Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Li, L.; Qin, J. Advances in Permeation of Solutes into Hair. Encyclopedia. Available online: https://encyclopedia.pub/entry/45173 (accessed on 07 February 2026).
Li L, Qin J. Advances in Permeation of Solutes into Hair. Encyclopedia. Available at: https://encyclopedia.pub/entry/45173. Accessed February 07, 2026.
Li, Lingyi, Jiahao Qin. "Advances in Permeation of Solutes into Hair" Encyclopedia, https://encyclopedia.pub/entry/45173 (accessed February 07, 2026).
Li, L., & Qin, J. (2023, June 04). Advances in Permeation of Solutes into Hair. In Encyclopedia. https://encyclopedia.pub/entry/45173
Li, Lingyi and Jiahao Qin. "Advances in Permeation of Solutes into Hair." Encyclopedia. Web. 04 June, 2023.
Copy Citation
The permeation and absorption of solutes into human hair are highly relevant to various applications, including the formulation of hair-care products, the development of water pollution control and remediation, and the risk assessment of environmental exposure.
human hair
permeation and absorption
influencing factor
1. Introduction
The permeation and absorption of solutes in human hair are closely related to a wide range of applications, including the formulation of hair-care products [1][2][3], the development of water pollution control and remediation [4], and the risk assessment of environmental exposure [5][6][7]. According to the latest report released by the U.S. market research firm Transparency Market Research, the market scale of global hair products in 2015 was 81.3 billion USD, and it is expected to reach 105.3 billion USD by 2024. Ingredients in hair products can be adsorbed on the surface of hair or penetrate into hair through permeation and absorption to meet consumers’ many daily hair-care needs, such as cleaning, repair, coloring, and styling [8][9][10][11][12]. Studies have shown that hair, as a natural keratin adsorbent, can be used to remove organic pollutants, such as formaldehyde [13][14][15], phenol [16], and heavy-metal ions [17], from wastewater. The treatment of wastewater with this method has the advantages of low treatment cost, easy design and operation, insensitivity to toxic pollutants, and no secondary pollution [4][13]. In addition, due to the lipophilicity and low biological activity of hair [6][18][19][20], it can also be used as a bioindicator to evaluate the exogenous exposure of humans to environmental pollutants and to assess exogenous exposure to chemicals in media, such as care agents, pesticides, sewage, and dust [7][21].
The permeation and absorption properties of solutes in the hair are the most important parameters for assessing their absorption amount and speed. Therefore, studying the mechanism of permeation and absorption of solutes in hair and constructing a corresponding permeation mechanism model can provide theoretical guidance for the formula design of hair products and shorten the product development cycle. It can also enrich wastewater treatment and chemical exogenous risk assessment, which have important theoretical and practical significance.
The permeation and absorption properties of solutes, such as surfactants [22][23][24], phenols [25][26], dyes [27][28][29][30], fats [31][32][33], and metal ions [17][34], have been investigated, and several different permeation mechanism models have been proposed. However, owing to the complex mechanism of solute absorption in hair and the many influencing factors, existing models have certain limitations. Based on a detailed introduction to the structure and composition of hair, the main factors that influence hair permeation and absorption are summarized, including the physical and chemical properties of hair (structure, composition, and charge characteristics), the physical and chemical properties of solutes (molecular size, shape, and hydrophobicity), and the solvent conditions (composition, temperature, and pH). Several major permeation models of solutes in hair are described in detail, and, based on the current research status, suggestions are made for future research directions to establish a theoretical foundation for further optimization of hair permeation models.
2. Hair Structure and Composition
2.1. Hair Structure
Hair consists of a hair follicle, hair root, and hair shaft. The hair follicle is a cystic tissue surrounding the hair root; the inner layer is an epithelial tissue hair follicle, which is connected with the epidermis; and the outer layer is a connective tissue hair follicle, which is connected with the dermis. The hair roots are hidden in the scalp, and their main function is biosynthesis and directional growth. The hair shaft is the part exposed outside of the skin, and it can be divided into three layers known as the cuticle, the cortex, and the medulla, from outside to inside. The cuticle cells, cortex cells, and the space between them are connected by the cell membrane complex (CMC) (Figure 1) [35][36].
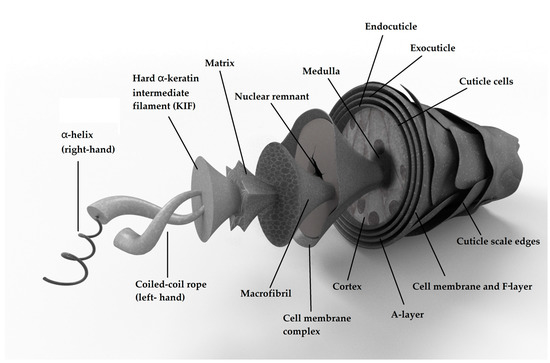
Figure 1. Structure of the human hair shaft.
The cuticle covers the outermost layer of the hair shaft, and it is covered with a stratum corneum layer. It is generally composed of lipid layers and 7 to 10 layers of flat cuticle cells that are staggered and overlapped into fish-scale or tile-like structures. Each cuticle cell has a thickness of 0.5 μm, a length of approximately 5 to 10 μm, and a total thickness of 5 μm in the cuticle layer [37]. The cuticle structure can be divided into 3 parts [11]: the epidermis (cystine content of ~12%), the exocuticle (cystine content of ~15%), and the endo-cuticle (cystine content of ~3%). The epidermis consists of a thin fat layer and a high-sulfur, protein-rich layer (a-layer, cystine content of ~30%) [38]. At the molecular level, the cuticle is a protein–lipid structure that is severely crosslinked by cystine, and it is not highly organized. This structure provides a barrier to prevent hair damage.
The cortex, which accounts for 70% to 90% of the hair’s weight, is the main part of the hair shaft, and it is an important component in determining the physical properties of hair [11]. The cortex cells are spindle-shaped with thicknesses of approximately 1–6 μm and lengths of approximately 50–100 μm [39]. The cells are closely arranged along the direction parallel to the axial direction of the hair, and the edges of the cells are serrated [38]. The cortex cells comprise many macrofibrils, hard α- keratin intermediate filaments, and coiled-coil ropes. They are arranged spirally to each other, embedded in a cell matrix composed of high-sulfur proteins, and form a very tight fiber bundle, giving hair a certain degree of elasticity, strength, and toughness [40].
The medulla is located in the center of the hair shaft. Due to different hair types, the medulla may be continuous, intermittent, or absent [36]. Morphologically, the medulla is a loose structure composed of spongy keratin and amorphous substances, and it is generally considered to have no important physiological function.
The CMC is the only continuous phase in the hair structure [36], and it acts as an adhesive between cuticle cells and cortex cells and has an important influence on the physical and chemical properties of hair. The main components of the CMC are low-sulfur protein and fat, which is the main structural lipid in hair and accounts for 5% to 7% of the hair mass [41]. The CMC lipids are similar to human epidermal keratin lipids, and their main components are ceramide, cholesterol sulfate, cholesterol, fatty alcohols, and free fatty acids [42].
2.2. Chemical Composition of Hair
The main chemical constituents of hair are keratin, fat, water, pigments, and trace elements.
Keratin is the most important component of hair, accounting for more than 90% of dry hair weight [26][43]. Keratin consists of 22 amino acids [11], of which cysteine has the highest content (up to 15.5%). The keratin peptide chain contains many cysteine residues, whose disulfide bonds act as crosslinks, allowing keratin molecules to bind tightly to form a dense structure that can resist the influence of external physical and chemical factors [39]. The mechanism of action of many hair cosmetics and treatment agents is to weaken or break the crosslinking effect of disulfide bonds, thus destroying the stability of the hair fiber structure and changing the curvature of the hair to achieve the effect of hairdressing.
3. Main Factors That Affect the Permeation and Absorption of Solutes in Hair
3.1. Hair
3.1.1. Geometry of Hair
Hair is a biological tissue composed of a variety of biological macromolecules and can be considered to be a heterogeneous porous medium [27]. Some researchers have proposed using porous media theory to describe the permeation properties of solutes in hair [27][28][44]. Chandrashekara et al. [27] studied the diffusion characteristics of solutes in hair using positron annihilation lifetime spectroscopy, and they found that the free-volume channels in hair fibers are the main pathway for solute diffusion. Morel et al. [28] proposed that in the porous structure of hair, the size, shape, and density of the pores all affect the permeation and absorption properties of solutes in hair. Molecules smaller than the pore size are easily transmitted, whereas molecules larger than the pore size are not transmitted. The permeation distance of solutes in damaged hair is generally higher because the pore size of the damaged hair becomes larger, making it easier for solutes to enter the hair.
Solutes penetrate into hair mainly through two channels: transcellular channels and intercellular channels [11][45]. The transcellular channel refers to the permeation of solutes through cuticle cells, which have a dense protein structure and a high degree of crosslinking, thus limiting the permeation of large solutes [1]. The intercellular channel refers to permeation through the glial structures (endocuticle and CMC) between the cuticle cells. As the components of the glial structures are mainly fats and low-sulfur proteins, which are prone to swelling, they are the preferred channel for lipophilic and non-polar solutes [1][11][38]. Gummer [46] found that hydrophilic substances can enter all of the structures in hair. The CMC can be considered to be a small compartment with the ability to swell and direct the solute from the hair surface to the cortex through capillary forces. It is believed that the outermost part of the cuticle is an open compartment structure, which explains the phenomenon of the rapid loss of dye in the rinsing process after hair dyeing, and it is inferred that the solute enters the cuticle from the external environment through the endocuticle. Kelch et al. [38] also found that the endocuticle and CMC are the main permeation pathways for solutes in hair.
3.1.2. Charge Characteristics of Hair
Keratin is the most important component of hair, and its isoelectric point is approximately 3.67 [11]. When the solvent pH is <3.67, the amino and carboxyl groups in the amino acids that make up keratin are protonated, and the hair appears to be positively charged externally, which increases the absorption of anionic solutes and decreases the absorption of cationic solutes. When the solvent pH = 3.67, the degree of protonation of the free amino groups is equal to the degree of dissociation of the carboxyl groups, the net charge is zero, and the hair is not charged. When the solvent pH is higher than 3.67, the free carboxyl group dissociates, the protonated amino group becomes deprotonated, and the hair appears to be negatively charged externally. Consequently, the absorption of cationic solutes increases while the absorption of anionic solutes decreases [23][26]. Therefore, the charge force can be considered one of the main driving forces for solute permeation and absorption in hair.
3.2. Solutes
3.2.1. Size and Shape of Solute Molecules
The size and shape of solute molecules affect their permeation in hair [28][44][47][48]. Assuming that the shape of the solute molecule is spherical, Holmes [44] determined that the maximum diameter of a molecule that can penetrate into hair is 1.48 nm. Sakai et al. [47] studied the penetrating distance of hair dyes of different sizes. The critical dimension of molecules that can enter hair was calculated to be 1 nm using the longest diagonal of the molecular minimum projected area as a measure of the size and shape of the molecule. However, the study had a single object of interest; the staining time was too long; and the permeation of cationic, anionic, and neutral molecules was not discussed separately. Morel et al. [28] considered the effect of the solute charge characteristics on the permeation and absorption of solutes based on the model of Sakai et al. [47]. They found that the critical sizes for the permeation of anionic, cationic, and neutral solutes into hair are approximately 1.2–1.3, 1.4, and 0.95 nm, respectively.
3.2.2. Solute Hydrophobicity
Some studies have reported that the permeation and absorption characteristics of solutes in biological substrates (e.g., hair, skin, and nails) are mainly related to the hydrophobicity of the solute [26][49][50][51][52]. Hydrophobic interaction is an important way that solute molecules and hair interact. Steinhardt et al. [53] found that the adsorption capacity of wool fibers for cationic surfactants increases with increasing hydrophobic structure. Scott et al. [54] determined the absorption characteristics of cetyl trimethyl ammonium bromide (CTAB) and dodecyl trimethyl ammonium bromide (DTAB) in hair. The results showed that CTAB with strong hydrophobicity has greater absorption in hair than DTAB with weak hydrophobicity. Robbins et al. [55] found that when the solvent pH is 3.6 (close to the isoelectric point of hair), the hair fibers are neutral, the absorption of solutes in hair is mainly caused by the hydrophobic effect, and the absorption capacity increases with increasing molecular weight and hydrophobic structure of the solute.
3.3. Solvent
3.3.1. Solvent Composition
Robbins et al. [55] found that increasing the fat content of hair product formulations can increase the adsorbed amount of cationic surfactants. Han et al. [56] found that when water is used as a solvent, the absorption of HC RED3 (a cationic semipermanent dye) in hair is 5–15 times higher than when the water/ethanol volume ratio is 1:1. This is because when water is used as a solvent, the polarity of the solvent is higher than that of a mixed solvent of water/ethanol, and the solubility of HC RED3 in water is lower than that in a mixed solvent of water/ethanol. This increases the amount of HC RED3 absorbed in hair. Therefore, the amount of the solute absorbed by hair can be increased by controlling the formulation of the solvent.
3.3.2. Solvent Temperature
Increasing the temperature decreases the viscosity of the liquid and increases the permeation rate of solutes in hair [44]. Han et al. [56] studied the absorption and diffusion coefficients of HC RED3 in hair at solvent temperatures of 25, 42, and 60 °C. They found that the diffusion coefficient of HC RED3 in hair increases with increasing temperature, and absorption of HC RED3 in hair slightly decreases with increasing temperature. Wortmann et al. [23] studied the permeation and absorption properties of anionic and cationic surfactants in hair at different temperatures. They found that the diffusion coefficients of anionic and cationic surfactants in hair increase with increasing temperature. In addition, the variation in the diffusion coefficient and temperature was in accordance with the Arrhenius equation.
3.3.3. Solvent pH
Changing the pH of the solvent can change the charge characteristics of ionic solutes, and it can also change the type and density of the charges carried by hair, thereby changing the interaction between hair and the solute molecules, which ultimately affects the permeation and absorption properties of the solute in hair. By combining scanning electron microscopy and micro-X-ray fluorescence analysis, Wortmann et al. [23] found that the diffusion coefficients and diffusion activation energies of anionic surfactants in hair decrease with the increasing pH of the solvent, while that of cationic surfactants increase. Furthermore, they found that the diffusion coefficients and diffusion activation energies of cationic and anionic surfactants are approximately the same at pH = 3.5 (close to the isoelectric point of hair). Using solvents with pH of 5.1, 6.0, and 10.4, Han et al. [56] found that the amount of HC RED3 absorbed by hair decreases with increasing pH due to the difference in the degree of ionization of the cationic HC RED3 dye. Morel et al. [28] found that under alkaline conditions, due to the force of the charge, the permeation distances of anionic, cationic, and neutral dyes in hair are different, and cationic dyes have the largest permeation distance and can penetrate the endocuticle and the δ-layer of the CMC to the cortex. Robbins et al. [55] suggested that the permeation and absorption of solutes in hair are driven by both hydrophobic and charge forces. When the solvent pH is close to the isoelectric point of the hair, solute permeation is mainly driven by hydrophobic forces. Conversely, when the pH of the solvent is greater or less than the isoelectric point of the hair, solute permeation is mainly driven by the charge force.
The pH of the solvent can also affect the swellability of hair. Robbins [11] and Valko et al. [57] found that in an alkaline solvent, the degree of swelling of hair significantly increases, which leads to an increase in the pore size of the hair, which favors solute absorption in hair. Otsuka et al. [58] pointed out that hair dyeing is usually performed under alkaline conditions, as the expanded hair fibers facilitate the rapid permeation of dyes.
The wettability of the hair surface can be changed by absorbing solutes under different pH conditions. Vickerstaff [59] and Robbins et al. [55] found that dyes mainly adsorb to the surface of hair fibers through charge or hydrophobic interactions depending on the pH of the solvent, which in turn changes the hydrophilic and hydrophobic properties of the hair surface. For anionic dyes, when the pH is lower than the isoelectric point, hydrophobic fibers are formed on the hair surface after dye absorption. When the pH is higher than the isoelectric point, hydrophilic fibers are formed on the hair surface after dye absorption, and the reverse is true for cationic dyes.
Faucher et al. [60][61] found that the electrolyte components (e.g., sodium salt, potassium salt, and calcium salt) in the solvent occupy part of the adsorption sites of the cationic polymer on hair, resulting in competitive inhibition, thereby reducing the amount of adsorption under alkaline conditions.
Thus, the solvent pH is a key factor that affects the permeation and absorption of solutes in hair, and changing the solvent pH causes changes in the physicochemical properties of the solute and hair, which in turn affect the permeation and absorption of the solute.
In conclusion, the main factors that affect the permeation and absorption of solutes in hair are summarized in Table 1.
Table 1. Main factors that affect permeation and absorption of solutes in hair.
| Factors | References | |
|---|---|---|
| Hair | Pore size, shape, and density | [27][28][44] |
| Transcellular/intercellular channel | [1][11][38][45][46] | |
| Charge characteristics | [11][23][26] | |
| Swellability | [11][57][58] | |
| Wettability of surface | [55][59] | |
| Keratin binding | [51] | |
| Solute | Size and shape | [28][44][47][48] |
| Charge characteristics | [23][26][28][55] | |
| Hydrophobicity | [26][49][50][51][52] | |
| Solvent conditions | pH | [11][23][28][55][56][57][58][59][60][61] |
| Temperature | [23][44][56] | |
| Composition of solvent | [55][56] | |
References
- Gavazzoni Dias, M.F. Hair cosmetics: An overview. Int. J. Trichology 2015, 7, 2.
- Madnani, N.; Khan, K. Hair cosmetics. Indian J. Dermatol. Venereol. Leprol. 2013, 79, 654.
- Morel, O.J.X.; Christie, R.M. Current trends in the chemistry of permanent hair dyeing. Chem. Rev. 2011, 111, 2537–2561.
- Gupta, A. Human hair “waste” and its utilization: Gaps and possibilities. J. Waste Manag. 2014, 2014, 498018.
- Kang, Y.; Wang, H.S.; Cheung, K.C.; Wong, M.H. Polybrominated diphenyl ethers (PBDEs) in indoor dust and human hair. Atmos. Environ. 2011, 45, 2386–2393.
- Covaci, A.; Tutudaki, M.; Tsatsakis, A.M.; Schepens, P. Hair analysis: Another approach for the assessment of human exposure to selected persistent organochlorine pollutants. Chemosphere 2002, 46, 413–418.
- Schramm, K.; Kuettner, T.; Weber, S.; Lützke, K. Dioxin hair analysis as monitoring pool. Chemosphere 1992, 24, 351–358.
- Im, S.H.; Jeong, Y.H.; Ryoo, J.J. Simultaneous analysis of anionic, amphoteric, nonionic and cationic surfactant mixtures in shampoo and hair conditioner by RP-HPLC/ELSD and LC/MS. Anal. Chim. Acta 2008, 619, 129–136.
- Morelli, J.J.; Szajer, G. Analysis of surfactants: Part II. J. Surfactants Deterg. 2001, 4, 75–83.
- Turner, G.A.; Matheson, J.R.; Li, G.Z.; Fei, X.Q.; Zhu, D.; Baines, F.L. Enhanced efficacy and sensory properties of an anti-dandruff shampoo containing zinc pyrithione and climbazole. Int. J. Cosmet. Sci. 2013, 35, 78–83.
- Robbins, C.R. Chemical and Physical Behavior of Human Hair, 5th ed.; Springer: New York, NY, USA, 2012; p. 494.
- Mahajan, A. Advancements in polymers used in hair care: A review. Int. J. Res. Cosmet. Sci. 2016, 6, 6–16.
- Talaiekhozani, A.; Talaei, M.R.; Yazdan, M.; Mir, S.M. Investigation of formaldehyde removal from synthetic contaminated air by using human hair. Environ. Health Eng. Manag. J. 2016, 3, 191–196.
- Ghanbarnejad, P.; Goli, A.; Bayat, B.; Barzkar, H.; Talaiekhozani, A.; Bagheri, M.; Alaee, S. Evaluation of formaldehyde adsorption by human hair and sheep wool in industrial wastewater with high concentration. J. Environ. Treat. Tech. 2014, 2, 12–17.
- Talaie, A.R.; Bagheri, M.; Ghotbinasab, S.; Talaie, M.R. Evaluation of formaldehyde wastewater adsorption on human hair. Health Syst. Res. 2011, 6, 735–743.
- Banat, F.A.; Al-Asheh, S. The use of human hair waste as a phenol biosorbent. Adsorpt. Sci. Technol. 2001, 19, 599–608.
- Tan, T.C.; Chia, C.K.; Teo, C.K. Uptake of metal ions by chemically treated human hair. Water Res. 1985, 19, 157–162.
- Altshul, L.; Covaci, A.; Hauser, R. The relationship between levels of PCBs and pesticides in human hair and blood: Preliminary results. Environ. Health Persp. 2004, 112, 1193.
- Covaci, A.; Schepens, P. Chromatographic aspects of the analysis of selected persistent organochlorine pollutants in human hair. Chromatographia 2001, 53, S366–S371.
- Tsatsakis, A.; Tutudaki, M. Progress in pesticide and POPs hair analysis for the assessment of exposure. Forensic Sci. Int. 2004, 145, 195–199.
- Zheng, J.; Wang, J.; Luo, X.; Tian, M.; He, L.; Yuan, J.; Mai, B.; Yang, Z. Dechlorane Plus in human hair from an e-waste recycling area in South China: Comparison with dust. Environ. Sci. Technol. 2010, 44, 9298–9303.
- Ran, G.; Zhang, Y.; Song, Q.; Wang, Y.; Cao, D. The adsorption behavior of cationic surfactant onto human hair fibers. Colloids Surf. B Biointerfaces 2009, 68, 106–110.
- Wortmann, F.J.; Gotsche, M.; Schmidt-Lewerkuhne, H. Diffusion and distribution of element-labelled surfactants in human hair. Int. J. Cosmet. Sci. 2004, 26, 61–69.
- Jenneson, P.M.; Clough, A.S.; Keddie, J.L.; Lu, J.R.; Meredith, P. Non-ionic surfactant concentration profiles in undamaged and damaged hair fibres determined by scanning ion beam nuclear reaction analysis. Nucl. Instrum. Methods Phys. Res. 1997, 132, 697–703.
- Chan, K.L.A.; Tay, F.H.; Taylor, C.; Kazarian, S.G. A novel approach for study of in situ diffusion in human hair using Fourier transform infrared spectroscopic imaging. Appl. Spectrosc. 2008, 62, 1041–1044.
- Wang, L.; Chen, L.; Han, L.; Lian, G. Kinetics and equilibrium of solute diffusion into human hair. Ann. Biomed. Eng. 2012, 40, 2719–2726.
- Chandrashekara, M.N.; Ranganathaiah, C. Diffusion of permanent liquid dye molecules in human hair investigated by positron lifetime spectroscopy. Colloids Surf. B Biointerfaces 2009, 69, 129–134.
- Morel, O.; Christie, R.M.; Greaves, A.; Morgan, K.M. Enhanced model for the diffusivity of a dye molecule into human hair fibre based on molecular modelling techniques. Color. Technol. 2008, 124, 301–309.
- Silva, A.L.D.S.; Joekes, I. Rhodamine B diffusion in hair as a probe for structural integrity. Colloids Surf. B Biointerfaces 2005, 40, 19–24.
- Volkov, V.; Cavaco-Paulo, A. Enzymatic phosphorylation of hair keratin enhances fast adsorption of cationic moieties. Int. J. Biol. Macromol. 2016, 85, 476–486.
- Rele, A.S.; Mohile, R.B. Effect of mineral oil, sunflower oil, and coconut oil on prevention of hair damage. J. Cosmet. Sci. 2003, 54, 175–192.
- Keis, K.; Persaud, D.; Kamath, Y.K.; Rele, A.S. Investigation of penetration abilities of various oils into human hair fibers. Int. J. Cosmet. Sci. 2006, 28, 78.
- Ruetsch, S.B.; Kamath, Y.K.; Rele, A.S.; Mohile, R.B. Secondary ion mass spectrometric investigation of penetration of coconut and mineral oils into human hair fibers: Relevance to hair damage. J. Cosmet. Sci. 2001, 52, 169–184.
- Kempson, I.M.; Skinner, W.M.; Kirkbride, K.P. Advanced analysis of metal distributions in human hair. Environ. Sci. Technol. 2006, 40, 3423–3428.
- Pragst, F.; Balikova, M.A. State of the art in hair analysis for detection of drug and alcohol abuse. Clin. Chim. Acta 2006, 370, 17–49.
- Jones, L. Hair structure anatomy and comparative anatomy. Clin. Dermatol. 2001, 19, 25.
- Efremenko, I.; Zach, R.; Zeiri, Y. Adsorption of Explosive Molecules on Human Hair Surfaces. J. Phys. Chem. C 2007, 111, 11903–11911.
- Kelch, A.; Wessel, S.; Will, T.; Hintze, U.; Wepf, R.; Wiesendanger, R. Penetration pathways of fluorescent dyes in human hair fibres investigated by scanning near-field optical microscopy. J. Microsc. Oxford 2000, 200, 179–186.
- Wei, G.; Bhushan, B.; Torgerson, P.M. Nanomechanical characterization of human hair using nanoindentation and SEM. Ultramicroscopy 2005, 105, 248–266.
- Randebrook, R.J. Neue erkenntnisse über den morphologischen aufbau des menschlichen haares. J. Soc. Cosmet. Chem. 1964, 15, 691–706.
- Wortmann, F.; Wortmann, G.; Zahn, H. Pathways for dye diffusion in wool fibers. Text. Res. J. 1997, 67, 720–724.
- He, X. Relationship between the damage of hair and the structure and composition of hair. China Surfactant Deterg. Cosmet. 2000, 30, 34–36. (In Chinese)
- Masukawa, Y.; Tanamachi, H.; Tsujimura, H.; Mamada, A.; Imokawa, G. Characterization of hair lipid images by argon sputter etching—Scanning electron microscopy. Lipids 2006, 41, 197–205.
- Holmes, A.W. Diffusion processes in human hair. J. Soc. Cosmet. Chem. 1964, 15, 595–603.
- Leeder, J.D.; Rippon, J.A. Some observations on the dyeing of wool from aqueous formic acid. J. Soc. Dye. Colour. 1983, 99, 64–65.
- Gummer, C.L. Elucidating penetration pathways into the hair fiber using novel microscopic techniques. J. Cosmet. Sci. 2001, 52, 265–280.
- Sakai, M.; Nagase, S.; Okada, T.; Satoh, N.; Tsujii, K. A universal structural model for human hair to understand the physical properties. 2. mechanical and permeation behaviors. Bull. Chem. Soc. Jpn. 2000, 73, 2169–2177.
- Speakman, J.B. The micelle structure of the wool fibre. Nature 1931, 126, 167–191.
- Mertin, D.; Lippold, B.C. In-vitro permeability of the human nail and of a keratin membrane from bovine hooves: Influence of the partition coefficient octanol/water and the water solubility of drugs on their permeability and maximum flux. J. Pharm. Pharmacol. 1997, 49, 30–34.
- Chen, L.; Han, L.; Lian, G. Recent advances in predicting skin permeability of hydrophilic solutes. Adv. Drug Deliver. Rev. 2013, 65, 295–305.
- Hansen, S.; Selzer, D.; Schaefer, U.F.; Kasting, G.B. An extended database of keratin binding. J. Pharm. Sci. US 2011, 100, 1712–1726.
- Wang, L.; Chen, L.; Lian, G.; Han, L. Determination of partition and binding properties of solutes to stratum corneum. Int. J. Pharmaceut. 2010, 398, 114–122.
- Steinhardt, J.; Zaiser, E.M. Combination of wool protein with cations and hydroxyl ions. J. Biol. Chem. 1950, 183, 789–802.
- Scott, G.V.; Robbins, C.R.; Barnhurst, J.D. Sorption of quaternary ammonium surfactants by human hair. J. Soc. Cosmet. Chem. 1969, 20, 135–152.
- Robbins, C.R.; Reich, C.; Patel, A. Adsorption to keratin surfaces: A continuum between a charge-driven and a hydrophobically driven process. J. Soc. Cosmet. Chem. 1994, 45, 85–94.
- Han, S.K.; Kamath, Y.K.; Weigmann, H.D. Diffusion of semipermanent dyestuffs in human hair. J. Soc. Cosmet. Chem. 1985, 36, 1–16.
- Valko, E.I.; Barnett, G. A study of the swelling of hair in mixed aqueous solvents. J. Soc. Cosmet. Chem. 1952, 3, 108–117.
- Otsuka Saito, K.; Ikeda, R.; Endo, K.; Tsujino, Y.; Takagi, M.; Tamiya, E. Isolation of a novel alkaline-induced laccase from Flammulina velutipes and its application for hair coloring. J. Biosci. Bioeng. 2012, 113, 575–579.
- Vickerstaff, T. The Physical Chemistry of Dyeing, 2nd ed.; Oliver and Boyd: London, UK, 1954; p. 413.
- Faucher, J.A.; Goddard, E.D. Influence of surfactants on the sorption of a cationic polymer by keratinous substrates. J. Colloid Interface Sci. 1976, 55, 313–319.
- Faucher, J.A.; Goddard, E.D.; Hannan, R.B. Sorption and desorption of a cationic polymer by human hair: Effects of salt solutions. Text. Res. J. 1977, 47, 616–620.
More
Information
Subjects:
Engineering, Chemical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
987
Revisions:
2 times
(View History)
Update Date:
05 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




