercberg, S.; et al. Total and Added Sugar Intakes, Sugar Types, and Cancer Risk: Results from the Prospective NutriNet-Santé Cohort. Am. J. Clin. Nutr. 2020, 112, 1267–1279.
- Tsigalou, C.; Konstantinidis, T.; Paraschaki, A.; Stavropoulou, E.; Voidarou, C.; Bezirtzoglou, E. Mediterranean Diet as a Tool to Combat Inflammation and Chronic Diseases. An Overview. Biomedicines 2020, 8, 201.
- Cárdeno, A.; Sánchez-Hidalgo, M.; Alarcón-de-la-Lastra, C. An Up-Date of Olive Oil Phenols in Inflammation and Cancer: Molecular Mechanisms and Clinical Implications. Curr. Med. Chem. 2013, 20, 4758–4776.
- Aparicio-Soto, M.; Sánchez-Hidalgo, M.; Rosillo, M.Á.; Castejón, M.L.; Alarcón-de-la-Lastra, C. Extra Virgin Olive Oil: A Key Functional Food for Prevention of Immune-Inflammatory Diseases. Food Funct. 2016, 7, 4492–4505.
- Cárdeno, A.; Sánchez-Hidalgo, M.; Rosillo, M.A.; de la Lastra, C.A. Oleuropein, a Secoiridoid Derived from Olive Tree, Inhibits the Proliferation of Human Colorectal Cancer Cell Through Downregulation of HIF-1α. Null 2013, 65, 147–156.
- Cháirez-Ramírez, M.H.; de la Cruz-López, K.G.; García-Carrancá, A. Polyphenols as Antitumor Agents Targeting Key Players in Cancer-Driving Signaling Pathways. Front. Pharmacol. 2021, 12, 710304.
- Amawi, H.; Ashby, C.; Samuel, T.; Peraman, R.; Tiwari, A. Polyphenolic Nutrients in Cancer Chemoprevention and Metastasis: Role of the Epithelial-to-Mesenchymal (EMT) Pathway. Nutrients 2017, 9, 911.
- Sharma, A.; Kaur, M.; Katnoria, J.K.; Nagpal, A.K. Polyphenols in Food: Cancer Prevention and Apoptosis Induction. Curr. Med. Chem. 2018, 25, 4740–4757.
- Jiang, Q.; Yang, M.; Qu, Z.; Zhou, J.; Zhang, Q. Resveratrol Enhances Anticancer Effects of Paclitaxel in HepG2 Human Liver Cancer Cells. BMC Complement Altern. Med. 2017, 17, 477.
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618.
- Arnan, X.; Claramunt-López, B.; Martinez Vilalta, J.; Estorach, M.; Poyatos, R. The Age of Monumental Olive Trees (Olea Europaea) in Northeastern Spain. Dendrochronologia 2012, 30, 11–14.
- Finicelli, M.; Squillaro, T.; Galderisi, U.; Peluso, G. Polyphenols, the Healthy Brand of Olive Oil: Insights and Perspectives. Nutrients 2021, 13, 3831.
- Serreli, G.; Deiana, M. Biological Relevance of Extra Virgin Olive Oil Polyphenols Metabolites. Antioxidants 2018, 7, 170.
- Surachmanto, E.E.; Datau, E.A. The Role of Omega-3 Fatty Acids Contained in Olive Oil. Acta Med. Indones. 2011, 43, 138–143.
- Rodriguez, M.G.; Caleja, C.; Nuñez-Estevez, B.; Pereira, E.; Fraga-Corral, M.; Reis, F.S.; Simal-Gandara, J.; Ferreira, I.C.F.R.; Prieto, M.A.; Barros, L. Flavonoids: A Group of Potential Food Additives with Beneficial Health Effects. In Natural Food Additives; Prieto, M.A., Otero, P., Eds.; IntechOpen: Rijeka, Croatia, 2021.
- Sun, L.; Luo, C.; Liu, J. Hydroxytyrosol Induces Apoptosis in Human Colon Cancer Cells through ROS Generation. Food Funct. 2014, 5, 1909–1914.
- Luo, G.; Huang, Y.; Mo, D.; Ma, N.; Gao, F.; Song, L.; Sun, X.; Xu, X.; Liu, L.; Huo, X.; et al. Tyrosol Attenuates Pro-Inflammatory Cytokines from Cultured Astrocytes and NF-ΚB Activation in in Vitro Oxygen Glucose Deprivation. Neurochem. Int. 2018, 121, 140–145.
- Kim, Y.-Y.; Lee, S.; Kim, M.-J.; Kang, B.-C.; Dhakal, H.; Choi, Y.-A.; Park, P.-H.; Choi, H.; Shin, T.-Y.; Choi, H.G.; et al. Tyrosol Attenuates Lipopolysaccharide-Induced Acute Lung Injury by Inhibiting the Inflammatory Response and Maintaining the Alveolar Capillary Barrier. Food Chem. Toxicol. 2017, 109, 526–533.
- Scotece, M.; Gómez, R.; Conde, J.; Lopez, V.; Gómez-Reino, J.J.; Lago, F.; Smith, A.B.; Gualillo, O. Further Evidence for the Anti-Inflammatory Activity of Oleocanthal: Inhibition of MIP-1α and IL-6 in J774 Macrophages and in ATDC5 Chondrocytes. Life Sci. 2012, 91, 1229–1235.
- Castejón, M.L.; Rosillo, M.Á.; Montoya, T.; González-Benjumea, A.; Fernández-Bolaños, J.M.; Alarcón-de-la-Lastra, C. Oleuropein Down-Regulated IL-1β-Induced Inflammation and Oxidative Stress in Human Synovial Fibroblast Cell Line SW982. Food Funct. 2017, 8, 1890–1898.
- Hormozi, M.; Salehi Marzijerani, A.; Baharvand, P. Effects of Hydroxytyrosol on Expression of Apoptotic Genes and Activity of Antioxidant Enzymes in LS180 Cells. Cancer Manag. Res. 2020, 12, 7913–7919.
- Sun, D.-W.; Zhang, H.-D.; Mao, L.; Mao, C.-F.; Chen, W.; Cui, M.; Ma, R.; Cao, H.-X.; Jing, C.-W.; Wang, Z.; et al. Luteolin Inhibits Breast Cancer Development and Progression In Vitro and In Vivo by Suppressing Notch Signaling and Regulating MiRNAs. Cell Physiol. Biochem. 2015, 37, 1693–1711.
- Jeon, Y.W.; Ahn, Y.E.; Chung, W.S.; Choi, H.J.; Suh, Y.J. Synergistic Effect between Celecoxib and Luteolin Is Dependent on Estrogen Receptor in Human Breast Cancer Cells. Tumor Biol. 2015, 36, 6349–6359.
- Park, S.-H.; Kim, J.-H.; Lee, D.-H.; Kang, J.-W.; Song, H.-H.; Oh, S.-R.; Yoon, D.-Y. Luteolin 8-C-β-Fucopyranoside Inhibits Invasion and Suppresses TPA-Induced MMP-9 and IL-8 via ERK/AP-1 and ERK/NF-ΚB Signaling in MCF-7 Breast Cancer Cells. Biochimie 2013, 95, 2082–2090.
- Kloesch, B.; Becker, T.; Dietersdorfer, E.; Kiener, H.; Steiner, G. Anti-Inflammatory and Apoptotic Effects of the Polyphenol Curcumin on Human Fibroblast-like Synoviocytes. Int. Immunopharmacol. 2013, 15, 400–405.
- Takayanagi, H. Mechanistic Insight into Osteoclast Differentiation in Osteoimmunology. J. Mol. Med. 2005, 83, 170–179.
- Liu, H.-T.; Ho, Y.-S. Anticancer Effect of Curcumin on Breast Cancer and Stem Cells. Food Sci. Hum. Wellness 2018, 7, 134–137.
- Abraham, J. PI3K/AKT/MTOR Pathway Inhibitors: The Ideal Combination Partners for Breast Cancer Therapies? Expert Rev. Anticancer Ther. 2015, 15, 51–68.
- Hu, S.; Xu, Y.; Meng, L.; Huang, L.; Sun, H. Curcumin Inhibits Proliferation and Promotes Apoptosis of Breast Cancer Cells. Exp. Med. 2018, 16, 1266–1272.
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Viljoen, A.M. Gingerols and Shogaols: Important Nutraceutical Principles from Ginger. Phytochemistry 2015, 117, 554–568.
- de Lima, R.M.T.; dos Reis, A.C.; de Menezes, A.-A.P.M.; de Oliveira Santos, J.V.; de Oliveira Filho, J.W.G.; de Oliveira Ferreira, J.R.; de Alencar, M.V.O.B.; da Mata, A.M.O.F.; Khan, I.N.; Islam, A.; et al. Protective and Therapeutic Potential of Ginger (Zingiber Officinale) Extract and -Gingerol in Cancer: A Comprehensive Review: Ginger Extract and -Gingerol as Anticancer Agents. Phytother. Res. 2018, 32, 1885–1907.
- Elmali, N.; Baysal, O.; Harma, A.; Esenkaya, I.; Mizrak, B. Effects of Resveratrol in Inflammatory Arthritis. Inflammation 2007, 30, 1–6.
- Zhang, Y.; Wang, G.; Wang, T.; Cao, W.; Zhang, L.; Chen, X. Nrf2–Keap1 Pathway–Mediated Effects of Resveratrol on Oxidative Stress and Apoptosis in Hydrogen Peroxide–Treated Rheumatoid Arthritis Fibroblast-like Synoviocytes. Ann. N. Y. Acad. Sci. 2019, 1457, 166–178.
- Nakayama, H.; Yaguchi, T.; Yoshiya, S.; Nishizaki, T. Resveratrol Induces Apoptosis MH7A Human Rheumatoid Arthritis Synovial Cells in a Sirtuin 1-Dependent Manner. Rheumatol. Int. 2012, 32, 151–157.
- Jang, Y.-G.; Go, R.-E.; Hwang, K.-A.; Choi, K.-C. Resveratrol Inhibits DHT-Induced Progression of Prostate Cancer Cell Line through Interfering with the AR and CXCR4 Pathway. J. Steroid Biochem. Mol. Biol. 2019, 192, 105406.
- Aires, V.; Limagne, E.; Cotte, A.K.; Latruffe, N.; Ghiringhelli, F.; Delmas, D. Resveratrol Metabolites Inhibit Human Metastatic Colon Cancer Cells Progression and Synergize with Chemotherapeutic Drugs to Induce Cell Death. Mol. Nutr. Food Res. 2013, 57, 1170–1181.




