1. Introduction
Honey is a sweet natural product that is produced by managed and wild bees, derived from the nectar of flowers. It is made up of various components such as sugar, protein, vitamins, minerals, aromatic substances, polyphenols, pigments, beeswax, and pollen that contribute to its color, smell, and flavor
[1][2]. However, honey adulteration is a growing concern, including the production of honey by feeding bees with commercial industrial sugar
[3], the addition of foreign sugar, as well as mislabeling. The botanical, geographical, and entomological origin of honey greatly influences its price
[4][5][6][7][8][9][10]. Among honeybees, the European honeybee,
Apis mellifera, and the Eastern honeybee,
A. cerana, are two dominant species for honey production
[11].
Apis cerana is native to temperate and tropical Asia and is widespread from Afghanistan to far east Russia and Japan, north into the foothills of the Himalayan mountains, and south through Indonesia
[12][13]. This species was introduced to New Guinea in the late 1970s from Indonesia and became invasive to Australia in 1998
[14], and eradication attempts against it remain unsuccessful
[14][15][16]. European honeybee,
A. mellifera, is native to Europe, the Middle East, and Africa, and was introduced to other continents in the 17th century. It is now widespread around the world
[17][18]. Because
A. mellifera is more productive and suitable for modern beekeeping, it has been introduced into many
A. cerana habitats, such as China, South Korea, and some Southeast Asian countries
[19][20]. Afterwards, the number of
A. cerana colonies declined due to high interspecific competition for the same niche as
A. mellifera. Other than managed honeybees, honey can be harvested from the colonies of wild
Apis bees and stingless bees. As a result of recent discoveries about the benefits of honey consumption, the demand for natural honey has increased and the price of natural honey is five to seven times higher than
A. mellifera honey
[8][11]. Therefore, honey from
A. cerana and wild bees is very prone to adulteration, either by mislabeling or mixing the natural honey with cheaper
A. mellifera honey to gain a higher profit
[8][11].
2. Identification Methods of Entomological Origin of Honey
2.1. Protein Based Method
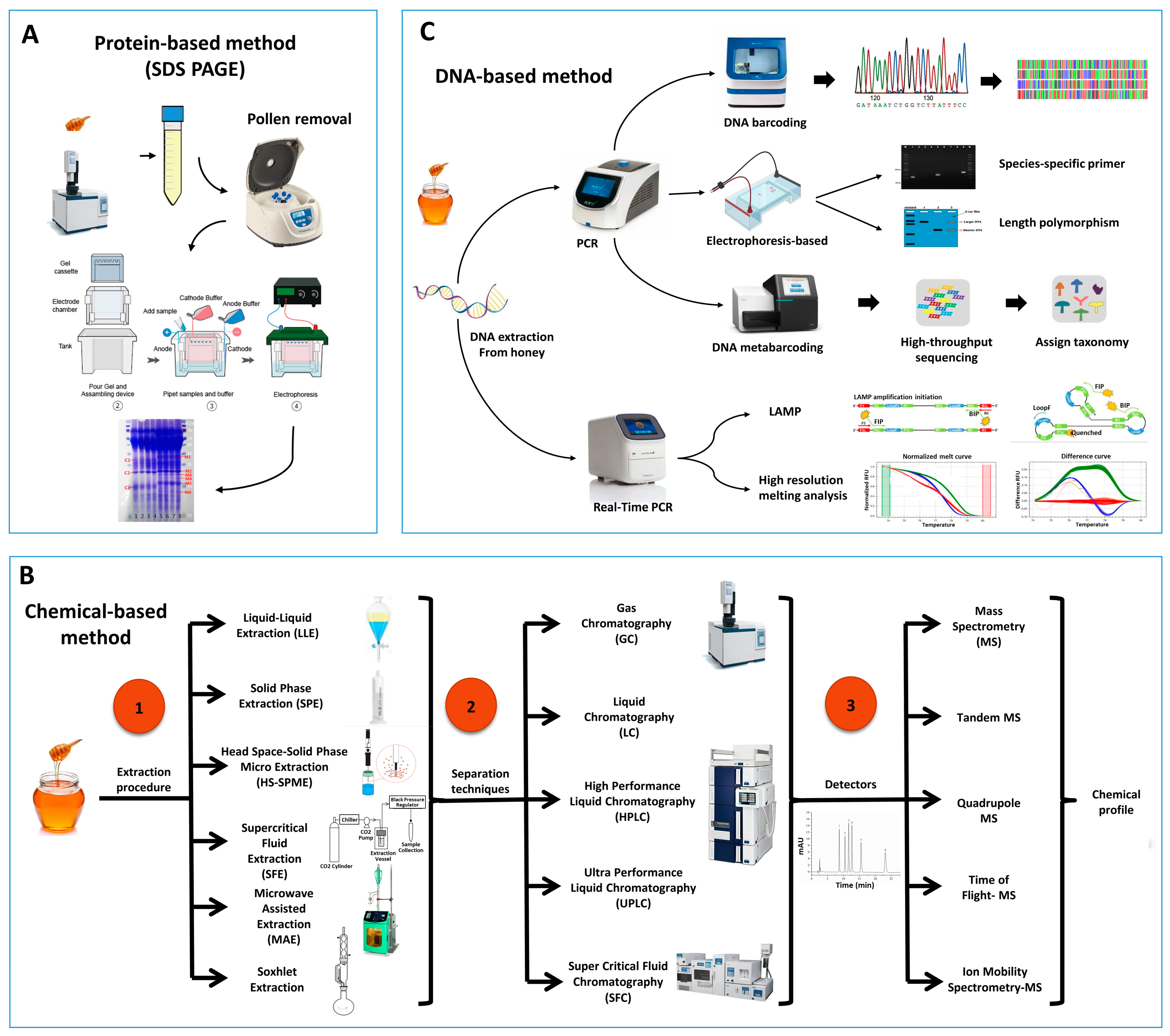
Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) is a popular technique for separating proteins based on their mass. In an electric field, proteins migrate through the gel from the negatively charged electrode toward the positively charged electrode, allowing for separation of proteins based on molecular weight. SDS-PAGE, as depicted in
Figure 1A, has frequently been used to identify the entomological origin of honey from both managed honeybees and stingless bees
[21][22]. Despite the limited amount of protein in honey, some studies have been conducted on its protein profile using methods such as Matrix-Assisted Laser Desorption Ionization Time of Flight Mass Spectrometry (MALDI-TOF-MS) and Liquid Chromatography Mass Spectrometry (LC-MS/MS)
[1][23][24]. However, in the majority of cases, these studies have not specifically focused on distinguishing the entomological source of honey. The Major Royal Jelly Proteins (MRJPs) are a group of nine homologous proteins (MRJP1–MRJP9)
[25][26][27] secreted by worker bees. Along with pollen proteins, they are significant protein resources in honey
[1][28]. As the MRJPs of different bee species are specific and they remain in the honey, there is a possibility of using such a protein to identify the entomological origin of honey. Previous studies have shown that the protein profile of SDS-PAGE is different between honey samples from
A. cerana and
A. mellifera [21]. Moreover, Won et al.
[1] showed that MRJP1 has different molecular weights in different honeybees (56 kDa in
A. cerana and 59 kDa in
A. mellifera) despite having similar primary structure. By producing an artificial marker protein from
Escherichia coli and co-electrophoresing it with honey samples, they successfully discriminated the entomological origin of honey samples by SDS–PAGE and found several adulterated honey samples. Additionally, Zhang et al.
[29] indicated that three species-specific bands with molecular weight between 15.0 and 29.4 KDa are present in
A. cerana honey, while
A. mellifera honey is characterized by the appearance of six species-specific bands with molecular weights between 13.8 and 33.1 KDa.
Figure 1. Schematic representation of the commonly used sample processing and techniques for the study of the entomological origin of honey. (A) Protein-based method, (B) Chemical-based methods emphasizing on different extraction methods, separation techniques, and Detection of identification of compounds, (C) DNA-based methods.
2.2. Physicochemical Properties and Bioactive Characterization of Honey to Determine Entomological Origin
The chemical properties of honey have been used to identify the entomological origin of honey. Zannat et al.
[30] showed that the physicochemical profile of honey such as moisture, formaldehyde and Hydroxymethylfurfural (HMF), pH, and color were key determinants to identify the entomological origin of Bangladesh honey samples, such as
A. cerana,
A. dorsata, and
A. mellifera honey. This method can be applied to detect the mislabeling of honey. Furthermore, it seems that Principle Component Analysis (PCA) involving the physicochemical properties of honey can be useful for identification of the entomological origin of honey samples regionally, but the results cannot always be extended to other regions due to the high variation in the chemical properties of honey from different localities. For instance, the moisture content of honey samples from Bangladesh were 21.3, 19, and 17% from
A. dorsata,
A. cerana and
A. mellifera, respectively. However, the reported amount of moisture for
A. cerana honey from Thailand was 21.2%
[31] which is comparable with the amount of moisture in
A. dorsata honey from Bangladesh. Furthermore, moisture content of
A. mellifera honey from Thailand was reported as 18.8%, which was similar to the amount of moisture in
A. cerana honey from Bangladesh. On the other hand, using the color of honey as a method of recognition cannot be recommended due to its variation based on the floral source of honey, storage duration, and storage conditions. However, since multidimensional analysis, such as PCA, is required for categorization of honey samples with different origins, more studies are required to understand the possibility of the usage of this method in identification of the entomological origin of honey.
Another attempt to identify the entomological origin of honey is bioactive characterization
[32][33][34][35] and mineral content
[36][37]. Kelulut honey, produced by stingless bees
Heterotrigona itama, was found distinguishable from
Apis spp. honey based on characteristics such as high moisture, free acidity, color intensity, and antioxidant capacities measured by AEAC and FRAP assays
[33]. Findings of the study by Wu et al.
[35] illustrated that
Lepidotrigona flavibasis honey exhibited the highest antioxidant activity (in terms of DPPH and FRAP assay), proline content, flavonoid content, and phenolic content among
A. cerana cerana,
A. dorsata, and
L. flavibasis honey samples. Rodríguez-Malavera et al.
[32] characterized 16 honey samples from 10 stingless bee species (
Melipona crinita,
M. eburnea,
M. grandis,
M. illota,
Nannotrigona melanocera,
Partamona epiphytophila,
Ptilotrigona lurida,
Scaptotrigona polystica,
Scaura latitarsis, and
Tetragonisca angustula) based on physicochemical properties (color and moisture), biochemical components (such as flavonoid, polyphenol, nitrites and protein contents), and bioactive properties (including antibacterial and antioxidant activities). Although the Trolox Equivalent Antioxidant Capacities (TEAC) measured by decoloration of the ABTS + ● radical cation were found to be different for honey samples from different bee species, the sample number of individual honey types was quite limited
[32]. Biluca et al.
[38] demonstrated the difference in moisture content and acidity on the 35 honey samples from stingless bees in Brazil compared to values of
Apis mellifera. Although the study indicated higher antioxidant activities and phenolic contents of the stingless bee honey, there was no specific marker for identifying its entomological origin
[38]. A review by Ávila et al.
[34] also demonstrated the broader biological activities of stingless bees compared to
Apis mellifera honey. Kek et al.
[36] indicated the possibility of distinguishing honey from different bee species such as
Apis spp. and
Heterotrigona spp. based chemical composition and mineral contents. However, although honey of different bee species is characterized with different physicochemical properties, bioactive characteristics, and minerals profile, it is often influenced by geographical location, floral origin, and storage conditions.
2.3. Chemical Profiling to Authenticate Entomological Origin of Honey
Due to intricate chemical makeup of honey with variations of physicochemical properties, integration of chemical analyses with compound profiling are employed in scientific studies on the authentication of honey. Figure 1B represents the typical approach of sample processing and chromatographic techniques used widely in scientific studies.
The analysis of volatile compounds is an alternative and promising approach in the authentication of honey samples of different entomological origin. Volatile compounds have a high vapor pressure at room temperature, and therefore, easily evaporate and turn into gas. Besides contributing to the unique fragrance, flower volatiles play vital role in attracting pollinators. Several studies have indicated that these volatile compounds and metabolites remain consistent in flowers, nectar, or honeydew which can be traced back to the specific floral source of the honey. For instance, 2-methoxybenzoic acid and 3-phenyllactic acid are biomarkers for Manuka honey, and methyl anthranilate is a marker for citrus honey
[37][39]. Volatile compounds also have been reported for identifying the entomological differences of honey
[40][41]. Gas Chromatography Mass Spectrometry (GC-MS) is a widely used technique which requires complex sample processing and pretreatment. A well-accepted method for the extraction of volatile compounds prior to separation and identification is Headspace Solid Phase Micro Extraction (HS-SPME). However, this technique requires rigorous testing and optimization of various factors, such as the type of fibers and ionic strength of the extraction medium, to ensure accurate and reliable results. Sharin et al.
[42] discriminated Malaysian stingless bee honey according to the different entomological origin (
Heterotrigona bakei,
Geniotrigona thoracica,
Tetrigona binghami) based on volatile compound profiling. Partial Least Square Discriminant Analysis (PLS-DA) model based on physicochemical properties such as pH, moisture, total soluble solid, ash, electric conductivity, and 13 significant volatile compounds, clearly differentiated the honey samples (n = 75) according to three stingless bee species.
T. binghami honey is marked by significantly higher electrical conductivity, moisture and ash content, and high abundance of 2,6,6-trimethyl-1-cyclohexene-1-carboxaldehyde,2,6,6-trimethyl-1-cyclohexene-1-acetaldehyde and ethyl 2-(5-methyl-5-vinyltetrahydrofuran-2-yl) propan-2-yl carbonate. Copaene was proposed as a chemical marker for
G. thoracica honey. In this study, prior to GC-MS analysis, chemical methods were carried out to isolate maximum number of potential volatile compounds from the complex honey matrices. One of the challenges of using GC-MS is inconsistency in results due to variations in the complexity in the type of food samples, including honey, and the pretreatment process. On the other hand, Headspace Gas Chromatography Ion mobility Spectrometry (HS-GC-IMS) is a method with no prior complex pretreatment of the sample. Mass spectrometry separates compounds by their mass-to-charge ratio, while Ion Mobility Spectrometry (IMS) is a post-ionization separation technique that separates ions by their size, shape, and charge. Briefly, the separation method works by driving ions through a gas-filled chamber using an electric field, and the ions separate based on their shape, with the more compact ions moving faster than those with an open structure. In a study with lychee honey, 20 from
Apis cerana and 20 from
Apis mellifera, from Guangdong province in southern China, the presence of volatiles such as 1-nonanol, phenethyl acetate, and 1-heptanol were significantly higher in
Apis cerana honey and therefore, can be used as markers for
cerana honey
[41]. Differences of volatile compounds were found between
Apis cerana and
Apis mellifera honeycomb
[43]. Some scientific studies used beeswax to differentiate the entomological origin of honeybees and stingless bees. Xu et al.
[44] demonstrated that the differences between the beeswax of
A. cerana cerana and
A. mellifera ligustica were mainly based on the content and composition of hydrocarbons, monoesters, and free acids. After GC-MS/MS analysis of honey from
A. mellifera and
A. cerana colonies, Zhang et al.
[29] found that Hentriacontane and 17-Pentatriacontene were the characteristic components of
A. mellifera and
A. cerana, respectively.
Metabolomics approaches are also used to discriminate honey of different entomological origin. A comprehensive LC-MS based metabolomics approach revealed that 3-amino-2-naphthoic acid and methyl indole-3-acetate are potential markers for
Apis cerana honey, as these compounds were present in higher amounts in
Apis cerana honey. On the other hand, kynurenic acid was determined as a marker for
Apis mellifera honey
[45]. In this study
[45], Electrospray Ionization Quadrupole Orbitrap High-Resolution Mass Spectrometry was used.
1H NMR-based metabolomics approach is also used to authenticate entomological as well as floral origin of honey
[46][47][48]. Aqueous (in D
2O) as well as chloroform extracts of honey were prepared, subjected to
1H NMR analysis followed by chemometric analysis Orthogonal Projection to Latent Structure Discrimination Analysis (OPLS-DA)
[46]. Unique metabolic fingerprints were obtained for different honey types such as
Apis mellifera,
Geotrigona-Trigona,
Melipona, and
Scaptotrigona [46].
Geotrigona-Trigona honeys contained relatively low quantities of fructose and glucose, and higher contents of di- and tri-saccharides such as raffinose, isomaltose, isomaltotriose, melibiose, and palatinose;
Melipona pot-honey contained relatively low amount of turanose and of di- and tri-saccharides in comparison to the other honeys. On the other hand,
Apis mellifera honey was characterized by higher contents of glucose and fructose. Concerning to the minor chemical compounds,
Geotrigona-Trigona honey differed from other types of honeys in the content of 3-hydroxy-2-butanone and 2,3-butanediol
[46]. Schievano et al.
[47] used NMR-based metabolomics approach to 67 samples to classify them according to entomological origins i.e.,
Apis mellifera,
Melipona aff. Fuscopilosa, and
Trigona clavipes. Another study
[48] employed
1H NMR Spectroscopy and Ultra-High Performance Liquid Chromatography coupled with Quadrupole Time of Flight Mass Spectrometry (UHPLC-QTOF-MS) followed by chemometrics analysis to classify raw stingless bee honey samples based on entomological origin using. The NMR spectral data indicated presence of
d-Fructofuranose in
Heterotrigona itama honey, β-
d-Glucose,
d-Xylose, and α-
d-Glucose in
Geniotrogona thoracica honey, and
l-Lactic acid, Acetic acid, and
l-Alanine in
Trigona apicalis honey
[48].
Fourier Transform Infrared (FTIR) spectroscopy is also used for entomological differentiation of honey. FTIR spectral data subjected to PCA showed distinguished clusters for
A. mellifera,
A.dorsata,
A. cerana, and
Trigona honey samples collected from different provinces of Philippine
[49]. FTIR spectroscopy was also applied in order to authenticate honey i.e., differentiation between real and fake honey
[50]. The study
[50] also depicted that the wavelength range 1600–1700 cm
−1 was the best to classify the
Apis spp. and
Tetragonula spp. honey samples.
2.4. DNA-Based Method
Recently, DNA-based methods are considered as a rapid, accurate, and suitable tool for species identification in animal products and processed foods
[51][52][53][54]. This method relies on the identification of insect DNA present within the honey sample, as represented in
Figure 1C. It is more precise, reliable, quick, and cost effective for analyzing large sample sizes compared to previously described methods
[10]. Most DNA is located in the cell nucleus (nuclear DNA), but a small amount of DNA can also be found in the mitochondria (mitochondrial DNA or mtDNA). The method is based on Polymerase Chain Reaction (PCR) and the different honey samples can be identified according to DNA barcoding or by analyzing the pattern of bands on gel electrophoresis.
2.4.1. DNA Barcoding
DNA barcoding is a molecular method that involves amplifying a short region of the target genome through PCR and comparing the resulting sequences with reference sequences available in public databases, such as GenBank, to identify any biological species
[55]. It has been widely used for species-level identification and has contributed to the genetic traceability of livestock, agricultural crops, and their food products
[55][56]. Recently, DNA barcoding has been developed to identify the entomological origin of honey from
A. dorsata,
A. mellifera,
A. cerana, and
Heterotrigona itama [57]. Several sets of primers have been designed to amplify the Cytochrome Oxidase I (COI) or 16S rRNA part of mitochondrial DNA (mtDNA) (
Table 1). After sequencing, the entomological origin of honey can be identified
[57][58]. Furthermore, this Sanger sequencing-based technique has been used to distinguish honey samples derived from different mitotypes (C1 and C2) of the C lineage of
Apis mellifera based on the sequence of 170 bp obtained from the primer set targeting tRNAleu-COX2 intergenic region of mtDNA
[59]. Although this method has been successful in identifying the species of honeybees, it has limitations in identifying the entomological origin of honey in counterfeit mixed honey samples due to the constraints of the Sanger sequencing approach.
Table 1. The primer sets which have been applied in the identification of the entomological origin of honey samples through DNA barcoding or metabarcoding approach.
2.4.2. Usage of Species-Specific Primers
Zhang et al.
[10] developed a DNA-based method for identifying honey samples from
A. cerana and
A. mellifera using two sets of species-specific primers that target MRJP2. The method involves a double PCR followed by gel electrophoresis, which results in an amplicon size of 212 bp for
A. cerana and 560 bp for
A. mellifera (
Table 2). The specificity of the primer sets also enables the detection of honey fraud using a multiplex PCR, where both sets of primers are used simultaneously in one PCR reaction to detect DNA from both species. This method successfully differentiated honey originated from
A. cerana javana and
A. mellifera in Indonesia. However, the authors mentioned that M-F and M-R primer set has to be used with caution since PCR amplicons were observed while using these primers and the DNA extracted from
A. cerana honey in low annealing temperature
[61].
Table 2. Species-specific primer sets for identification of honey samples through PCR or Real-Time PCR.
Mitochondrial DNA is present in most cells of the organism in relatively high copy numbers. mtDNA is inherited maternally and characterized by a high genetic variation between related species and a low intraspecific variation
[63][64][65]. Therefore, it is suitable for taxonomic purposes, such as species identification through DNA barcoding and phylogenetic analysis. Among all mitochondrial genes, COI has shown to be the most interesting and largely used, given its lower mutation rates and high incidence of nucleotide substitution at the third codon position when compared to other protein-coding genes
[66][67]. Kim et al.
[8] developed two sets of species-specific primers targeting 133 bp and 178 bp of COI gene for
A. mellifera and one set of primers with amplicon size of 178 bp for amplification of only
A. cerana DNA (
Table 2). Although the sizes of the amplicons are suitable enough for relatively old honey samples as well, Zhang et al.
[10] claimed that the
A. mellifera primer sets are not specific enough for distinguishing of
A. cerana honey originated from China. Later, Soares et al.
[11] designed species-specific primers targeting 111 bp of the tRNAleu-cox2 intergenic region to detect
A. cerana DNA through PCR. Recently, Mohamadzade Namin et al.
[62] developed three sets of species-specific primers targeting NADH Dehydrogenase 2 (ND2) region of mtDNA (
Table 2) in order to distinguish entomological origin of major types of honey in Asian market from
A. cerana,
A. dorsata, and
A. mellifera. The results of the specificity test indicated the possibility of differentiation of honey samples based on gel electrophoresis pattern (223, 301, and 376 bp for
A. cerana,
A. dorsata, and
A. mellifera, respectively).
2.4.3. Real-Time PCR-Based Methods
High-Resolution Melting Analysis
Quantitative analysis of DNA fragment melt curves after PCR amplification is known as High-Resolution Melt Analysis (HRM Analysis). HRM Analysis is a modern version of amplicon melting analysis. To perform HRM, a real-time PCR detection system with exceptional thermal stability and sensitivity, as well as dedicated software, is required. The use of high-quality qPCR instruments and DNA-binding dyes has enabled the identification of even minor variations in nucleic acid sequences by precisely melting double-stranded PCR amplicons
[68]. The species-specific primers developed by Zhang et al.
[10] to distinguish
A. cerana honey and
A. mellifera honey through gel electrophoresis pattern are also applicable in Real-Time PCR assay through melting curve analysis as a single melt peak for each species was observed (76.4 °C for
A. cerana and 82.9 °C for
A. mellifera). In addition, the melting curve analysis demonstrated the possibility of using the primer sets developed by Mohamadzade Namin et al.
[62] to identify the entomological origin of honey from
A. cerana (Tm: 71.9 °C),
A. dorsata (Tm: 69.2 °C), and
A. mellifera (Tm: 72.4 °C). Another set of primer was developed by Soares et al.
[11] to amplify 140 bp of 16S rRNA gene of the mtDNA to differentiate
A. cerana and
A. mellifera honey through high-resolution melting analysis of Real-time PCR.
This method has been used by Soares et al.
[69] to distinguish the entomological origin of honey from different lineages of European honeybee,
A. mellifera. In their study
[69], a two-step method was employed that involved a PCR approach for identifying the A lineage through agarose gel electrophoresis, followed by a real-time PCR that distinguished between the M and C lineages using their high-resolution melting profile. The developed primers targeting 150 bp of the COI gene can be applied to differentiate lineage A, C, and M from various European countries. Since the nucleotide substitutions is the source of the differences between different clusters in the high-resolution melting curve analysis, the sequencing of PCR amplicons can be the alternative method when the Real-Time PCR is not available
[70].
Loop-Mediated Isothermal Amplification (LAMP) Technology
LAMP is another Real-Time PCR (qPCR) technique which has been used to distinguish the entomological origin of honey. Introduced in 2000, LAMP improves the sensitivity and specificity of nucleic acid amplification efficacy
[71]. Besides, the ability of LAMP to synthesize DNA using auto-cycling strand displacement activity at a constant temperature (60–65 °C) has rendered expensive thermocyclers and laborious technical optimization of cycling conditions unnecessary and significantly shortened the amplification duration as well
[71][72]. As a result of these distinctive characteristics, LAMP has been integrated into the development of diagnostic assays for detecting a wide range of medically significant communicable diseases
[73]. Recently, Gao et al.
[74] designed specific LAMP primers that target the MRJP2 gene. The developed primers effectively amplify the target gene and successfully distinguish between the
A. cerana and
A. mellifera honey with the detection sensitivity of 4 ng/μL and 1 ng/μL for
A. cerana honey and
A. mellifera honey, respectively.
2.4.4. Length Polymorphism of PCR Product
Variation of the amplicon sizes resulting from the PCR reaction can also be applied as a technique to identify the entomological origin of honey. Utzeri et al.
[75] applied a primer set that targeted the tRNAleu-COX2 intergenic mtDNA to identify the lineage origin of European honeybee from which honey samples were collected. The primers produced fragments of 85 bp for
A. mellifera C lineage (including
A. mellifera ligustica), 138 bp for M lineage (including
A. mellifera mellifera), or 152 bp for A lineage (honeybee subspecies of African origin and
A. mellifera siciliana). These different fragment sizes can be distinguished through a high percentage agarose gel electrophoresis. Honey originated from
A. mellifera iberiensis showed either 152 bp or 138 bp or both fragment sizes, which is consistent with the hybrid origin of Iberian honeybee populations
[75].
2.4.5. DNA Metabarcoding Approach
DNA metabarcoding is a next-generation sequencing approach allowing the identification of the origin of samples with multiple sources
[76][77][78]. To prepare the metabarcoding library, two rounds of PCR amplification are needed. The first PCR involves the use of primers with overhanging sequences for amplification, followed by the attachment of a specific tag to the amplicons during the second PCR. These tags serve as a unique identifier for each sample, making it easy to track and distinguish them. The resulting sequences then undergo various bioinformatics processes, including quality control, trimming, merging, dereplication, and chimera checking. The filtered sequences are then compared to reference libraries to facilitate identification. Prosser and Hebert
[60] recently developed a metabarcoding-based approach to identify the entomological origin of honey using COI gene (
Table 1). This method was successfully applied to distinguish the entomological origin of
A. mellifera honey and stingless bee,
Melipona beecheii, honey, and there is perhaps a possibility of using the same primer set to detect the other
Apis honeybee species. The methodology was assessed using pure honey samples and it would be interesting to assess the possibility of using this method to understand the level of adulteration in the case of mixed honey samples. Further research is required to determine the possibility of using the same primer set to distinguish other
Apis and non-
Apis honeybees. One potential limitation of this approach is the possibility of mismatches in the amplification site of phylogenetically distant groups, which could affect its accuracy. Despite these limitations, DNA metabarcoding has significant potential as a tool for identifying the origin of honey samples from different bees.