Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Takahiro Yamasaki | -- | 2266 | 2023-06-01 11:03:39 | | | |
| 2 | Catherine Yang | -1160 word(s) | 1106 | 2023-06-02 03:32:30 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Yamasaki, T.; Saeki, I.; Kotoh-Yamauchi, Y.; Sasaki, R.; Tanabe, N.; Oono, T.; Matsuda, T.; Hisanaga, T.; Matsumoto, T.; Hidaka, I.; et al. Hepatic Arterial Infusion Chemotherapy for Advanced Hepatocellular Carcinoma. Encyclopedia. Available online: https://encyclopedia.pub/entry/45097 (accessed on 07 February 2026).
Yamasaki T, Saeki I, Kotoh-Yamauchi Y, Sasaki R, Tanabe N, Oono T, et al. Hepatic Arterial Infusion Chemotherapy for Advanced Hepatocellular Carcinoma. Encyclopedia. Available at: https://encyclopedia.pub/entry/45097. Accessed February 07, 2026.
Yamasaki, Takahiro, Issei Saeki, Yurika Kotoh-Yamauchi, Ryo Sasaki, Norikazu Tanabe, Takashi Oono, Takashi Matsuda, Takuro Hisanaga, Toshihiko Matsumoto, Isao Hidaka, et al. "Hepatic Arterial Infusion Chemotherapy for Advanced Hepatocellular Carcinoma" Encyclopedia, https://encyclopedia.pub/entry/45097 (accessed February 07, 2026).
Yamasaki, T., Saeki, I., Kotoh-Yamauchi, Y., Sasaki, R., Tanabe, N., Oono, T., Matsuda, T., Hisanaga, T., Matsumoto, T., Hidaka, I., Ishikawa, T., Takami, T., Suehiro, Y., & Sakaida, I. (2023, June 01). Hepatic Arterial Infusion Chemotherapy for Advanced Hepatocellular Carcinoma. In Encyclopedia. https://encyclopedia.pub/entry/45097
Yamasaki, Takahiro, et al. "Hepatic Arterial Infusion Chemotherapy for Advanced Hepatocellular Carcinoma." Encyclopedia. Web. 01 June, 2023.
Copy Citation
Systemic therapeutic agents, including combination immunotherapy, could promote a change in the treatment strategy in patients with advanced hepatocellular carcinoma (HCC). Hepatic arterial infusion chemotherapy (HAIC) is highly effective in patients with vascular invasion compared with sorafenib.
advanced hepatocellular carcinoma
hepatic arterial infusion chemotherapy
sorafenib
vascular invasion
1. Overview of HAIC
1.1. Concept
Hepatic arterial infusion chemotherapy (HAIC) involves two procedures as follows: as scheduled, chemotherapeutic regimens are administered through a reservoir port connected to a catheter, which is implanted under the skin, and a catheter is inserted each time without implantation of the reservoir port. As HAIC is expected to accumulate drug concentrations in the local liver and reduce systemic toxicity of anti-cancer drugs, it is considered to have a more favorable antitumor effect and less influence on other organs than systemic chemotherapy. However, currently, no randomized controlled trials (RCTs) have compared HAIC with systemic chemotherapy in a large number of HCC patients.
1.2. Regimens
As the anti-cancer drugs that can be used in HAIC differ across countries, it may be difficult to adopt these HAIC regimens. In Japan, three regimens have been used for HAIC treatment: 5-fluorouracil (5-FU) combined with low-dose cisplatin (CDDP) (low-dose FP) [1][2][3], 5-FU combined with interferon (FAIT) [4][5][6][7], and CDDP alone [8][9][10] (Table 1). The response rates (complete response [CR] + partial response [PR]/all patients) of the regimens comprising low-dose FP or FAIT and the CDDP regimen were approximately 30–40% and 20–30%, respectively. Recently, HAIC regimens comprising low-dose FP or CDDP alone have been generally used in Japan [11].
Table 1. Regimens of hepatic arterial infusion chemotherapy.
| Authors [Reference] | Publishing Year | Regimens | Case Number | Vascular Invasion (%) | Response Rate (%) | Median Survival Time (Months) |
|---|---|---|---|---|---|---|
| Low-dose FP | ||||||
| Saeki, et al. [1] | 2015 | Low-dose FP including the combination of LV/IV or IV plus IFN |
90 | ND | 34.4 | 10.6 |
| Nouso, et al. [2] | 2013 | CDDP+5FU | 476 | 44.1 | 40.5 | 14.0 (341 patients) |
| Ueshima, et al. [3] | 2010 | Low-dose FP | 52 | 80.8 | 38.5 | 15.9 |
| FAIT | ||||||
| Monden, et al. [4] | 2012 | IFNα, 5-FU | 34 | 90.0 | 26.7 | 8.4 |
| Low-dose FP/CDDP | 35 | 90.3 | 25.8 | 11.8 | ||
| Yamashita, et al. [5] | 2011 | IFNα, CDDP, 5-FU | 57 | 26.7 | 45.6 | 17.6 |
| IFNα, 5-FU | 57 | 50.0 | 24.6 | 10.5 | ||
| Nagano, et al. [6] | 2011 | IFNα, 5-FU | 102 | 100.0 | 39.2 | 9.0 |
| Obi, et al. [7] | 2006 | IFNα, 5-FU | 116 | 100.0 | 52.0 | 6.9 |
| CDDP | ||||||
| Ikeda, et al. [8] | 2013 | CDDP powder (IA call) | 25 | 100.0 | 28.0 | 7.6 |
| Kim, et al. [9] | 2011 | CDDP | 41 | 83.3 | 12.2 | 7.5 |
| CDDP, 5-FU | 97 | 27.8 | 12.0 | |||
| Yoshikawa, et al. [10] | 2008 | CDDP powder (IA call) | 80 | 27.5 | 33.8 | ND |
ND, not described; Low-dose FP, low-dose 5-fluorouracil plus cisplatin; LV, leucovorin; IV, isovorin; IFN, interferon; CDDP, cisplatin; 5-FU, 5-fluorouracil.
1.3. Indications
HAIC is commonly used to treat advanced HCC, whether naive or recurrent tumors. According to the Clinical Practice Guidelines for Hepatocellular Carcinoma (2017 version) established by the Japan Society of Hepatology (JSH) [12], HAIC or MTA is recommended as a second-line treatment in HCC patients with ≥4 nodules, without vascular invasion and extrahepatic metastasis (EHM); whereas transcatheter arterial chemoembolization (TACE), hepatectomy, HAIC, and MTA are recommended as first-line treatments in HCC patients with vascular invasion, without EHM. Furthermore, patients with Child–Pugh A or B HCC are candidates for HAIC [12]. In this regard, the guidelines from Korea and Taiwan demonstrated that HAIC may be considered an optional treatment in a subpopulation of patients [13][14].
1.4. Clinical Outcomes
As shown in Table 1, the median survival time (MST) was different based on the degree of vascular invasion. Radiological responders (CR or PR) show significantly longer survival than radiological non-responders (stable or progressive disease). A Japanese nationwide survey reported that the MST was significantly longer in patients who received HAIC (n = 341, 14 months) than in those who did not receive active treatment (n = 341, 5.2 months) in a propensity score-matched analysis [2]. In Child–Pugh A or B HCC patients with portal vein tumor thrombus, the MST was similarly significantly longer in patients receiving HAIC (7.9 months) than in those without therapy (3.1 months) [2]. A recent report demonstrated that none of the HAIC regimens (low-dose FP, FAIT, and CDDP alone) had no effect on survival in patients with advanced HCC [15].
2. Clinical Benefits and Disadvantages of HAIC
The clinical benefits of HAIC for advanced HCC are as follows: (1) even a patient with Child–Pugh B HCC (7 or 8 points) is a candidate for HAIC [16], (2) Child–Pugh scores barely decline after HAIC [17][18], (3) HAIC is highly effective in patients with vascular invasion compared with sorafenib [15][19], and 4) survival in patients receiving HAIC may not be associated with skeletal muscle volume [20]. In contrast, the disadvantages of HAIC for advanced HCC are as follows: (1) a highly technical procedure is needed to implant a catheter with a reservoir port; (2) hospitalization is needed to continue HAIC treatments; (3) patients have to return for follow-up visits every 2 weeks to maintain the reservoir system; and (4) adverse events related to the reservoir system, such as port migration, catheter dislocation, arterial occlusion, reservoir system occlusion, subcutaneous hematomas, or infection [21].
Atezolizumab combined with bevacizumab was recently approved, and this combination will be recommended as the first-line therapy for advanced HCC. However, a comparison between atezolizumab plus bevacizumab and HAIC has not been performed. Patients with macrovascular invasion, including an invasion of the main portal trunk, accounted for 38% of those in the atezolizumab plus bevacizumab group; however, the details were not shown [22]. Therefore, as there has been no information regarding this combination therapy in real-world practice, further studies are required.
The treatment proposal for HAIC for advanced HCC was drafted in Figure 1. The combination of atezolizumab and bevacizumab will be shifted to the first-line therapy in patients with Child–Pugh A HCC, regardless of EHM, and currently used MTAs will be shifted to later lines of therapy [23]. HAIC may be an optional treatment in patients with Child–Pugh A HCC and vascular invasion, especially Vp3 or Vp4, without EHM [15][19]. MTAs are generally used in patients with Child–Pugh A HCC, whereas the use of MTAs in patients with Child–Pugh B HCC remains controversial. Some Asian guidelines recommended that sorafenib is considered in selected patients with Child–Pugh B (e.g., score, 7 points) [13][14][24][25][26], although sorafenib treatment significantly worsened survival in patients with Child–Pugh B HCC compared to those with Child–Pugh A HCC [27]. In contrast, patients with Child–Pugh B HCC (score 7 or 8 points) are candidates for HAIC [16]. The medical needs of patients receiving second-line therapy for Child–Pugh B HCC without EHM and those who have EHM with Child–Pugh B HCC, are yet to be met. However, HAIC may be considered in a subpopulation of both Child–Pugh B HCC and EHM patients if the intrahepatic tumor is directly linked to prognosis. Therefore, patients in clinical trials who can tolerate deteriorated liver function would be candidates for the novel therapy [28][29]. However, deferasirox, an oral iron chelator, has limited efficacy due to associated adverse effects, especially renal dysfunction [29]. In the future, systemic therapeutic agents would be expected to be developed for the unmet medical needs of patients undergoing advanced HCC treatment.
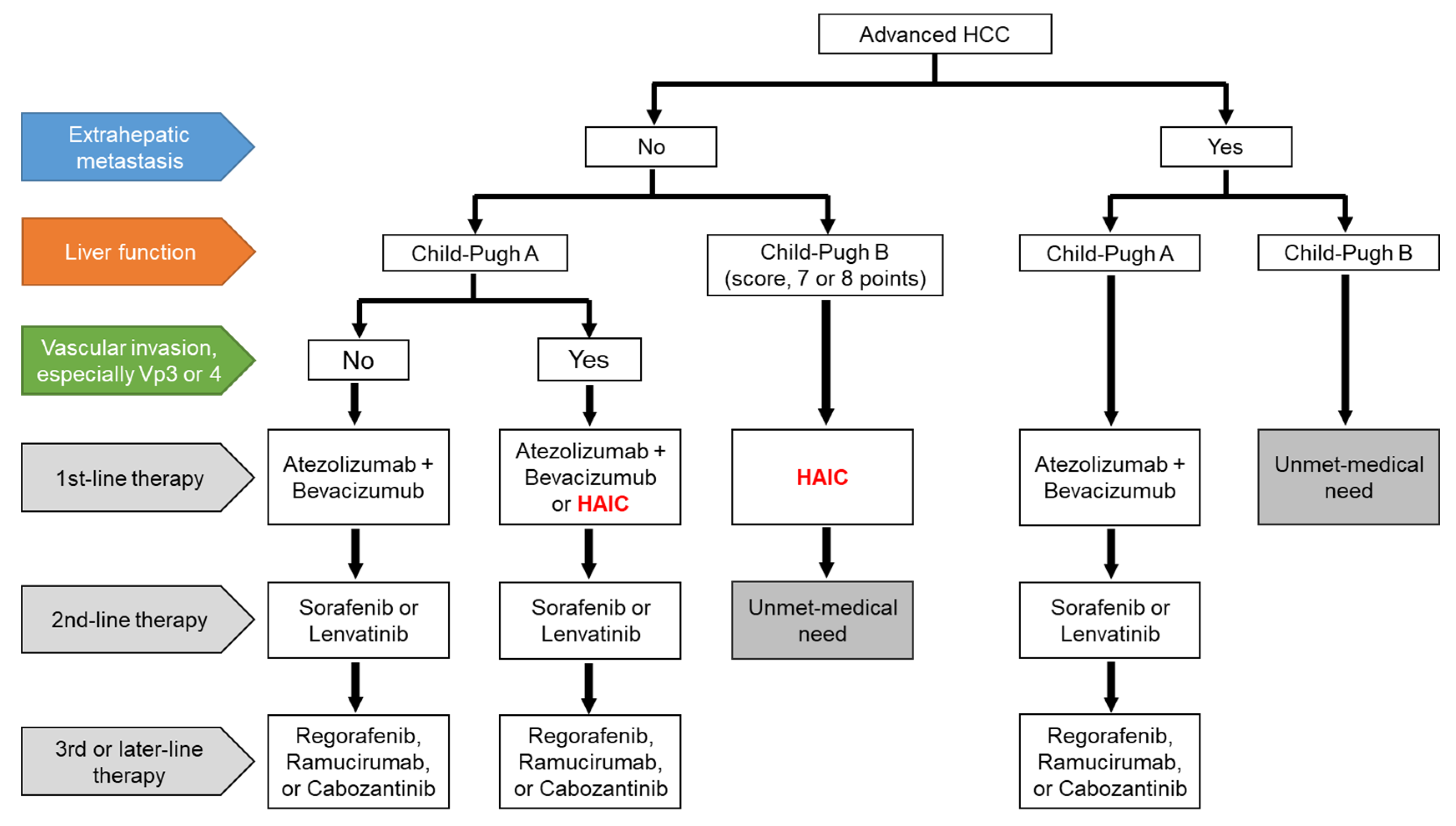
Figure 1. A draft of the treatment proposal for advanced HCC. HCC, hepatocellular carcinoma; HAIC, hepatic arterial infusion chemotherapy; Vp3, primary branch portal vein invasion; Vp4, main portal vein invasion.
References
- Saeki, I.; Yamasaki, T.; Tanabe, N.: Iwamoto, T.; Matsumoto, T.; Urata, Y.; Hidaka, I.; Ishikawa, T.; Takami, T.; Yamamoto, N.; et al. A new therapeutic assessment score for advanced hepatocellular carcinoma patients receiving hepatic arterial infusion chemotherapy. PLoS One. 2015, 10, e0126649. doi: 10.1371/journal.pone.0126649.
- Nouso, K.; Miyahara, K.; Uchida, D.; Kuwaki, K.; Izumi, N.; Omata, M.; Ichida, T.; Kudo, M.; Ku, Y.; Kokudo, N.; et al. Effect of hepatic arterial infusion chemotherapy of 5-fluorouracil and cisplatin for advanced hepatocellular carcinoma in the Nationwide Survey of Primary Liver Cancer in Japan. J. Cancer. 2013, 109, 1904-1907. doi: 10.1038/bjc.2013.542.
- Ueshima, K.; Kudo, M.; Takita, M.; Nagai, T.; Tatsumi, C.; Ueda, T.; Kitai, S.; Ishikawa, E.; Yada, N.; Inoue, T.; et al. Hepatic arterial infusion chemotherapy using low-dose 5-fluorouracil and cisplatin for advanced hepatocellular carcinoma. Oncology. 2010, 78 Suppl 1, 148-153. doi: 10.1159/000315244.
- Monden, M.; Sakon, M.; Sakata, Y.; Ueda, Y.; Hashimura, E.; FAIT Research Group. 5-fluorouracil arterial infusion + interferon therapy for highly advanced hepatocellular carcinoma: A multicenter, randomized, phase II study. Res. 2012, 42, 150-165. doi: 10.1111/j.1872-034X.2011.00905.x.
- Yamashita, T.; Arai, K.; Sunagozaka, H.; Ueda, T.; Terashima, T.; Mizukoshi, E.; Sakai, A.; Nakamoto, Y.; Honda, M.; Kaneko, S. Randomized, phase II study comparing interferon combined with hepatic arterial infusion of fluorouracil plus cisplatin and fluorouracil alone in patients with advanced hepatocellular carcinoma. Oncology. 2011, 81, 281-290. doi: 10.1159/000334439.
- Nagano, H.; Wada, H.; Kobayashi, S.; Marubashi, S.; Eguchi, H.; Tanemura, M.; Tomimaru, Y.; Osuga, K.; Umeshita, K.; Doki, Y.; et al. Long-term outcome of combined interferon-α and 5-fluorouracil treatment for advanced hepatocellular carcinoma with major portal vein thrombosis. Oncology. 2011, 80, 63-69. doi: 10.1159/000328281.
- Obi, S.; Yoshida, H.; Toune, R.; Unuma, T.; Kanda, M.; Sato, S.; Tateishi, R.; Teratani, T.; Shiina, S.; Omata M. Combination therapy of intraarterial 5-fluorouracil and systemic interferon-alpha for advanced hepatocellular carcinoma with portal venous invasion. Cancer. 2006, 106, 1990-1997. doi: 10.1002/cncr.21832.
- Ikeda, M.; Okusaka, T.; Furuse, J.; Mitsunaga, S.; Ueno, H.; Yamaura, H.; Inaba, Y.; Takeuchi, Y.; Satake, M.; Arai, Y. A multi-institutional phase II trial of hepatic arterial infusion chemotherapy with cisplatin for advanced hepatocellular carcinoma with portal vein tumor thrombosis. Cancer Chemother. Pharmacol. 2013, 72, 463-470. doi: 10.1007/s00280-013-2222-x.
- Kim, B.K.; Park, J.Y.; Choi, H.J.; Kim, d.Y.; Ahn, S.H.; Kim, J.K.; Lee, d.Y.; Lee, K.H.; Han, K.H. Long-term clinical outcomes of hepatic arterial infusion chemotherapy with cisplatin with or without 5-fluorouracil in locally advanced hepatocellular carcinoma. Cancer Res. Clin. Oncol. 2011, 137, 659-667. doi: 10.1007/s00432-010-0917-5.
- Yoshikawa, M.; Ono, N.; Yodono, H.; Ichida, T.; Nakamura, H. Phase II study of hepatic arterial infusion of a fine-powder formulation of cisplatin for advanced hepatocellular carcinoma. Res. 2008, 38, 474-483. doi: 10.1111/j.1872-034X.2008.00338.x.
- Kudo, M.; Izumi, N.; Kokudo, N.; Sakamoto, M.; Shiina, S.; Takayama, T.; Tateishi, R.; Nakashima, O.; Murakami, T.; Matsuyama, Y.; et al. Report of the 21th Nationwide follow-up survey of primary liver cancer in Japan (2010-2011).Hepatol Res. 2020 Dec 31. doi: 10.1111/hepr.13612.
- Kokudo, N.; Takemura, N.; Hasegawa, K.; Takayama, T.; Kubo, S.; Shimada, M.; Nagano, H.; Hatano, E.; Izumi, N.; Kaneko, S.; et al. Clinical practice guidelines for hepatocellular carcinoma: The Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol Res. 2019, 49, 1109-1113. doi: 10.1111/hepr.13411.
- Korean Liver Cancer Association; National Cancer Center. 2018 Korean Liver Cancer Association-National Cancer Center Korea Practice Guidelines for the Management of Hepatocellular Carcinoma. Gut Liver. 2019, 13, 227-299. doi: 10.5009/gnl19024.
- Surveillance group; Diagnosis group; Staging group; Surgery group; Local ablation group; TACE/TARE/HAI group; Target therapy/systemic therapy group; Radiotherapy group; Prevention group; Drafting group. Management consensus guideline for hepatocellular carcinoma: 2016 updated by the Taiwan Liver Cancer Association and the Gastroenterological Society of Taiwan. Formos. Med. Assoc. 2018, 117, 381-403. doi: 10.1016/j.jfma.2017.09.007.
- Ueshima, K.; Ogasawara, S.; Ikeda, M.; Yasui, Y.; Terashima, T.; Yamashita, T.; Obi, S.; Sato, S.; Aikata, H.; Ohmura, T.; et al. Hepatic Arterial Infusion Chemotherapy versus Sorafenib in Patients with Advanced Hepatocellular Carcinoma. Liver Cancer. 2020, 9, 583-595. doi: 10.1159/000508724.
- Terashima, T.; Yamashita, T.; Arai, K.; Kawaguchi, K.; Kitamura, K.; Yamashita, T.; Sakai, Y.; Mizukoshi, E.; Honda, M.; Kaneko, S. Response to chemotherapy improves hepatic reserve for patients with hepatocellular carcinoma and Child-Pugh B cirrhosis. Cancer Sci. 2016, 107, 1263-1269. doi: 10.1111/cas.12992.
- Terashima, T.; Yamashita, T.; Arai, K.; Kawaguchi, K.; Kitamura, K.; Yamashita, T.; Sakai, Y,.; Mizukoshi, E.; Honda, M.; Kaneko, S. Beneficial Effect of Maintaining Hepatic Reserve during Chemotherapy on the Outcomes of Patients with Hepatocellular Carcinoma. Liver Cancer. 2017, 6, 236-249. doi: 10.1159/000472262.
- Saeki, I.; Yamasaki, T.; Maeda, M.; Hisanaga, T.; Iwamoto, T.; Matsumoto, T.; Hidaka, I.; Ishikawa, T.; Takami, T.; Sakaida, I. Evaluation of the "assessment for continuous treatment with hepatic arterial infusion chemotherapy" scoring system in patients with advanced hepatocellular carcinoma. Hepatol. Res. 2018, 48, E87-E97. doi: 10.1111/hepr.12932.
- Liu, M.; Shi, J.; Mou, T.; Wang, Y.; Wu, Z.; Shen, A. Systematic review of hepatic arterial infusion chemotherapy versus sorafenib in patients with hepatocellular carcinoma with portal vein tumor thrombosis. J. Gastroentero.l Hepatol. 2020, 35, 1277-1287. doi: 10.1111/jgh.15010.
- Saeki, I.; Yamasaki, T.; Maeda, M.; Hisanaga, T.; Iwamoto, T.; Matsumoto, T.; Hidaka, I.; Ishikawa, T.; Takami, T.; Sakaida, I. Effect of body composition on survival benefit of hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma: A comparison with sorafenib therapy. PLoS One. 2019, 14, e0218136. doi: 10.1371/journal.pone.0218136.
- Obi, S; Sato, S; Kawai, T. Current Status of Hepatic Arterial Infusion Chemotherapy. Liver Cancer. 2015, 4, 188-199. doi: 10.1159/000367746.
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. Engl. J. Med. 2020, 382, 1894-1905. doi: 10.1056/NEJMoa1915745.
- Kudo, M. A Paradigm Change in the Treatment Strategy for Hepatocellular Carcinoma. Liver Cancer. 2020, 9, 367–377. doi:10.1159/000507934.
- Omata, M.; Cheng, A.L.; Kokudo, N.; Kudo, M.; Lee, J.M.; Jia, J.; Tateishi, R.; Han, K.H.; Chawla, Y.K.; Shiina, S.; et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Int. 2017, 11, 317-370. doi:10.1007/s12072-017-9799-9.
- Shao, Y.Y.; Wang, S.Y.; Lin, S.M.; Diagnosis Group,; Systemic Therapy Group. Management consensus guideline for hepatocellular carcinoma: 2020 update on surveillance, diagnosis, and systemic treatment by the Taiwan Liver Cancer Association and the Gastroenterological Society of Taiwan. Formos. Med. Assoc. 2020, S0929-6646(20)30531-3. doi: 10.1016/j.jfma.2020.10.031.
- Zhou, J.; Sun, HC.; Wang, Z.; Cong, W.M.; Wang, J.H.; Zeng, M.S.; Yang, J.M.; Bie, P.; Liu, L.X.; Wen, T.F.; et al. Guidelines for Diagnosis and Treatment of Primary Liver Cancer in China (2017 Edition). Liver Cancer. 2018, 7, 235-260. doi: 10.1159/000488035.
- Hollebecque, A.; Cattan, S.; Romano, O.; Sergent, G.; Mourad, A.; Louvet, A.; Dharancy, S.; Boleslawski, E.; Truant, S.; Pruvot, F.R.; et al. Safety and efficacy of sorafenib in hepatocellular carcinoma: the impact of the Child-Pugh score. Pharmacol. Ther. 2011, 34, 1193-1201. doi: 10.1111/j.1365-2036.2011.04860.x.
- Yamasaki, T; Terai, S; Sakaida, I. Deferoxamine for advanced hepatocellular carcinoma. Engl. J. Med. 2011, 365, 576-578. doi: 10.1056/NEJMc1105726.
- Saeki, I; Yamamoto, N; Yamasaki, T; Takami, T; Maeda, M; Fujisawa, K; Iwamoto, T; Matsumoto, T; Hidaka, I; Ishikawa, T; et al. Effects of an oral iron chelator, deferasirox, on advanced hepatocellular carcinoma. World J. Gastroenterol. 2016, 22, 8967-8977. doi: 10.3748/wjg.v22.i40.8967.
More
Information
Subjects:
Gastroenterology & Hepatology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
626
Revisions:
2 times
(View History)
Update Date:
02 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




