Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Gianluca Spitaleri | -- | 1698 | 2023-06-06 08:34:36 | | | |
| 2 | Conner Chen | + 6 word(s) | 1704 | 2023-06-07 07:15:25 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Spitaleri, G.; Trillo Aliaga, P.; Attili, I.; Del Signore, E.; Corvaja, C.; Corti, C.; Crimini, E.; Passaro, A.; De Marinis, F. First-Line Randomized Clinical Trials of ALK-Is. Encyclopedia. Available online: https://encyclopedia.pub/entry/45218 (accessed on 07 February 2026).
Spitaleri G, Trillo Aliaga P, Attili I, Del Signore E, Corvaja C, Corti C, et al. First-Line Randomized Clinical Trials of ALK-Is. Encyclopedia. Available at: https://encyclopedia.pub/entry/45218. Accessed February 07, 2026.
Spitaleri, Gianluca, Pamela Trillo Aliaga, Ilaria Attili, Ester Del Signore, Carla Corvaja, Chiara Corti, Edoardo Crimini, Antonio Passaro, Filippo De Marinis. "First-Line Randomized Clinical Trials of ALK-Is" Encyclopedia, https://encyclopedia.pub/entry/45218 (accessed February 07, 2026).
Spitaleri, G., Trillo Aliaga, P., Attili, I., Del Signore, E., Corvaja, C., Corti, C., Crimini, E., Passaro, A., & De Marinis, F. (2023, June 06). First-Line Randomized Clinical Trials of ALK-Is. In Encyclopedia. https://encyclopedia.pub/entry/45218
Spitaleri, Gianluca, et al. "First-Line Randomized Clinical Trials of ALK-Is." Encyclopedia. Web. 06 June, 2023.
Copy Citation
Anaplastic lymphoma kinase (ALK) translocation amounts to around 3–7% of all non-small-cell lung cancers (NSCLCs). The clinical features of ALK+ NSCLC are an adenocarcinoma histology, younger age, limited smoking history, and brain metastases. The activity of chemotherapy and immunotherapy is modest in ALK+ disease. Several randomized trials have proven that ALK inhibitors (ALK-Is) have greater efficacy with respect to platinum-based chemotherapy and that second/third generation ALK-Is are better than crizotinib in terms of improvements in median progression-free survival and brain metastases management.
NSCLC
ALK
ALK inhibitors
1. Introduction
Anaplastic lymphoma kinase (ALK) was first detected in a subset of anaplastic large-cell lymphomas in 1994 [1]. The first description of echinoderm microtubule-associated protein-like 4 (EML-4)-ALK rearrangement in non-small-cell lung cancer (NSCLC) was reported in a Japanese male former smoker in 2007 [2]. Physiologically ALK is expressed only in the brain and spinal cord of embryos, and it is essential for neurological development [3]. In adulthood, ALK is constitutively expressed in limited nervous tissues. The aberrant expression and activation of ALK fusion proteins in cells leads to cellular transformation through a signaling network which involves the activation of the JAK/STAT3, PI3K/AKT/mTOR, and RAS/ERK pathways, which are essential for cell proliferation, cycling, and survival (Figure 1) [4]. In addition, several EML4-ALK variants and new partner genes (e.g., KIF5B, HIP1, BIRC6, MYT1L, and MPRIP, among others) have been identified [5][6][7][8][9] (Figure 2).
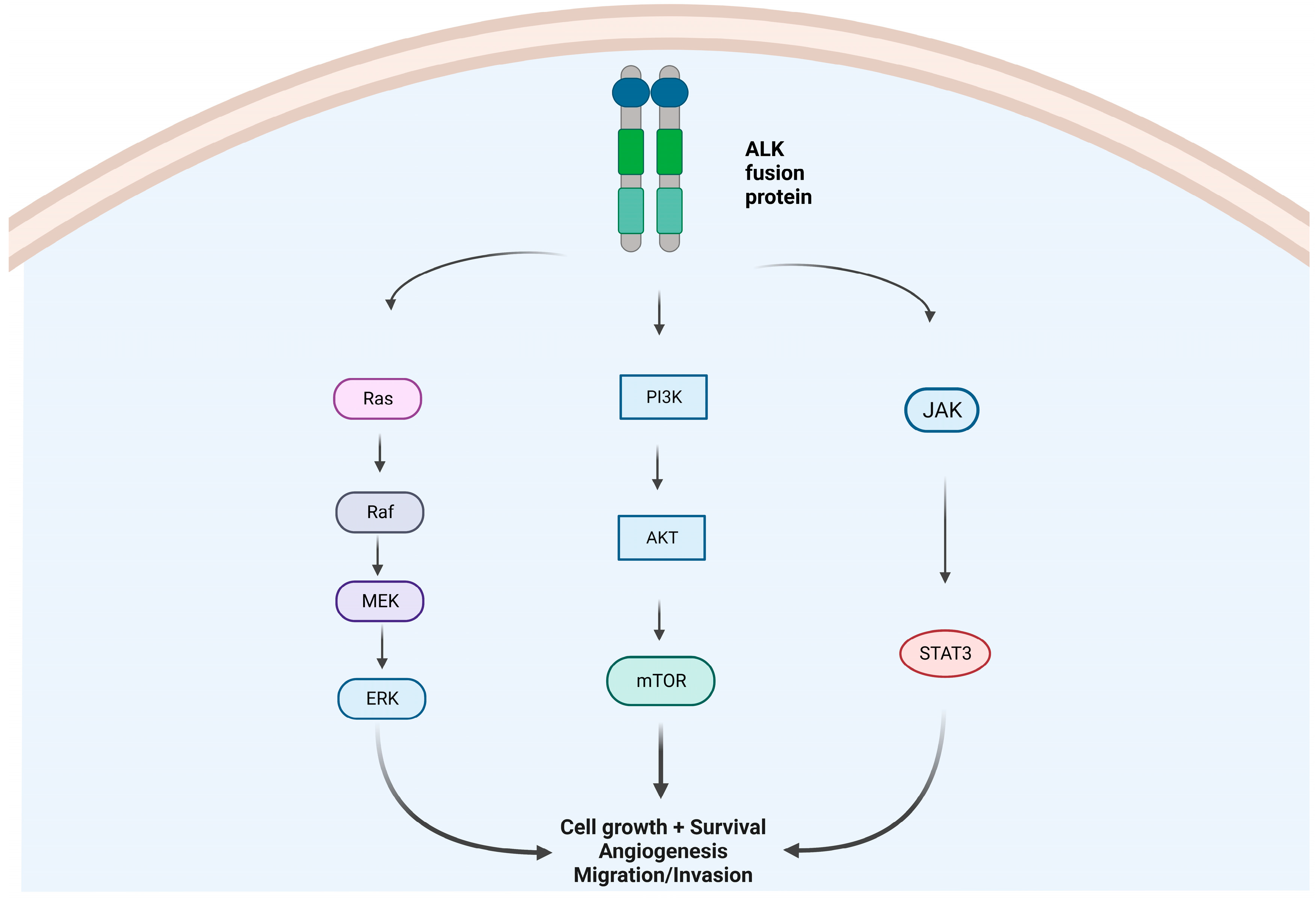
Figure 1. Intracellular molecular pathways due to an aberrant ALK-fusion protein oncogene: MAPK/ERK pathway, IK3/AKT/mTOR pathway, and JAK/STAT pathway. Abbreviations: Ras = Rat sarcoma virus proteins; Braf = serine/threonine-protein kinase B-Raf (v-Raf murine sarcoma viral oncogene homolog B); MEK = mitogen-activated protein kinase kinase; ERK = extracellular-signal-regulated kinases; PIK3 = phosphatidylinositol 3-kinases; AKT = protein kinase B (also called PKB); mTOR= mammalian target of rapamycin protein. JAK = janus kinase. STAT3 = signal transducer and activator of transcription 3. Created by biorender.com.
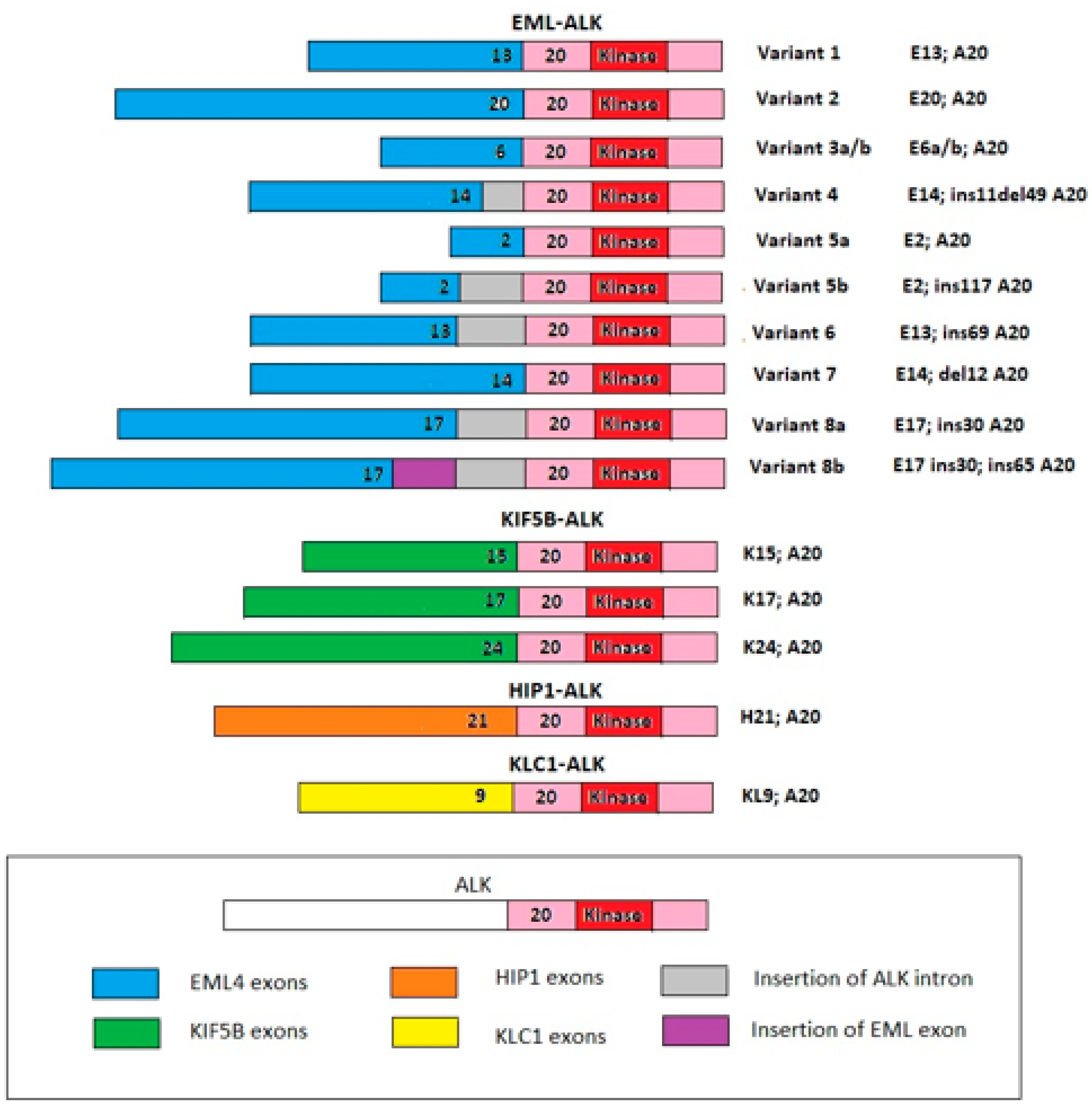
Figure 2. The most common ALK fusion genes reported in NSCLC. EML/ALK fusion is the most well-known. There are several variants for this fusion, variant 1 being the most common, followed by variant 3 and variant 2. There are also other fusion partners for ALK (some are drawn in the figure). Abbreviations: KIF5B = kinesin 5B family member; HIP1 = huntingtin interacting protein; KLC1 = kinesin light chain 1.
ALK amounts to around 3–7% of all NSCLCs and around 12% of all lung adenocarcinomas [10][11][12]. Moreover, it is associated with a more aggressive histologic grade. The frequency of ALK is higher in younger patients (median age around 50 years old), in females, and in patients with a limited smoking history (never or <10 pack-years) [10][11][12]. The incidence of ALK seems to be the same, irrespective of ethnicity [10]. Nevertheless, all non-squamous NSCLCs, regardless of these clinical features, should be tested for ALK, either by Fluorescence In Situ Hybridization (FISH) and/or by immunohistochemistry (IHC) [13]. It has been reported that concomitant mutations of other molecular gene drivers are extremely rare, representing less than 2% [14][15].
Moreover, patients with ALK+ NSCLC are more likely to receive a diagnosis of metastatic disease, and the most frequent sites of metastases are the pericardium, pleura, and liver [16]. Patients with ALK+ NSCLC are more likely to have brain metastases, particularly when they have advanced disease [17]. At the time of diagnosis of advanced disease, the incidence of brain metastases is around 25%, and their occurrence can increase to up to 45% within three years of survival with the use of non-penetrating brain barrier targeted therapies [18].
The presence of ALK seems to worsen the clinical outcomes of patients, particularly in the advanced setting [19][20][21]. In the pre-targeted therapy era, the 5-year OS rate for molecularly unselected stage IV NSCLC was approximately 2% [22].
The impact of chemotherapy on ALK+ NSCLC is modest, even though this disease seems to be more sensitive to pemetrexed-based regimens with respect to ALK- NSCLC [23][24][25], maybe due to the fact that ALK+ adenocarcinoma has the lowest levels of thymidylate synthase [25][26][27]. Three randomized clinical trials have confirmed the good performance of pemetrexed in ALK+ NSCLC, both in the second-line setting (PROFILE-1007) and in the first-line setting (PROFILE-1014 and ASCEND-4): in the second-line setting, the overall response rate (ORR) to pemetrexed was 29% with a median-progression-free survival (mPFS) of 4.2 months; in the first-line setting, the ORR was 27–45% with a mPFS of 7.0–8.1 months [28][29][30]. However, the activity of pemetrexed seems to vanish in patients who have been heavily pre-treated, as reported in the ASCEND-5 (post-crizotinib and up two lines of chemotherapy) and ALURA (post-crizotinib and one line of chemotherapy) trials, where the mPFS was 2.9 and 1.6 months, respectively [31][32].
The IMMUNOTARGET registry shows that the activity of immunotherapy (IO) in ALK+ NSCLC is poor: in 23 patients with ALK+ NSCLC, none responded to IO with mPFS of 3.1 months. Neither smoking exposure nor PD-L1 expression augmented the activity of this treatment in this subset of patients [33]. Hence, immunotherapy is not a promising treatment.
Instead, since the advent of ALK inhibitors (ALK-I), the treatment landscape and prognosis of ALK+ NSCLC patients have been radically revolutionized. Two different retrospective analyses have shown longer median survival lengths of 6.8 and 4.3 years, respectively [34][35].
2. First-Line Randomized Clinical Trials of ALK-Is
PROFILE-1014 was the first randomized clinical trial of an ALK-I. It was designed to compare crizotinib versus first-line chemotherapy (cisplatin or carboplatin plus pemetrexed) in 343 patients with advanced ALK+ (as determined centrally with the use of a Vysis ALK Break Apart FISH Probe Kit) non-squamous NSCLC who had not received previous systemic treatment for advanced disease [29]. Crossover to crizotinib treatment after disease progression was permitted for patients who had previously been assigned to the control arm. The primary endpoint was PFS as assessed by independent radiologic review (BIRC-PFS). The ORR of crizotinib was 74% versus 45% of the control arm. The mPFS was statistically significantly longer for crizotinib (10.9 months) respect to chemotherapy (7 months) with a hazard ratio (HR) of 0.45 (95% CI, 0.35 to 0.60; p < 0.001). This benefit was observed across all the subgroups. The safety profile of crizotinib was acceptable. The most common adverse events (AEs) with crizotinib were vision disorders, diarrhea, nausea, and edema. Grade (G) 3–4 AEs were 54% and 5% resulted in permanent drug discontinuation. The 4-year overall survival (OS) rate was 56.6% (95% CI, 48.3% to 64.1%) in the arm assigned to crizotinib [36]. In the ASCEND-4 trial, 376 patients with stage IIIB/IV ALK+ (centrally tested by VENTANA anti-ALK, D5F3 IHC assay) non-squamous NSCLC were randomized 1:1 to ceritinib (n = 189) or chemotherapy (n = 187) [30]. Patients randomized to chemotherapy were allowed to crossover to ceritinib at the disease progression. The primary endpoint was BIRC-PFS. The mPFS was 16.6 months in the ceritinib group versus 8.1 months in the chemotherapy group with a HR 0.55 (95% CI 0.42–0.73, p < 0.00001). Regarding toxicity: the G3/4 AEs rate was 78%, and dose reductions and discontinuation were 28% and 2%, respectively. The most common AEs related to ceritinib were diarrhea (85%), nausea (69%), vomiting (66%), and an increase in alanine aminotransferase (ALT) (60%). The performance of ceritinib was jeopardized by two findings in two scales of quality of life ‘QLQ-C30 instrument’: chemotherapy was more favorable than ceritinib for diarrhea and nausea/vomiting scales.
The Global ALEX trial was the first one to compare two ALK-Is in treatment-naïve patients with ALK+ (centrally tested by VENTANA anti-ALK, D5F3 IHC assay) NSCLC [37]. The investigators randomized 303 patients to receive either alectinib or crizotinib. Unlike the prior trials, the primary endpoint was investigator-assessed PFS. Cross-over was not allowed. The BIRC mPFS was significantly longer with alectinib (25.7 months) than with crizotinib (10.4 months) with a HR 0.50 (95% CI 0.36 to 0.70, p < 0.001). The benefit with Alectinib was consistent for all subgroups save for active smokers and patients with an ECOG PS of 2. As for the principal endpoint of the trial, the investigator assessed mPFS with alectinib was 34.8 months versus 10.9 months with crizotinib (HR 0.43, 95% CI 0.32–0.58). The OS, after a median follow-up of 48 months, was still immature with an estimated 5-year OS rate of 62.5% with alectinib and 45.5% with crizotinib [38]. The safety profile of alectinib was good: the G3-4 AEs rate was 52%, adverse events leading dose reductions and treatment discontinuation were 52% and 20%, respectively [38]. The most common AEs of alectinib were anemia (20%), myalgia (16%), increased blood bilirubin (15%), increased weight (10%), musculoskeletal pain (7%), and photosensitivity reaction (5%).
In ALTA 1L trial, 275 patients with advanced ALK + (locally tested by Ventana IHC assay and/or FISH) NSCLC, who had not previously received ALK-I, were randomized to receive brigatinib or crizotinib [39]. In the crizotinib group, crossover to brigatinib was permitted after disease progression. The primary endpoint was BIRC-PFS. After a follow-up period of 40 months, the BIRC m-PFS with brigatinib was 24 months versus 11.1 months with crizotinib (HR = 0.48, 95% CI: 0.35–0.66). The mPFS with brigatinib reached 30.8 months; the estimated 4-year OS with brigatinib was 66% [40][41]. The most common AEs were gastrointestinal events, increased blood creatine phosphokinase, cough, and increased aminotransferases. The main indicators of brigatinib toxicity were a G3/4 rate (78%) and adverse events leading to a dose reduction and treatment discontinuation (44% and 13%, respectively) [41].
In the eXalt3 trial, 290 naïve patients with ALK+ (locally tested by IHC and or FISH and after protocol amendment was centrally confirmed) NSCLCs were randomized to receive ensartinib or crizotinib [42]. The principal endpoint was BIRC-PFS, which was assessed in all randomized patients and in patients enrolled after a major protocol amendment (mITT). Crossover was not permitted. After a median follow-up of 24 months, the BIRC mPFS with ensartinib was 25.8 months versus 12.7 months with crizotinib with an HR of 0.51 (95% CI 0.35–0.72, p < 0.001). In the mITT population, the median PFS in the ensartinib group was not reached, and the median PFS in the crizotinib group was 12.7 months (HR 0.45; 95% CI, 0.30–0.66; p < 0.001). The G3-4 AE rate with ensartinib was 50%, while the rates of adverse events leading to dose reduction and treatment discontinuation were 24% and 9%, respectively. The most common events with ensartinib were skin rash, aspartate aminotransferase (AST) increase, ALT increase, pruritus, nausea, and edema [42].
In the CROWN trial, 296 naïve patients with advanced ALK+ (locally tested by FISH or IHC assay) NSCLC were randomized to receive lorlatinib or crizotinib [43]. The principal endpoint was BIRC mPFS. Crossover was not allowed. At the median follow-up of 33 months, the BIRC mPFS with lorlatinib was still not reached while the mPFS with crizotinib was 9.1 months (HR, 0.19; 95% CI, 0.131–0.274) [44]. The G3-4 toxicity rate was 75% (63% treatment-related), while the rates of adverse events leading to a dose reduction or treatment discontinuation were 21% and 11%, respectively [44]. The most common adverse events with lorlatinib were hyperlipidemia, edema, increased weight, peripheral neuropathy, and cognitive effects. Importantly, in a post-hoc analysis, the high incidence of dyslipidemia did not translate to a higher risk of cardiovascular events [44].
References
- Morris, S.W.; Kirstein, M.N.; Valentine, M.B.; Dittmer, K.G.; Shapiro, D.N.; Saltman, D.L.; Look, A.T. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science 1994, 263, 1281–1284.
- Soda, M.; Choi, Y.L.; Enomoto, M.; Takada, S.; Yamashita, Y.; Ishikawa, S.; Fujiwara, S.-I.; Watanabe, H.; Kurashina, K.; Hatanaka, H.; et al. Identification of the transforming EML4–ALK fusion gene in non-small-cell lung cancer. Nature 2007, 448, 561–566.
- Vernersson, E.; Khoo, N.K.; Henriksson, M.L.; Roos, G.; Palmer, R.H.; Hallberg, B. Characterization of the expression of the ALK receptor tyrosine kinase in mice. Gene Expr. Patterns 2006, 6, 448–461.
- Huang, H. Anaplastic Lymphoma Kinase (ALK) Receptor Tyrosine Kinase: A Catalytic Receptor with Many Faces. Int. J. Mol. Sci. 2018, 19, 3448.
- Takeuchi, K.; Choi, Y.L.; Soda, M.; Inamura, K.; Togashi, Y.; Hatano, S.; Enomoto, M.; Takada, S.; Yamashita, Y.; Satoh, Y.; et al. Multiplex Reverse Transcription-PCR Screening for EML4-ALK Fusion Transcripts. Clin. Cancer Res. 2008, 14, 6618–6624.
- Shan, L.; Jiang, P.; Xu, F.; Zhang, W.; Guo, L.; Wu, J.; Zeng, Y.; Jiao, Y.; Ying, J. BIRC6-ALK, a Novel Fusion Gene in ALK Break-Apart FISH-Negative Lung Adenocarcinoma, Responds to Crizotinib. J. Thorac. Oncol. 2015, 10, e37–e39.
- Tsou, T.C.; Gowen, K.; Ali, S.M.; Miller, V.A.; Schrock, A.B.; Lovly, C.M.; Reckamp, K.L. Variable Response to ALK Inhibitors in NSCLC with a Novel MYT1L-ALK Fusion. J. Thorac. Oncol. 2019, 14, e29–e30.
- Fang, W.; Gan, J.; Hong, S.; Lu, F.; Zhang, L. MPRIP-ALK, a Novel ALK Rearrangement That Responds to ALK Inhibition in NSCLC. J. Thorac. Oncol. 2019, 14, e148–e151.
- Li, M.; Tang, Q.; Chen, S.; Wang, Y. A novel HIP1-ALK fusion variant in lung adenocarcinoma showing resistance to Crizotinib. Lung Cancer 2021, 151, 98–100.
- Koivunen, J.P.; Mermel, C.; Zejnullahu, K.; Murphy, C.; Lifshits, E.; Holmes, A.J.; Choi, H.G.; Kim, J.; Chiang, D.; Thomas, R.; et al. EML4-ALK Fusion Gene and Efficacy of an ALK Kinase Inhibitor in Lung Cancer. Clin. Cancer Res. 2008, 14, 4275–4283.
- Shaw, A.T.; Yeap, B.Y.; Mino-Kenudson, M.; Digumarthy, S.R.; Costa, D.B.; Heist, R.S.; Solomon, B.; Stubbs, H.; Admane, S.; McDermott, U.; et al. Clinical Features and Outcome of Patients with Non–Small-Cell Lung Cancer Who Harbor EML4-ALK. J. Clin. Oncol. 2009, 27, 4247–4253.
- Song, Z.; Zheng, Y.; Wang, X.; Su, H.; Zhang, Y.; Song, Y. ALK and ROS1 rearrangements, coexistence and treatment in epidermal growth factor receptor-wild type lung adenocarcinoma: A multicenter study of 732 cases. J. Thorac. Dis. 2017, 9, 3919–3926.
- Hendriks, L.; Kerr, K.; Menis, J.; Mok, T.; Nestle, U.; Passaro, A.; Peters, S.; Planchard, D.; Smit, E.; Solomon, B.; et al. Oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 339–357.
- Zhuang, X.; Zhao, C.; Li, J.; Su, C.; Chen, X.; Ren, S.; Li, X.; Zhou, C. Clinical features and therapeutic options in non-small cell lung cancer patients with concomitant mutations of EGFR, ALK, ROS1, KRAS or BRAF. Cancer Med. 2019, 8, 2858–2866.
- Passaro, A.; Attili, I.; Rappa, A.; Vacirca, D.; Ranghiero, A.; Fumagalli, C.; Guarize, J.; Spaggiari, L.; de Marinis, F.; Barberis, M.; et al. Genomic Characterization of Concurrent Alterations in Non-Small Cell Lung Cancer (NSCLC) Harboring Actionable Mutations. Cancers 2021, 13, 2172.
- Doebele, R.C.; Lu, X.; Sumey, C.; Bs, D.A.M.; Weickhardt, A.J.; Oton, A.B.; Bunn, P.A.; Barón, A.E.; Franklin, W.A.; Aisner, D.L.; et al. Oncogene status predicts patterns of metastatic spread in treatment-naive nonsmall cell lung cancer. Cancer 2012, 118, 4502–4511.
- Yang, B.; Lee, H.; Um, S.-W.; Kim, K.; Zo, J.I.; Shim, Y.M.; Kwon, O.J.; Lee, K.S.; Ahn, M.-J.; Kim, H. Incidence of brain metastasis in lung adenocarcinoma at initial diagnosis on the basis of stage and genetic alterations. Lung Cancer 2019, 129, 28–34.
- Rangachari, D.; Yamaguchi, N.; VanderLaan, P.A.; Folch, E.; Mahadevan, A.; Floyd, S.R.; Uhlmann, E.J.; Wong, E.T.; Dahlberg, S.E.; Huberman, M.S.; et al. Brain metastases in patients with EGFR -mutated or ALK -rearranged non-small-cell lung cancers. Lung Cancer 2015, 88, 108–111.
- Yang, P.; Kulig, K.; Boland, J.M.; Erickson-Johnson, M.R.; Oliveira, A.M.; Wampfler, J.; Jatoi, A.; Deschamps, C.; Marks, R.; Fortner, C.; et al. Worse Disease-Free Survival in Never-Smokers with ALK+ Lung Adenocarcinoma. J. Thorac. Oncol. 2012, 7, 90–97.
- Sun, J.-M.; Lira, M.; Pandya, K.; Choi, Y.-L.; Ahn, J.S.; Mao, M.; Han, J.; Park, K.; Ahn, M.-J.; Kim, J. Clinical characteristics associated with ALK rearrangements in never-smokers with pulmonary adenocarcinoma. Lung Cancer 2014, 83, 259–264.
- Blackhall, F.; Peters, S.; Bubendorf, L.; Dafni, U.; Kerr, K.M.; Hager, H.; Soltermann, A.; O’Byrne, K.J.; Dooms, C.; Sejda, A.; et al. Prevalence and Clinical Outcomes for Patients with ALK-Positive Resected Stage I to III Adenocarcinoma: Results from the European Thoracic Oncology Platform Lungscape Project. J. Clin. Oncol. 2014, 32, 2780–2787.
- Cetin, K.; Ettinger, D.S.; Hei, Y.-J.; O’Malley, C.D. Survival by histologic subtype in stage IV nonsmall cell lung cancer based on data from the Surveillance, Epidemiology and End Results Program. Clin. Epidemiol. 2011, 3, 139–148.
- Camidge, D.R.; Kono, S.A.; Lu, X.; Okuyama, S.; Barón, A.E.; Oton, A.B.; Davies, A.M.; Varella-Garcia, M.; Franklin, W.; Doebele, R.C. Anaplastic lymphoma kinase Gene Rearrangements in Non-small Cell Lung Cancer are Associated with Prolonged Progression-Free Survival on Pemetrexed. J. Thorac. Oncol. 2011, 6, 774–780.
- Lee, J.-O.; Kim, T.M.; Lee, S.-H.; Kim, D.-W.; Kim, S.; Jeon, Y.-K.; Chung, D.H.; Kim, W.-H.; Kim, Y.T.; Yang, S.-C.; et al. Anaplastic Lymphoma Kinase Translocation: A Predictive Biomarker of Pemetrexed in Patients with Non-small Cell Lung Cancer. J. Thorac. Oncol. 2011, 6, 1474–1480.
- Shaw, A.; Varghese, A.; Solomon, B.; Costa, D.; Novello, S.; Mino-Kenudson, M.; Awad, M.; Engelman, J.; Riely, G.; Monica, V.; et al. Pemetrexed-based chemotherapy in patients with advanced, ALK-positive non-small cell lung cancer. Ann. Oncol. 2013, 24, 59–66.
- Gandara, D.R.; Huang, E.; Desai, S.; Mack, P.C.; Beckett, L.; Stephens, C.; Zeger, G.; Danenberg, K.D.; Maus, M.K.H.; Li, T. Thymidylate synthase (TS) gene expression in patients with ALK positive (+) non-small cell lung cancer (NSCLC): Implications for therapy. J. Clin. Oncol. 2012, 30, 7582.
- Xu, C.-W.; Wang, G.; Wang, W.-L.; Gao, W.-B.; Han, C.-J.; Gao, J.-S.; Zhang, L.-Y.; Li, Y.; Wang, L.; Zhang, Y.-P.; et al. Association between EML4-ALK fusion gene and thymidylate synthase mRNA expression in non-small cell lung cancer tissues. Exp. Ther. Med. 2015, 9, 2151–2154.
- Shaw, A.T.; Kim, D.-W.; Nakagawa, K.; Seto, T.; Crinó, L.; Ahn, M.-J.; De Pas, T.; Besse, B.; Solomon, B.J.; Blackhall, F.; et al. Crizotinib versus Chemotherapy in AdvancedALK-Positive Lung Cancer. N. Engl. J. Med. 2013, 368, 2385–2394.
- Solomon, B.J.; Mok, T.; Kim, D.-W.; Wu, Y.-L.; Nakagawa, K.; Mekhail, T.; Felip, E.; Cappuzzo, F.; Paolini, J.; Usari, T.; et al. First-Line Crizotinib versus Chemotherapy in ALK-Positive Lung Cancer. N. Engl. J. Med. 2014, 371, 2167–2177.
- Soria, J.-C.; Tan, D.S.W.; Chiari, R.; Wu, Y.-L.; Paz-Ares, L.; Wolf, J.; Geater, S.L.; Orlov, S.; Cortinovis, D.; Yu, C.-J.; et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK -rearranged non-small-cell lung cancer (ASCEND-4): A randomised, open-label, phase 3 study. Lancet 2017, 389, 917–929.
- Shaw, A.T.; Kim, T.M.; Crinò, L.; Gridelli, C.; Kiura, K.; Liu, G.; Novello, S.; Bearz, A.; Gautschi, O.; Mok, T.; et al. Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ASCEND-5): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2017, 18, 874–886.
- Novello, S.; Mazières, J.; Oh, I.-J.; de Castro, J.; Migliorino, M.; Helland, A.; Dziadziuszko, R.; Griesinger, F.; Kotb, A.; Zeaiter, A.; et al. Alectinib versus chemotherapy in crizotinib-pretreated anaplastic lymphoma kinase (ALK)-positive non-small-cell lung cancer: Results from the phase III ALUR study. Ann. Oncol. 2018, 29, 1409–1416.
- Mazieres, J.; Drilon, A.; Lusque, A.B.; Mhanna, L.; Cortot, A.; Mezquita, L.; Thai, A.A.; Mascaux, C.; Couraud, S.; Veillon, R.; et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: Results from the IMMUNOTARGET registry. Ann. Oncol. 2019, 30, 1321–1328.
- Pacheco, J.M.; Gao, D.; Smith, D.; Purcell, T.; Hancock, M.; Bunn, P.; Robin, T.; Liu, A.; Karam, S.; Gaspar, L.; et al. Natural History and Factors Associated with Overall Survival in Stage IV ALK-Rearranged Non–Small Cell Lung Cancer. J. Thorac. Oncol. 2019, 14, 691–700.
- Moskovitz, M.; Dudnik, E.; Shamai, S.; Rotenberg, Y.; Popovich-Hadari, N.; Wollner, M.; Zer, A.; Gottfried, M.; Mishaeli, M.; Rosenberg, S.K.; et al. ALK Inhibitors or Chemotherapy for Third Line in ALK-positive NSCLC? Real-world Data. Oncologist 2022, 27, e76–e84.
- Solomon, B.J.; Kim, D.-W.; Wu, Y.-L.; Nakagawa, K.; Mekhail, T.; Felip, E.; Cappuzzo, F.; Paolini, J.; Usari, T.; Tang, Y.; et al. Final Overall Survival Analysis From a Study Comparing First-Line Crizotinib Versus Chemotherapy in ALK-Mutation-Positive Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 2251–2258.
- Peters, S.; Camidge, D.R.; Shaw, A.T.; Gadgeel, S.; Ahn, J.S.; Kim, D.W.; Ou, S.H.I.; Pérol, M.; Dziadziuszko, R.; Rosell, R.; et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 829–838.
- Mok, T.; Camidge, D.; Gadgeel, S.; Rosell, R.; Dziadziuszko, R.; Kim, D.-W.; Pérol, M.; Ou, S.-H.; Ahn, J.; Shaw, A.; et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann. Oncol. 2020, 31, 1056–1064.
- Camidge, D.R.; Kim, H.R.; Ahn, M.-J.; Yang, J.C.-H.; Han, J.-Y.; Lee, J.-S.; Hochmair, M.J.; Li, J.Y.-C.; Chang, G.-C.; Lee, K.H.; et al. Brigatinib versus Crizotinib in ALK-Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2027–2039.
- Camidge, D.R.; Kim, H.R.; Ahn, M.-J.; Yang, J.C.H.; Han, J.-Y.; Hochmair, M.J.; Lee, K.H.; Delmonte, A.; Campelo, M.R.G.; Kim, D.-W.; et al. Brigatinib versus Crizotinib in Advanced ALK Inhibitor–Naive ALK-Positive Non–Small Cell Lung Cancer: Second Interim Analysis of the Phase III ALTA-1L Trial. J. Clin. Oncol. 2020, 38, 3592–3603.
- Camidge, D.R.; Kim, H.R.; Ahn, M.-J.; Yang, J.C.; Han, J.-Y.; Hochmair, M.J.; Lee, K.H.; Delmonte, A.; Campelo, M.R.G.; Kim, D.-W.; et al. Brigatinib Versus Crizotinib in ALK Inhibitor–Naive Advanced ALK-Positive NSCLC: Final Results of Phase 3 ALTA-1L Trial. J. Thorac. Oncol. 2021, 16, 2091–2108.
- Horn, L.; Wang, Z.; Wu, G.; Poddubskaya, E.; Mok, T.; Reck, M.; Wakelee, H.; Chiappori, A.A.; Lee, D.H.; Breder, V.; et al. Ensartinib vs Crizotinib for Patients with Anaplastic Lymphoma Kinase−Positive Non–Small Cell Lung Cancer. JAMA Oncol. 2021, 7, 1617.
- Shaw, A.T.; Bauer, T.M.; de Marinis, F.; Felip, E.; Goto, Y.; Liu, G.; Mazieres, J.; Kim, D.-W.; Mok, T.; Polli, A.; et al. First-Line Lorlatinib or Crizotinib in Advanced ALK-Positive Lung Cancer. N. Engl. J. Med. 2020, 383, 2018–2029.
- Solomon, B.J.; Bauer, T.M.; Mok, T.S.K.; Liu, G.; Mazieres, J.; de Marinis, F.; Goto, Y.; Kim, D.-W.; Wu, Y.-L.; Jassem, J.; et al. Efficacy and safety of first-line lorlatinib versus crizotinib in patients with advanced, ALK-positive non-small-cell lung cancer: Updated analysis of data from the phase 3, randomised, open-label CROWN study. Lancet Respir. Med. 2022, 11, 354–366.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
07 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




