Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Miryam Palacios-Pérez | -- | 4246 | 2023-06-01 07:45:45 | | | |
| 2 | Rita Xu | -18 word(s) | 4228 | 2023-06-01 07:59:17 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Smith, D.; Jheeta, S.; López-Cortés, G.I.; Street, B.; Fuentes, H.V.; Palacios-Pérez, M. Inheritance of Microbiome-Deficiency. Encyclopedia. Available online: https://encyclopedia.pub/entry/45093 (accessed on 07 February 2026).
Smith D, Jheeta S, López-Cortés GI, Street B, Fuentes HV, Palacios-Pérez M. Inheritance of Microbiome-Deficiency. Encyclopedia. Available at: https://encyclopedia.pub/entry/45093. Accessed February 07, 2026.
Smith, David, Sohan Jheeta, Georgina I. López-Cortés, Bernadette Street, Hannya V. Fuentes, Miryam Palacios-Pérez. "Inheritance of Microbiome-Deficiency" Encyclopedia, https://encyclopedia.pub/entry/45093 (accessed February 07, 2026).
Smith, D., Jheeta, S., López-Cortés, G.I., Street, B., Fuentes, H.V., & Palacios-Pérez, M. (2023, June 01). Inheritance of Microbiome-Deficiency. In Encyclopedia. https://encyclopedia.pub/entry/45093
Smith, David, et al. "Inheritance of Microbiome-Deficiency." Encyclopedia. Web. 01 June, 2023.
Copy Citation
Like the majority of non-communicable diseases that have recently gained attention, functional gastrointestinal (GI) disorders (FGID) in both children and adults are caused by a variety of medical conditions. In general, while it is often thought that common conditions such as obesity may cause other problems, for example, asthma or mental health issues, more consideration needs to be given to the possibility that they could both be brought on by a single underlying problem. Based on the variations in non-communicable disease, in recent years, researchers group has been revisiting the exact role of the intestinal microbiome within the Vertebrata.
microbiome
non-communicable disease
maternal inheritance
1. Functional Gastrointestinal Disorders: Non-Communicable Disease
Functional gastrointestinal disorders (FGID) are a subset of non-communicable diseases, constituting a wide range of apparently random, seemingly unprovoked disturbances of the gut. They include disorders of hypersensitivity, gut motility, and altered immune function. The development of the field has been overseen by a not-for-profit group called the Rome Foundation, which has issued information and guidance at intervals. The most recent, Rome IV, was published in 2016 and included information on the microenvironment of the gut, the microbiome (albeit mostly the bacteriome), and the gut–brain axis [1]. Interestingly, the connection with the gut–brain axis could perhaps help to explain the superficial similarity of the complex Rome IV deliberations with those pertaining to the brain, the equally complex Diagnostic and Statistical Manual of Mental Disorders, currently in its 5th Edition (DSM-5) [2]. Paediatric FGID represents a subset of such diseases with their own challenges of diagnosis and treatment [3].
The disease term “non-communicable” represents a set of conditions that have recently been increasing in extent and variety but does not seem to be directly related to any infectious agent. While commonly encountered in developed countries, they are an increasing problem in the developing world, albeit starting from a low base [4]. However, what confuses the issue is when seemingly similar conditions arise following an infection, for example, the so-called “long COVID” [5]. Indeed, it may be possible that many immune-related conditions have a hidden trigger such as the potential role of the Epstein–Barr virus in multiple sclerosis [6]. Another feasible cause of non-communicable disease is dietary deficiency, which is especially problematic in otherwise undiagnosed coeliac disease [7] or in dietary intolerance and allergy [8]. Interestingly, the latest idea to explain the growth of microbiome-related disease relies on a combination of dietary deficiency, potentially toxic additives, and high energy, the so-called “ultra-processed foods” [9]. As implied by the title of this research, however, the view of non-communicable disease is as a form of deficiency disease, specifically a lack of function of researchers' mutualistic partner, the intestinal microbiome [10]. In this view, while there is undoubtably a role for a poor diet in non-communicable health problems, it is as an accelerant of disease, rather than as a cause in its own right. Unfortunately, in the absence of a definitive diagnosis, the full extent of such microbiome-deficiency disease is not clear.
Needless to say, many adult weight-related diseases are considered to be some kind of moral failure, and are often blamed on lifestyle factors, albeit incorrectly [11]. As the spread seems to be too fast for classic genetic diseases, this raises the question of maternal microbial inheritance: the microbes transfer from the mother to the neonate during the birth process; additionally, the role of seminal microbiome has more recently been investigated not only as an epigenetic factor [12], but also for the optimal development of the preconception environment [13][14].
2. Investigating the Cause of Disease: Denis Burkitt and Dysbiosis
The first person to document the profound difference between the health of people living a traditional lifestyle and those in the high-income Westernised world was Denis Burkitt. Born in 1911 in Enniskillen, Northern Ireland, UK, Burkitt trained as a surgeon in Edinburgh, UK, and served in Africa during the Second World War. After the end of the conflict, he went back to the African continent, travelled extensively, and made copious notes about the diet and health of people living their traditional modes of life. He discovered a transmissible viral cancer, now known as Burkitt’s lymphoma [15], but was surprised to find a complete absence of the plethora of non-communicable diseases found in “modern Western civilisation” [16]. The diseases identified by Burkitt are listed in Table 1, and are composed of conditions associated with a malfunctioning immune system including cancer and assorted disorders of blood circulation, weight gain, and cholesterol deposition. A product of his time, Burkitt unfortunately did not report any information on mental health issues, but he did report a significant observation, that the faecal output of people in “Westernised” societies was only about one third that of people living in traditional societies, who remained free from non-communicable diseases. The relative retention of energy in people exhibiting low faecal output is the most succinct explanation for weight gain in modern societies [17].
Table 1. Selected diseases characteristic of modern Western civilization [16].
| Appendicitis | Coeliac Disease * | Coronary Heart Disease |
|---|---|---|
| Deep vein thrombosis | Diabetes, type 2 | Diverticular disease |
| Gall stones | Haemorrhoids | Hiatus hernia |
| Multiple Sclerosis * | Obesity | Pernicious anaemia * |
| Pulmonary embolism | Rheumatoid arthritis * | Thyrotoxicosis * |
| Tumours of the bowel * | Ulcerative colitis * | Varicose veins |
* Diseases having a significant immune system component.
Observing that the onset of these diseases followed exposure to something in modern life, Burkitt suggested an environmental cause rather than an infectious agent. Lacking knowledge of the microbiota–gut–brain axis, he identified the low fibre intake of modern diets as leading to poor cholesterol metabolism and reduced faecal output, thus giving rise to the bulk of the other conditions listed. However, he admitted that he could not account for the observed immune system deficits simply on the basis of inadequate dietary fibre [16]. As Burkitt himself noted, however, as the cattle herding, steppe dwelling Maasai (Masai in his day) enjoyed a dietary regime similar to that of Westernised peoples, and did not suffer from non-communicable diseases [16], it could be that Burkitt’s “Westernised” diseases are not due to dietary factors at all.
The rapid spread of obesity and related diseases nevertheless raised the possibility of some kind of infection. Accordingly, the discovery that the gut microbes of genetically obese mice could transfer enhanced metabolic potential to germ-free mice briefly raised interest [18]. In contrast, around this time, the term dysbiosis was being used to imply a malfunction of the microbiome due to the absence of specific bacteria, the idea being to replace these entities with so-called probiotics. However, it has been pointed out that these ideas have not, as yet, been scientifically validated [19]. The recent publications have emphasised the lack of microbiome functionality consequently upon reduced microbial diversity as opposed to a lack of specific bacteria, and have therefore sought to introduce heavy metal pollution as a Burkitt-like environmental factor affecting the microbiome. Accordingly, obesity is a consequence of a failure of microbial growth and consequent excretion [17][20].
While the microbiota–gut–brain axis has been related to the onset of obesity [21], it has also been linked to mental health [22]. The own view is that the microbiome evolved as an “intergenerational” component of the vertebrate immune system in order to calibrate the neonate to recognise the microbial environment of its mother [23]. The main danger of an uncalibrated immune system is the misinterpretation of harmless antigens as representing harmful substances, leading to an epidemic of immune-related disease. Conversely, the immune system may also fail to recognise precancerous behaviour, thereby accounting for the observed rise in cancers apparently resulting from a wide variety of causes [24]. In this view, the function of the microbiota–gut–brain axis is to supply the microbiome with nutrition by controlling gut motility, while at the same time retaining sufficient nutrition for the survival of the host. The link with obesity follows from the breakdown of this axis, while mental health problems arise from the lack of interoception, the connection between body and brain [25]. In the view, therefore, the appearance of paediatric functional gastrointestinal disorders follows from the inheritance of a malfunctioning maternal microbiome, in combination with the genetic inheritance of the child. While there are elements of diagnosis in this research, its fundamental purpose is the prevention of disease by the assembly of a “birth probiotic” and its use in the repair of the human microbiome.
3. Summary of Microbiome-Related Concepts and Terminology
Researchers used the term microbiota to indicate the microbial entities themselves, while the term microbiome included the genetic abilities relevant to the host organism. It is important to note that a multicellular entity may coexist with several seemingly independent microbial communities, but it is possible that one is preferred, for example, the reported dominance of the gut microbiome within the gut–brain–skin axis that has been associated with acne vulgaris-induced depression [26]. Similarly, the terms holobiont and hologenome refer to all entities and/or their genes acting together to give each other survival advantages across multiple generations. It is generally considered that the many forms of non-communicable disease is largely a consequence of the reduced diversity of the intestinal microbiome seen across the developed world [27].
As further described below, communication between the two separate entities of the multicellular host and microbial community is by hormone-like semiochemicals, molecules passing a message between different species. The microbiome concept makes most sense from an evolutionary perspective, where a microbial community drives the immune status of the multicellular entity [23], possibly via eukaryotic microbial sentinel cells, potential precursors of the antigen-presenting dendritic cells of the immune system [28]. Researchers note that the immune system is involved with the gut–brain axis, in what researchers have termed an immune/semiochemical system [29]. Finally, researchers used the computer science term handshaking to describe the initial interaction between the genetics of the neonate host and its newly inherited “guest” microbiome, possibly even within the first few hours [25]. Significantly, the concept of neonate-handshaking accounts for the difficulty experienced in attempts to ameliorate non-communicable diseases such as allergy, for example, by increasing the microbial diversity in either adults or children [30].
4. Evolution: Lynn Margulis and Carl Woese; A Vertebrate Holobiont
At about the same time that Denis Burkitt was describing his African experiences, Lynn Margulis, writing as Lynn Sagan, was describing her ground-breaking work on the symbiotic origin of eukaryotes [31]. Taking the concept further, she later introduced the “symbiosis in evolution” concept as the holobiont, the idea that a species should be treated as a combination of every living entity involved in its biology [32]. A logical development of this theme is the hologenome, in which the critical component is the expression of genes providing functions of some value to the host [33]. While the bulk of symbiosis work is being undertaken on commercially sensitive invertebrates [34], it could be that such animals interact too easily with their environment, introducing unhelpful complexity. As an example of such flexibility, the ability of invertebrates to gain potentially valuable genes by horizontal gene transfer (HGT) continues to surprise. A recent example is the extensive uptake of plant genes into the genome of the whitefly herbivore Bemisi tabaci [35].
Paradoxically, it may be that the microbiome of vertebrate animals will prove to afford a clearer example of symbiosis than the nominally less complicated invertebrates. While Darwin espoused the idea of the survival of the fittest, this idea has dominated the discussion of genetic evolution for decades, with symbiosis and other forms of change largely relegated to an afterthought. Instead, it seems that “evolution” on the unicellular level occurs largely by HGT, a concept championed by Carl Woese using the expression “Darwinian threshold” to distinguish the difference between the two forms of evolutionary change [36]. Researchers' argument has been that the animal-microbe holobiont gains the best of both sides of Woese’s threshold, the stability of the multicellular host on one hand, allied to the flexibility of its diverse microbial guest on the other [23].
Logic suggests that the basic function of a symbiotic microbiome guest is to support the immune system of its multicellular host while obtaining nutrition in return [10]. In this context, while the development of the first vertebrate animals across the Ediacaran–Cambrian boundary may have contributed to their efficiency as predators [37], the elongation of their body plan would have posed problems for the heritability of key microbes from within their microbiomes. Accordingly, it seems that the outcome of this challenge was a division of function: while the major cognitive apparatus lies at the front of the animal, under the control of the brain, the primary microbiome resides at the end of the digestive tract, allowing relevant microbial constituents to be passed on to the next generation. Accordingly, the most succinct explanation for the immune system component of non-communicable disease is that a form of microbial sentinel cell exists within the properly functioning microbiome, and that this class of cell has been lost under the conditions of modern life [23]. While the microbiome has often been treated as an ad hoc collection of environmental bacteria introduced via our food, more recent evidence has traced an origin in humans back to our African origins [38]. Of course, a sentinel cell will be more complex than simple prokaryotes, and although unicellular eukaryotes are known to exist inside the microbiome, their function is not completely characterised. One example of such entities includes members of the genus Blastocystis, classified as parasites, but known to be associated with normal weight, apparently healthy individuals [39]. The fungal “mycobiome” has also been studied, especially within early childhood [40], and a suggestion has been made that microbial eukaryotes could be the missing link in gut microbiome studies [41]. Note that researchers have dealt with these concepts in greater detail in a recent publication covering both the evolutionary aspects of vertebrate animal–microbe symbiosis as well as its loss in the polluted environment of high-income countries [23].
5. Epidemiology: David Strachan and David Barker; An Infant Origins Hypothesis
In September 1989, David Strachan published the results of his observations concerning the spectacular rise of diseases such as asthma and seasonal allergic rhinitis: “hay fever, hygiene, and household size” [42]. In essence, what became known as his “Hygiene Hypothesis” suggested that atopic and autoimmune diseases start in children that live in modern environments, the key point being that the absence of microbes is capable of training their immune systems. This idea was taken up by Graham Rook and his team as the “Old Friends” concept of external training agents. In spite of intensive efforts, by 2013, it was clear that such an external agent could not be found [43]. Nevertheless, levels of atopic disease kept increasing, with seemingly new conditions such as food allergy raising concern [44]. The most important feature about these immune system disorders is that they originated in the young, typically being observed in a characteristic sequence described as the “atopic march” [45]. In addition, it also became clear that animals associated with humans suffer from a very similar series of immune system problems, suggesting a common mechanism, at least across the Mammalia [46].
At almost the same time as Strachan was writing, in November 1990, David Barker published his own article “The foetal and infant origins of adult disease” [47]. Using the subtitle “The womb may be more important than the home”, his idea later became known as “The Foetal Origins Hypothesis”. Although he was mostly describing weight gain leading to stroke and heart disease, it is important to note that he specifically mentioned schizophrenia in his original article [47]. Although successful when studied from an economic perspective [48], its fundamental problem is the absence of an acceptable underlying mechanism, as confirmed by a later review [49]. Whatever the fundamental cause, obesity and related issues are increasing, as illustrated by a study of sport-related statistics measured in different batches of 10-year-old schoolchildren in 1998, 2008, and 2014. Although the overall BMI did not change, the height and weight both increased over this period, and vital attributes such as handgrip and leg strength both declined [50]. Similar results were found in an independent study [51]. It is important to note, however, that Barker’s initial concept applies to the birth process in general and could therefore be termed as an “Infant Origins Hypothesis”, laying the emphasis on the second part of the title of his original paper [47].
6. A Microbiome-Health Hypothesis: “Handshaking” and the Birth Process
Interestingly, there are a variety of conceptual overlaps between biology and engineering. In particular, “computer viruses” possess characteristics similar to biological viruses. Perhaps analogously, as described in the Concepts and Terminology section, while the engineering term “handshaking” refers to the exchange of protocols of communication between two independent electronic devices, it may be that the same term could be employed to indicate interactions between the microbiome and the gut wall of the newborn animal. In its current format, our Microbiome-Health Hypothesis suggests that this “handshaking” requires a fully functional microbiome to be in place immediately after birth (or after hatching from an egg), in order to allow for the proper development of the gut–brain axis. Of course, in humans, there are many years between neonate and adult, and it is likely that this axis will need a supplement to the handshaking process after key events such as puberty. There is little doubt, however, that the early days are the most important, quite possibly the first hours. It seems clear that the period immediately after maternal microbial inheritance is of great significance to the health of both the child and adult [52][53]. While it is likely that the microbiome has its effect through epigenetic mechanisms [54], it is clear that much remains to be done [55].
Modern antiseptic methods ensure that babies can be safely delivered by caesarean section (C-section) under a sterile environment. Interestingly, the same procedures can be used for the obtention of gnotobiotic, so-called germ-free, laboratory animals [56]. It is known that laboratory mice have modified physiological responses to stress under these conditions, but that post-natal reintroduction of gut microbes eliminates many of their problems [57]. Altered gut microbes have a similar effect in humans, as a later study involving children experiencing problems with caregiving has shown [58]. The relationship between C-section delivery and obesity has been studied most often, usually finding a positive correlation. For example, a study by Yuan et al. showed a 15% greater chance of the development of obesity after C-section birth, with “additional research needed to clarify the mechanism” [59]. When a swabbing procedure called “vaginal inoculation” took place, Dominguez-Bello and her team demonstrated that the microbiota of those children showed some similarity to their vaginally delivered peers. Health studies are now underway [60]. A similar study by Chu et al. compared the maturation rate of the microbiome between C-section or vaginal methods of delivery, showing that there was no apparent difference six weeks post-natal [61]. Likewise, the role of birth mode in the possible transmission of obesity from mother to child was followed by bacterial 16S rRNA, suggesting that species from the phylum Firmicutes were most likely to be responsible [62], but they did not check for the presence of unicellular eukaryotes. In contrast, a similar study following the transfer of vaginal fungi in addition to bacteria identified lower mycobiome diversity as contributing to type 1 diabetes in children [63].
Of course, this vaginal inoculation procedure can only transfer the maternal microbiota that are already present and is therefore of no use to the baby if such microbiota were not functioning properly in the mother. In addition, while simpler cells such as bacteria can be picked up from the environment more-or-less readily, unicellular eukaryotic sentinel cells are more likely to be specific to the individual and would not be expected to be recovered once degraded. In this fashion, microbiome quality can only worsen across the generations, an observation that is consistent with the epidemiology of non-communicable disease [64]. However, the C-section procedure is convenient and often necessary, and is increasing worldwide [65]. Furthermore, the incidence of C-section delivery is predicted to significantly increase in the future [66]. Finally, regardless of how “natural” the concept of vaginal inoculation/seeding, there remains a reluctance to carry out a procedure that has neither been subjected to stringent clinical trials nor has a robust “theory of microbiome action” to guide its practitioners [67].
At this point, it is worth bearing in mind the comments of Brüssow on the continuing need to confirm the relevance of suspect microbes by reference to the principles exemplified by Koch’s postulates [19].
6.1. Natural Birth: Efficient Microbial Transfer
Three components common to all the Vertebrata include the brain (acting with its body), the intestinal microbiome, and a connecting gut–brain axis (Figure 1, left hand side). As semiochemicals act on the gut wall, the gut–brain axis induces peristalsis, passing nutrition down to the microbiome. Along with the nutrition, environmental antigens prime the hypothetical microbial sentinel cells ready for the next stage, as the animal creates its offspring. The centre section indicates the natural birth process, where the neonate becomes an independent entity, either via live birth or egg. It is at this stage that direct “contamination” takes place, transferring the microbiome to the infant [68]. Interestingly, bacteria are present in many of the structures associated with birth, but it is not known as to what degree they contribute to either the success of the pregnancy or the health of the baby [69]. The birth process is completed as illustrated on the right-hand side, as semiochemical production initiates “handshaking”, and microbial sentinel cells calibrate the immune system relative to the microbial environment of the mother. At this stage, mammals have evolved an extra layer of assistance, as mother’s milk provides nutrients [70] and possibly supplementary microbes [71].
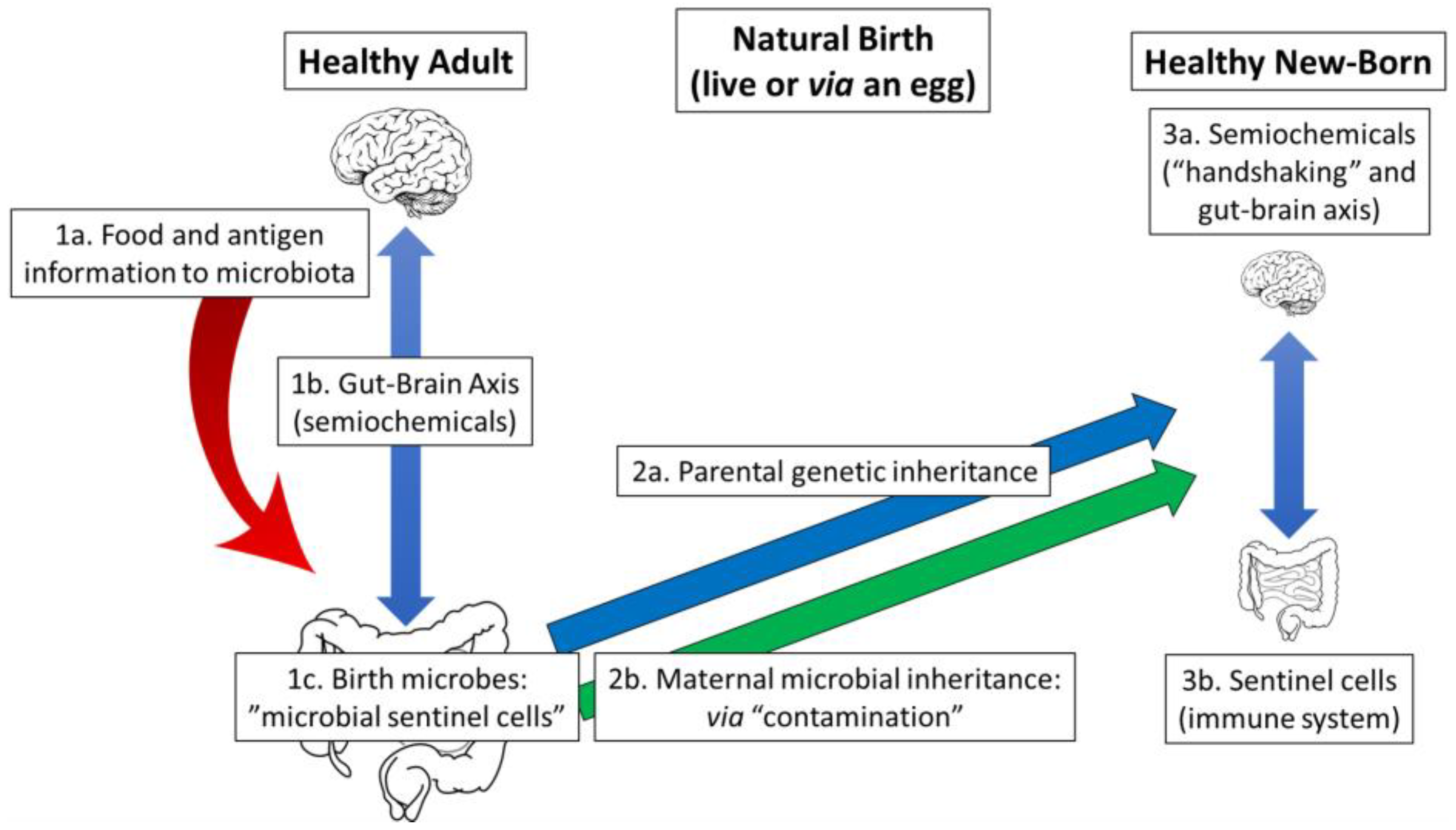
Figure 1. Natural birth. Left hand side: Indicates the function of the gut–brain axis in the healthy vertebrate adult. In essence, this axis acts to partition nutrition between the body (the host) and the microbiome (the guest). Box 1a. Alongside food, antigen information regarding the external microbial environment is passed down to the microbiome for subsequent transfer to the next generation, with microbial sentinel cells acting as an “intergenerational” component of the immune system. Box 1b. The microbiome produces semiochemicals, hormone-like molecules that pass information between different species, in this case, “interkingdom”, from prokaryotes to eukaryotes, acting to stimulate the gut wall, and therefore the gut–brain axis. Finally, Box 1c represents hypothetical microbial sentinel cells ready to be transferred to the neonate by apparently accidental contamination. Centre: This represents the vertebrate birth process that has evolved since the Precambrian. Box 2a. The foetus develops according to the genetic inheritance of the parents and Box 2b is contaminated by the microbes from the intestine of the mother on birth: the “maternal microbial inheritance”. Right hand side: Illustrates the newborn animal, with complete parental genetic and maternal microbial inheritances. Box 3a. Semiochemicals produced by the microbiota in the intestine interact with the gut wall in a “handshaking” process, allowing the growth of the gut–brain axis. Box 3b. The sentinel cells transferred from the maternal microbiome interact with the gut wall in their own version of the handshaking process. Their output is part of the combined immune/semiochemical system interacting with the gut–brain axis.
6.2. Delivery by Sterile Caesarean Section: Limited Microbiota Transfer
The situation described in Figure 2 represents a healthy adult, with a fully functioning microbiome and gut–brain axis (left hand side), being delivered of her baby under sterile hospital settings (centre section). Nevertheless, hospital “sterility” is not as severe as for the birth of germ-free animals, and slow contamination will occur under these conditions. The key point here is that, as stated above, the microbiome of the child will eventually establish itself, according to the findings of Chu et al., but, of course, it may be too late for a completely effective handshaking function or immune system support [61]. Disease results from either the absence of an effective gut–brain axis such as poor mental health or inadequate “calibration” of the immune system, leading to problems such as autoimmune disease (right hand side). A final point is that any microbes that do not manage to spread to the infant will not be present in future births, affording what can be described as a “snowball effect” of increasing problems across generations.
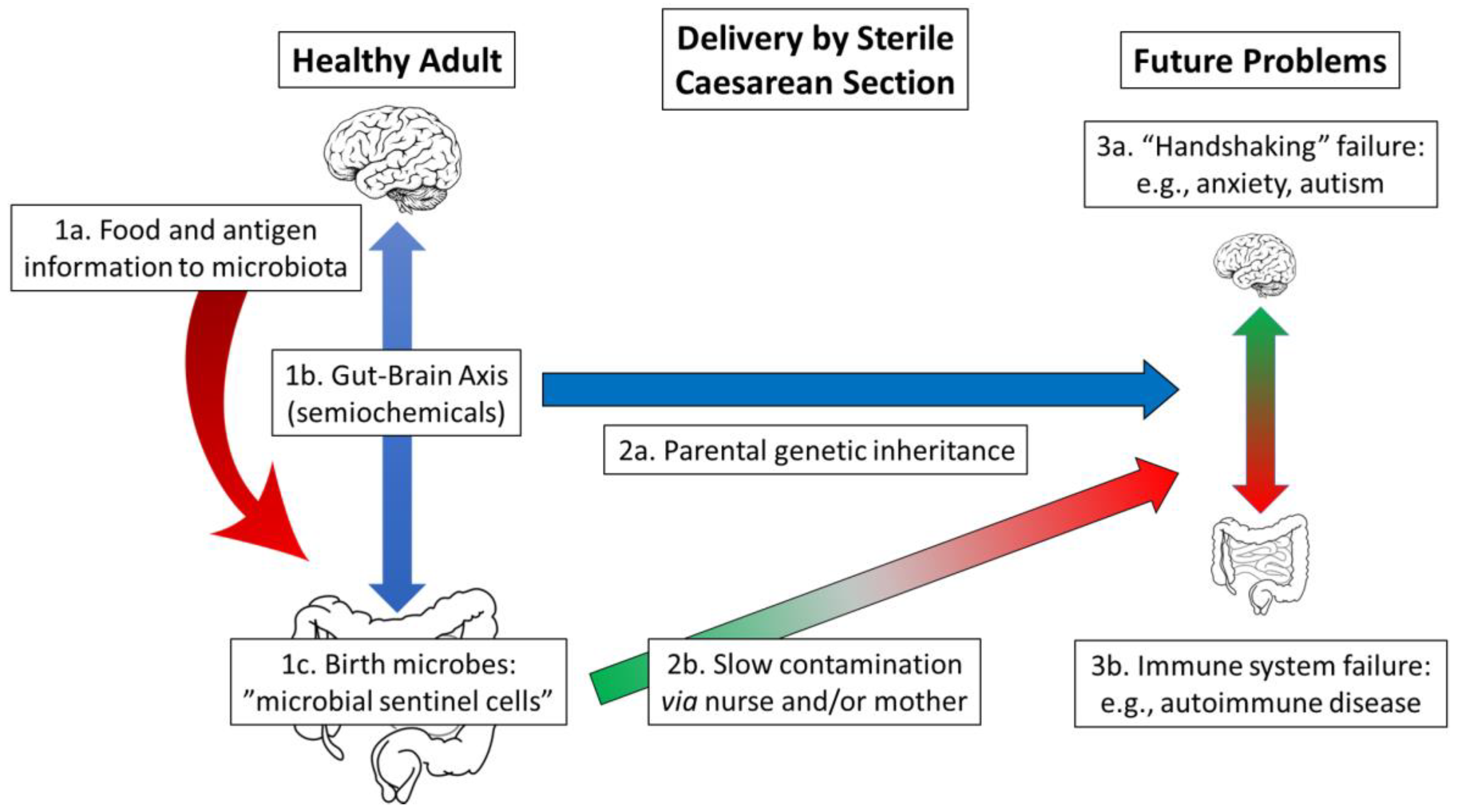
Figure 2. Delivery by caesarean section under hospital conditions. Left hand side: Represents a healthy vertebrate adult with, Box 1a, the transfer of food and antigen information to the microbiome, Box 1b, semiochemicals produced by the intestinal microbiota, and Box 1c, “birth microbes” ready to be delivered to the baby. Centre: Indicates that the two halves of the natural birth process have become separated. Box 2a represents the parental genetic inheritance of the baby while Box 2b illustrates the difficulty experienced by the maternal microbiota to reach the baby. Right hand side: Represents the chance of disease as the child grows into an adult. Box 3a describes the effect of the loss of interoception due to the absence of a fully functioning gut–brain axis on the developing brain, with the potential for conditions such as anxiety and autism. Box 3b indicates problems with the immune system, potentially autoimmune disease, as it is no longer calibrated by the presence of microbial sentinel cells mainly from the mother.
Of course, the birth of twins poses special dangers for the mother, and planned C-section deliveries are often carried out. Unless the vaginal inoculation procedure [60] is carried out, each twin will have different levels of contamination, and hence a different chance of disease. While twin studies (genetically identical versus fraternal twins) have been used to study the vexed question of “genes versus environment”; only a few decades ago, delivery by C-section was not normally considered, implying that similar studies carried out today would yield different results.
References
- Drossman, D.A. Functional gastrointestinal disorders: History, pathophysiology, clinical features and Rome IV. Gastroenterology 2016, 150, 1262–1279.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Arlington, VA, USA, 2013.
- Hyams, J.S.; Di Lorenzo, C.; Saps, M.; Shulman, R.J.; Staiano, A.; van Tilburg, M. Childhood functional gastrointestinal disorders: Child/adolescent. Gastroenterology 2016, 150, 1456–1468.
- Haregu, T.N.; Byrnes, A.; Singh, K.; Sathish, T.; Pasricha, N.; Wickramasinge, K.; Thankappan, K.R.; Oldenburg, B. A scoping review of non-communicable disease research capacity strengthening initiatives in low and middle income countries. Glob. Health Res. Policy 2019, 4, 31.
- Davis, H.E.; McCorkell, L.; Moore, J.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–148.
- Soldan, S.S.; Lieberman, P.M. Epstein-Barr virus and multiple sclerosis. Nat. Rev. Microbiol. 2023, 21, 51–64.
- Kreutz, J.M.; Adriaanse, M.P.M.; van der Ploeg, E.M.C.; Vreugdenhil, A.C.E. Narrative review: Nutrient deficiencies in adults and children with treated and untreated celiac disease. Nutrients 2020, 12, 500.
- Gargano, D.; Appanna, R.; Santonicola, A.; De Bartolomeis, F.; Stellato, C.; Cianferoni, A.; Casolaro, V.; Iovino, P. Food Allergy and Intolerance: A Narrative Review on Nutritional Concerns. Nutrients 2021, 13, 1638.
- Elizabeth, L.; Machado, P.; Zinöcker, M.; Baker, P.; Lawrence, M. Ultra-processed foods and health outcomes: A narrative review. Nutrients 2020, 12, 1955.
- Jheeta, S.; Smith, D. Seeing the wood for the trees: A new way to view the human intestinal microbiome and its connection with non-communicable disease. Med. Hypotheses 2019, 125, 70–74.
- Donkin, I.; Barrès, R. Sperm Epigenetics and Influence of Environmental Factors. Mol. Metab. 2018, 14, 1–11.
- Altmäe, S.; Franasiak, J.M.; Mändar, R. The Seminal Microbiome in Health and Disease. Nat. Rev. Urol. 2019, 16, 703–721.
- Marcho, C.; Oluwayiose, O.A.; Pilsner, J.R. The Preconception Environment and Sperm Epigenetics. Andrology 2020, 8, 924–942.
- Vallgårda, S. Why the concept “lifestyle diseases” should be avoided. Scand. J. Public Health 2011, 39, 773–775.
- Burkitt, D. A sarcoma involving the jaws in African children. Br. J. Surg. 1958, 46, 218–223.
- Burkitt, D.P. Some diseases characteristic of modern western civilization. Br. Med. J. 1973, 1, 274–278.
- Smith, D.; Jheeta, S. Microbiome-gut dissociation: Investigating the origins of obesity. Gastrointest. Disord. 2021, 3, 156–172.
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nat. Cell Biol. 2006, 444, 1027–1031.
- Brüssow, H. Problems with the concept of gut microbiota dysbiosis. Microb. Biotechnol. 2019, 13, 423–434.
- Smith, D.; Palacios-Pérez, M.; Jheeta, S. Microbiome-gut dissociation in the neonate: Obesity and coeliac disease as examples of microbiome-function deficiency disorder. Gastrointest. Disord. 2022, 4, 108–128.
- Torres-Fuentes, C.; Schellenkens, H.; Dinan, T.G.; Cryan, J.F. The microbiota-gut-brain axis in obesity. Lancet Gastroenterol. Hepatol. 2017, 2, 747–756.
- Dinan, T.G.; Cryan, J.F. Gut microbiota: A missing link in psychiatry. World Psychiatry 2020, 19, 111–112.
- Smith, D.; Palacios-Pérez, M.; Jheeta, S. The enclosed intestinal microbiome: Semiochemical signals from the Precambrian and their disruption by heavy metal pollution. Life 2022, 12, 287.
- Blackadar, C.B. Historical review of the causes of cancer. World J. Clin. Oncol. 2016, 7, 54–86.
- Smith, D.; Jheeta, S.; Fuentes, H.V.; Street, B.; Palacios-Pérez, M. Microbiome-gut dissociation in the neonate: Autism-related developmental brain disease and the origin of the placebo effect. Gastrointest. Disord. 2022, 4, 291–311.
- Bowe, W.P.; Logan, A.C. Acne vulgaris, probiotics and the gut-brain-skin axis—Back to the future? Gut Pathog. 2011, 3, 1.
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179.
- Banchereau, J.; Briere, F.; Caux, C.; Davoust, J.; Lebecque, S.; Liu, Y.-J.; Pulendran, B.; Palucka, K. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000, 18, 767–811.
- Smith, D.; Jheeta, S.; Fuentes, H.V.; Palacios-Pérez, M. Feeding our microbiota: Stimulation of the immune/semiochemical system and the potential amelioration of non-communicable diseases. Life 2022, 12, 1197.
- Chernikova, D.; Yuan, I.; Shaker, M. Prevention of allergy with diverse and healthy microbiota: An update. Curr. Opin. Pediatr. 2019, 31, 418.
- Sagan, L. On the origin of mitosing cells. J. Theor. Biol. 1967, 14, 255–274.
- Margulis, L. Symbiogenesis and symbionticism. In Symbiosis as a Source of Evolutionary Innovation: Speciation and Morphogenesis; Margulis, L., Fester, R., Eds.; MIT Press: Cambridge, MA, USA, 1991; pp. 49–92.
- Rosenburg, E.; Zilber-Rosenburg, I. The hologenome concept of evolution after 10 years. Microbiome 2018, 6, 78.
- Yong, E. I Contain Multitudes: The Microbes within Us and a Grander View of Life; Penguin Random House LLC.: London, UK, 2016; ISBN 978-1-784-70017-1.
- Gilbert, C.; Maumus, F. Multiple horizontal acquisitions of plant genes in the whitefly Bemisia tabaci. Genome Biol. Evol. 2022, 14, evac141.
- Woese, C. On the evolution of cells. Proc. Natl. Acad. Sci. USA 2002, 99, 8742–8747.
- Budd, G.E. At the origin of animals: The revolutionary Cambrian fossil record. Curr. Genom. 2013, 14, 344–354.
- Suzuki, T.A.; Fitzstevens, J.L.; Schmidt, V.T.; Enav, H.; Huus, K.; Mbong, M.; Adegite, B.R.; Zinsou, J.F.; Esen, M.; Velavan, T.; et al. Codiversification of gut microbiota with humans. Science 2022, 377, 1328–1332.
- Stensvold, C.R.; van der Giezen, M. Associations between gut microbiota and common luminal intestinal parasites. Trends Parasitol. 2018, 34, 369–377.
- Ward, T.L.; Dominguez-Bello, M.G.; Heisel, T.; Al-Ghalith, G.; Knights, D.; Gale, C.A. Development of the human mycobiome over the first month of life and across body sites. mSystems 2018, 3, e00140.
- Laforest-Lapointe, I.; Arrieta, M.-C. Microbial eukaryotes: A missing link in gut microbiome studies. mSystems 2018, 3, e00201-17.
- Strachan, D.P. Hay fever, hygiene and household size. BMJ 1989, 299, 1259–1260.
- Rook, G.A.W.; Lowry, C.A.; Raison, C.L. Microbial ‘Old Friends’, immunoregulation and stress resilience. Evol. Med. Public Health 2013, 1, 46–64.
- Loh, W.; Tang, M.L.K. The epidemiology of food allergy in the global context. Int. J. Environ. Res. Public Health 2018, 15, 2043.
- Hill, D.A.; Spergel, J.M. The atopic march: Critical evidence and clinical relevance. Ann. Allergy Asthma Immunol. 2018, 120, 131–137.
- Marsella, R.; De Benedetto, A. Atopic dermatitis in animals and in people: An update and comparative review. Vet. Sci. 2017, 4, 37.
- Barker, D.J. The fetal and infant origins of adult disease. BMJ 1990, 301, 1111.
- Eriksson, J.G. The fetal origins hypothesis–10 years on. BMJ 2005, 330, 1096–1097.
- Almond, D.; Currie, J. Killing me softly: The fetal origins hypothesis. J. Econ. Perspect. 2011, 25, 153–172.
- Sandercock, G.R.H.; Cohen, D.D. Temporal trends in muscular fitness of English 10-year-olds 1998–2014: An allometric approach. J. Sci. Med. Sport 2019, 22, 201–205.
- Ðuric, S.; Sember, V.; Starc, G.; Soric, M.; Kovac, M.; Jurak, G. Secular trends in muscular fitness from 1983 to 2014 among Slovenian children and adolescents. Scand. J. Med. Sci. Sport. 2021, 31, 1853–1861.
- Mesa, D.M.; Loureiro, B.; Iglesia, I.; Gonzalez, S.F.; Olivé, E.L.; Algar, O.G.; Solana, M.J.; Cabero, M.J.; Sainz, T.; Martinez, L.; et al. The evolving microbiome from pregnancy to early infancy: A comprehensive review. Nutrients 2020, 12, 133.
- Robertson, R.C.; Manges, A.R.; Finlay, B.B.; Prendergast, A.J. The human microbiome and child growth—First 1000 days and beyond. Trends Microbiol. 2019, 27, 131–147.
- Waddington, C.H. Toward a Theoretical Biology; The basic ideas of biology; Edinburgh University Press: Edinburgh, Scotland, 1968; pp. 1–32.
- Qin, Y.; Wade, P.A. Crosstalk between the microbiome and the epigenome: Messages from bugs. J. Biochem. 2018, 163, 105–112.
- Basic, M.; Bleich, A. Gnotobiotics: Past, present and future. Lab. Anim. 2019, 53, 232–243.
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.-N.; Kubo, Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004, 558, 263–275.
- Callaghan, B.L.; Fields, A.; Gee, D.G.; Gabard-Durnam, L.; Caldera, C.; Humphreys, K.L.; Goff, B.; Flannery, J.; Telzer, E.H.; Shapiro, M.; et al. Mind and gut: Associations between mood and gastrointestinal distress in children exposed to adversity. Dev. Psychopathol. 2020, 32, 309–328.
- Yuan, C.; Gaskins, A.J.; Blaine, A.I.; Zhang, C.; Gillman, M.W.; Missmer, S.A.; Field, A.E.; Chavarro, J.E. Association between cesarean birth and risk of obesity in offspring in childhood, adolescence, and early adulthood. JAMA Pediatr. 2016, 170, e162385.
- Song, S.J.; Wang, J.; Martino, C.; Jiang, L.; Thompson, W.K.; Shenhav, L.; McDonald, D.; Marotz, C.; Harris, P.R.; Hernandez, C.D.; et al. Naturalization of the microbiota developmental trajectory of Cesarean-born neonates after vaginal seeding. Med 2021, 2, 951–964.e5.
- Chu, D.M.; Ma, J.; Prince, A.L.; Anthony, K.M.; Seferovic, M.D.; Aagaard, K.M. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat. Med. 2017, 23, 314–326.
- Tun, H.M.; Chari, R.; Field, C.J.; Guttman, D.S.; Becker, A.B.; Mandhane, P.J.; Turvey, S.E.; Subbarao, P.; Sears, M.R.; Scott, J.A.; et al. Roles of birth mode and infant gut microbiota in intergenerational transmission of overweight and obesity from mother to offspring. JAMA Pediatr. 2018, 172, 368–377.
- Ruotsalainen, A.L.; Tejesvi, M.V.; Vänni, P.; Suokas, M.; Tossavainen, P.; Pirttilä, A.M.; Talvensaari-Mattila, A.; Nissi, R. Child type 1 diabetes associated with mother vaginal bacteriome and mycobiome. Med. Microbiol. Immunol. 2022, 211, 185–194.
- Smith, D.; Jheeta, S. The epidemiology of the dysfunctional microbiome in animals and in humans: The propensity for the development of non-communicable disease. EC Gastroenterol. Dig. Syst. 2020, 7, 83–93.
- Chien, P. Global rising rates of caesarean sections. BJOG Int. J. Obstet. Gynaecol. 2021, 128, 781–782.
- Betran, A.P.; Ye, J.; Moller, A.-B.; Souza, J.P.; Zhang, J. Trends and projections of caesarean section rates: Global and regional estimates. BMJ Glob. Health 2021, 6, e005671.
- Cunnington, A.J.; Sim, K.; Deierl, A.; Kroll, S.; Brannigan, E.; Darby, J. “Vaginal seeding” of infants born by caesarean section. BMJ 2016, 352, i227.
- Valles-Colomer, M.; Blanco-Míguez, A.; Manghi, P.; Asnicar, F.; Dubois, L.; Golzato, D.; Armanini, F.; Cumbo, F.; Huang, K.D.; Manara, S.; et al. The person-to-person transmission landscape of the gut and oral microbiomes. Nature 2023, 614, 125–135.
- Di Simone, N.; Santamaria Ortiz, A.; Specchia, M.; Tersigni, C.; Villa, P.; Gasbarrini, A.; Scambia, G.; D’Ippolito, S. Recent Insights on the Maternal Microbiota: Impact on Pregnancy Outcomes. Front. Immunol. 2020, 11, 528202. Available online: https://www.frontiersin.org/articles/10.3389/fimmu.2020.528202 (accessed on 23 April 2023).
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74.
- Kim, S.Y.; Yi, D.Y. Analysis of the human breast milk microbiome and bacterial extracellular vesicles in healthy mothers. Exp. Mol. Med. 2020, 52, 1288–1297.
More
Information
Subjects:
Integrative & Complementary Medicine
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
575
Revisions:
2 times
(View History)
Update Date:
02 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




