Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Loredana Raciti | -- | 3009 | 2023-05-29 14:47:40 | | | |
| 2 | Rita Xu | Meta information modification | 3009 | 2023-05-30 03:47:46 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Raciti, L.; Formica, C.; Raciti, G.; Quartarone, A.; Calabrò, R.S. Gender and Neurosteroids. Encyclopedia. Available online: https://encyclopedia.pub/entry/44971 (accessed on 08 February 2026).
Raciti L, Formica C, Raciti G, Quartarone A, Calabrò RS. Gender and Neurosteroids. Encyclopedia. Available at: https://encyclopedia.pub/entry/44971. Accessed February 08, 2026.
Raciti, Loredana, Caterina Formica, Gianfranco Raciti, Angelo Quartarone, Rocco Salvatore Calabrò. "Gender and Neurosteroids" Encyclopedia, https://encyclopedia.pub/entry/44971 (accessed February 08, 2026).
Raciti, L., Formica, C., Raciti, G., Quartarone, A., & Calabrò, R.S. (2023, May 29). Gender and Neurosteroids. In Encyclopedia. https://encyclopedia.pub/entry/44971
Raciti, Loredana, et al. "Gender and Neurosteroids." Encyclopedia. Web. 29 May, 2023.
Copy Citation
Neurosteroids are synthesized de novo in the nervous system; they mainly moderate neuronal excitability, and reach target cells via the extracellular pathway. The synthesis of neurosteroids occurs in peripheral tissues such as gonads tissues, liver, and skin; then, because of their high lipophilia, they cross the blood–brain barrier and are stored in the brain structure. Neurosteroidogenesis occurs in brain regions such as the cortex, hippocampus, and amygdala by enzymes necessary for the in situ synthesis of progesterone from cholesterol. Neurosteroids could be considered the main players in both sexual steroid-induced hippocampal synaptic plasticity and normal transmission in the hippocampus.
neurosteroids
neuroplasticity
GABA-receptors
estrogen
gender
1. Introduction
Neurosteroids (NSs) were named in 1981 [1] and identified as steroids that are synthesized de novo in the nervous system, such as in the hippocampus and other brain structures, and are accumulated in the nervous system autonomously from the steroidogenic endocrine glands. Neurosteroids have been implicated in several neurological mechanisms, such as epileptogenesis, hepatic encephalopathy, neurodegeneration, neuroprotection, and psychiatric disorders. NSs’ origins from cholesterol or other steroidal precursors derive from circulating steroid hormones. They mainly moderate neuronal excitability [2][3] and reach target cells via extracellular pathways. These paracrine signals modulate neurotransmitter-gated ion channels and G-protein-coupled receptors, predominantly [4][5] γ-aminobutyric (GABA)A2 and the N-methyl-D’Aspartate (NMDA) receptors [4][5]. Excitability is carried by chemicals signals discharged from astrocytes, oligodendrocytes, Schwann cells, and neurons such as Purkinje cells, hippocampal neurons, and retinal amacrine and ganglion cells of the brain [6][7].
Following the structural characteristics, NSs can be classified as:
- (i)
-
pregnane, such as allopregnanolone (5α-pregnane-3α-ol-20-one) and allotetrahydrodeoxycorticosterone (THDOC, 5α-pregnane-3α,21-diol-20-one), whose precursors are progesterone and deoxycorticosterone, respectively;
- (ii)
-
androstane, such as androstanediol and etiocholanone, derived from testosterone and estradiol;
- (iii)
-
sulphated, such as pregnenolone sulfate (PS) and dehydroepiandrosterone sulfate (DHEAS).
The NSs cross the brain barrier and stimulate brain function. Neurons and glial cells display activity of 5α-Reductase [8][9], whereas neocortex and subcortical white matter, as well as hippocampal tissues, have 5α-reductase and 3α-HSOR enzymes activities [10][11].
Moreover, the brain astrocytes and glutamatergic principal neurons express cytochrome P450 cholesterol side-chain cleavage enzyme (CYP450scc) that transforms cholesterol to pregnenolone [12][13]. Therefore, a translocator protein (18 kD) present in the peripheral tissues and in the brain, acting as a peripheral or mitochondrial benzodiazepine receptor [14][15], supports the moving of cholesterol through to the inner mitochondrial membrane [16]. Successively, in the inner mitochondrial membrane the availability of cholesterol to the CYP450scc increases, so cholesterol is converted to pregnenolone, which is a key intermediate for NSs biosynthesis. Moreover, the presence of the 3β-hydroxysteroid dehydrogenase enzyme that converted the pregnenolone to progesterone has been shown in the brain [17] (Figure 1).
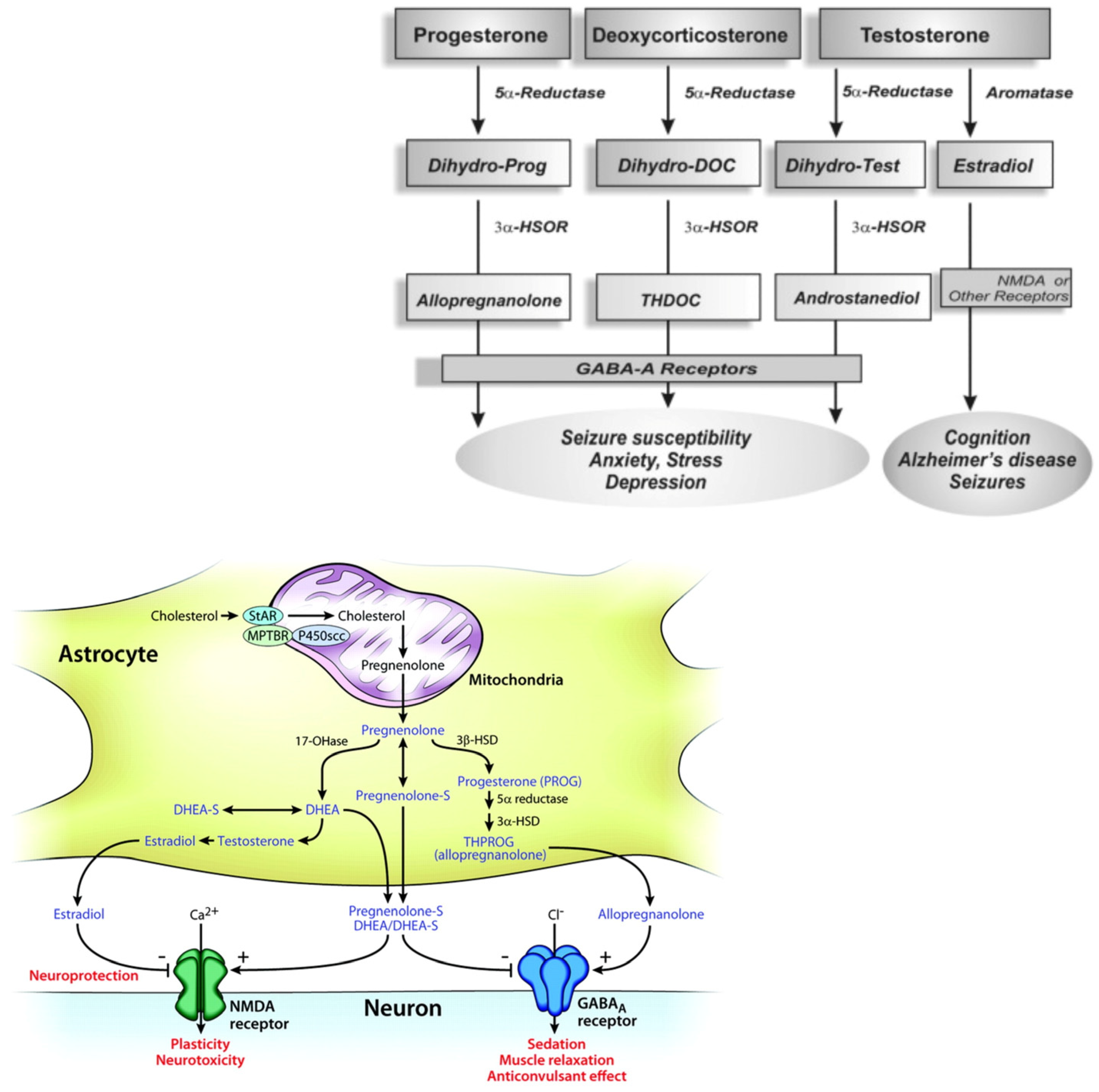
Figure 1. Biosynthetic pathways for steroidogenesis. The conversion of progesteron, deoxycoricosteron and testosterone in allopregnenolone, THDOC and androstanediol by the enzyme 5α-Reductase, aromatase and 3α-HSOR, impacting on brain functions (molecular view; schematic illustration); the GABA-A receptors composed of α, β and γ or δ subunits structure that form chloride ion channel.
Neurosteroidogenesis occurs in the brain regions such as the cortex, hippocampus, and amygdala by enzymes necessary for the in situ synthesis of progesterone from cholesterol [18][19]. NSs may act via multiple pathways, by regulating gene transcription or through a direct action on neurotransmitter-gated ion channel receptors and G-protein-coupled receptors. The most important targets are the γ-aminobutyric (GABA)A [20] and the NMDA receptors [21][22][23][24]. Nevertheless, the biosynthesis of NSs in the brain is still unclear.
2. Mechanism of Action of Neurosteroids
There are two chronic effects of NSs in the brain: genomic (classical intracellular steroid receptors), due to their metabolic interconversion to traditional steroids [3], and non-genomic rapid actions, mediated by ion channels and membrane receptors.
The effects of NSs on the brain excitability modulation depend on the interaction between neural membrane receptors and ion channels with rapid effects (within minutes), in contrast to the slow effects of steroid hormone via intracellular steroid receptors (even though a prolonged duration has been shown) [22]. Moreover, it has been shown that NSs directly and widely control the GABA-A receptors, the ligand-gated ion channels [23], in positive or negative phase depending on the type of the steroid molecule [24].
Structurally, GABA-A receptors are heteropentamers with five protein subunits that form the chloride ion channels to moderate the bulk of synaptic inhibition in the central nervous system. NSs increase the chance of opening the GABA-A receptor chloride channel, given that the closed time increases the chloride current through the channel with a reduction of neuronal excitability. The GABA function includes the opening of chloride ion channels, and the internalization of the chloride ion to facilitate the hyperpolarization of the membrane [25][26][27]. GABA-A receptors bypass the depolarization of the excitatory neurotransmission and avoid the action potential generation by two types of inhibitory neurotransmission: synaptic (phasic) and extrasynaptic (tonic) inhibition, that are modulated and potentiated by NSs. The GABA-A receptor subunit influences the NSs action modulation of GABA-A receptors [23][24][25][26][27][28][29]. Specifically, the α-subunit influences the NSs efficacy, whereas the γ-subunit may control both the efficacy and potency [27]. The precise site of NSs binding is currently unknown, although it seems to be placed at highly conserved glutamine (position 241 in the M1 domain of the α-subunit), playing a key role in NSs modulation. Therefore, the effect of NSs is probable due to their action on both synaptic and perisynaptic/extrasynaptic GABAA receptors.
The intermittent release of high levels of GABA, from presynaptic axon terminals of GABAergic interneurons, triggers the γ2-containing receptors at the synapse, inducing the phasic/synaptic inhibition. The tonic/inhibition results from the extrasynaptic continuous activation of δ-containing receptors, related to low levels of ambient GABA molecules that escape reuptake by GABA transporters [27][28][29]. The δ-subunits are located perisynaptically/extrasynaptically and they mediate the “tonic” GABA-A receptor current [20][28][29][30][31], producing a stable inhibition of neurons and decreasing their excitability.
Furthermore, the location of the δ-subunit on the dendrites of hippocampal dentate gyrus granule cells supports the GABA-A receptors to work as a controller of hippocampus excitability.
Allopregnanolone (ALLO), THDOC, and androstanediol are potent positive allosteric modulators of GABA-A receptors [4][5][32], binding the D ring at both C20 of the pregnane steroid side chain and C17 of the androstane of the ring A with a positive activity at GABA-A receptors [19].
ALLO has anxiolytic, sedative–hypnotic, and anticonvulsant effects. Alcohol, hydroxybutyrate, and diazepam may potentiate GABAergic inhibition directly or through the increased availability of ALLO [20]. Pregnanolone sulphate (PS) and DHEAS are sulphated at C3 (called “sulphated steroids”) and block GABA-A receptors at low micromolar concentrations, reduced the channel opening frequency [24][33][34][35][36] and inhibited actions [24][36]. They are also effective allosteric agonists at the NMDA receptor complex and negative non-competitive modulators of the GABA-A receptor [37]. High micromolar concentrations of PS and DHEAS accomplish actions on NMDA receptor-mediated currents and act on the σ1 receptors with presynaptic action, inducing glutamate release. Therefore, NSs can also modulate the NMDA type glutamate receptors [38]. The NMDA receptors exhibit two distinct sites for NSs modulation: one facilitates the effects of positive modulators, while the other pleads the effects of negative modulators. The results may have implications on mechanisms of cognition, neuroprotection, and neurotoxicity [21]. Such receptors may modulate the release of acetylcholine and dopamine, neurotransmitter systems involved in memory, motor control and behavior [39]. PS increases the fractional open time of NMDA-activated channels, by increasing the frequency and the duration of the channel opening based on the subunit ligand, and inhibits the NMDA-induced [3H] norepinephrine. The NR2A and NR2B subunits supported a potentiating effect, while NR2C and NR2D subunits sustain an inhibitor effect [40]. DHEAS and PS, as well as pregnenolone, cooperate with σ1 receptors in the brain [40]. DHEAS and PS perform an agonist action, while progesterone acts as an antagonist.
On the other hand, DHEAS potentiate the NMDA-evoked excitability of hippocampus neurons, an effect that could be blocked by the σ1 antagonist haloperidol and NE-100, as well as by progesterone [40].
Therefore, whereas pregnenolone sulphate exercises excitotoxic effects on cortical and retinal cells, DHEA and DHEA-S have a neuroprotective effect against glutamate toxicity in vitro. The brain NSs vary in concentrations through time with different physiological mechanisms, depending on aging, stress, menstrual cycle, pregnancy, menopause and neurologic and psychiatric disorders [41]. Mameli et al. showed that NSs are implicated in correct brain development: some NSs may act as retrograde messengers, encouraging plasticity in immature synapses during development [42]. In fact, the administration of some positive modulators of GABAergic function such as diazepam, a benzodiazepine agonist, cause several variations in GABA-mediated functions in adulthood, with impaired locomotion and exploration as consequences [43]. Moreover, it has been shown that the enzymes necessary for neurosteroidogenesis are expressed in the immature brain and that the treatment with NSs of neuronal cells in vitro induces trophic effects, especially in the case of progesterone. The result was the boost of dendritic outgrowth in Purkinje cells. At the same time, the authors showed that ALLO helps the formation of neuronal circuitry that supported the persistence of neurons’ development. On the other hand, ALLO administered in rat pups caused an impairment of the diffusion of interneurons in the adult prefrontal cortex due to a physiological age fluctuation of ALLO in the rat brain [42]. This latter finding showed a first prenatal peak of cortical levels of ALLO and a second peak in the second week of life [44]. The administration of 10 mg/kg of ALLO on the fifth postnatal day caused an impairment of the localization and function of prefrontal and dorsal thalamic GABAergic neurons in the adult rat brain [44][45]. Moreover, perinatal NSs administration might modify the normal development of the hippocampus and the striatal and cortical dopaminergic activity [46][47].
Other research in rats by Darbra et al. showed that the administration of ALLO, during neonatal age, manipulates the behavioral affects in adolescents and adults [48].
In particular, the administration of finasteride to inhibit neonatal ALLO diminished the novelty exploratory behavior, decreased unspecific body weight and increased anxiety-related behavior during adolescence. Moreover, the neonatal administration of ALLO progressively declines the prepulse inhibition (PPI) of the acoustic startle response in adulthood, indicating an impairment of the sensorimotor gating [48][49]. The deficiency of sensorimotor gating is characteristic of various neuropsychiatric disorders, such as schizophrenia [50]. Furthermore, the effects of NSs on the dopaminergic system have also been investigated. Li et al. showed that finasteride, administrated during adolescence, inhibited the dopaminergic system in late adolescent male rats [51]. The impairment of the dopaminergic system caused the inhibition of exploratory and motor behaviors, and a drop in dopamine metabolites in the frontal cortex, hippocampus, caudate putamen, and nucleus accumbens of late adolescent male rats. The inhibition of the 5α reductase II of these areas caused a block of dihydrotestosterone production and, consequently, androgen production, with a lack of stimulation of the dopaminergic system.
Moreover, a down-regulation of tyrosine hydroxylase mRNA and protein expressions in the substantia nigra and ventral tegmental area has been shown. No delayed dopaminergic effect has been demonstrated after the administration of finasteride during the first early post-natal period. This result suggested that the finasteride effect on the dopaminergic system is mediated by the inhibition of the activity of androgen. Consequently, androgen acts on central nervous system function [52][53], stimulating the activity of the dopaminergic system, and finasteride could be used as a therapeutic option for neuropsychiatric disorders such as schizophrenia or Tourette syndrome.
3. Gender Differences in NSs Action
Sex difference is one of the long-standing issues in neuroscience research concerning certain brain disorders.
Testosterone is either irreversibly converted to estradiol (E2) by the activity of aromatase or metabolized to DHT by the activity of 5α-reductase. The sexual NSs (SN) DHT and E2 concentrations in the hippocampus are significantly higher than in the serum of males and females, respectively [54]. E2 and DHT are synthesized de novo from cholesterol that is transported through the mitochondrial membranes by the steroidogenic acute regulatory protein (StAR), which is expressed in the hippocampus of male and female animals [55].
Ovariectomy and gonadectomization in males reduced the hippocampal dendritic spine density [56][57]. These mechanisms of action could be blocked by letrozole, an aromatase inhibitor, in females, as well as by finasteride, a 5α-reductase inhibitor, in males [58].
E2 enhances the cellular model for learning and memory in the hippocampus, the so-called long-term potentiation at the CA3-CA1 synapses, increases the number of NMDA receptor binding sites, without effect on AMPA receptor binding sites [55], and enhances the immunofluorescence of the NMDA receptor subunit NR1 in females. Therefore, the result is a block of the NR2B NMDA receptor subunit that abolishes E2-induced enhancement of LTP. Consequently, E2 induces the magnitude of LTP at the CA3-CA1 Schaffer collaterals in the hippocampus.
Fester et al. [58] showed that the receptors for Gonadotropin releasing hormone (GnRH) regulate E2 synthesis in the ovaries and, subsequently, SN synthesis in the hippocampus. Therefore, due to the estrous cycle, GnRH are released cyclically from the hypothalamus in females; thus, the brain SN levels are correlated to related to sex hormones and the reproductive state of the organism throughout life. On the other hand, after GnRH stimulation, similar effects in males were not shown [58] and it has been assumed that GnRH stimulates testosterone synthesis, which is converted to DHT in males. The double function of increasing spine density and enhancing LTP has been related to the memory-enhancing effects of sexual steroids. In ovariectomized animals, treatment with E2 and testosterone increased CA1 pyramidal spine synapse density [59]. The local release of E2 induces testosterone to the rescue of spine density [60]. On the other hand, in orchiectomized males, the only steroids that restore the orchiectomy-induced spine synapse loss are either testosterone or the non-aromatizable DHT. Therefore, as previously reported, it could be assumed that synaptic plasticity in the hippocampus is sex-dependent, with the E2 sex steroid in females and testosterone in males [61].
Moreover, Fester et al. [58] highlighted the role of SN as the principal player in SN, which induced hippocampal synaptic plasticity [61]. Additionally, these results provided evidence that the continuous synthesis of NSs is required for normal transmission in the hippocampus [55][58][61].
Treatment with E2 has led to a beneficial function of memory in women; meanwhile, androgens have shown positive results in working memory tests on male animals [61][62]. As researchers have seen, the mechanisms of action of NSs are different for gender. A study conducted in a healthy population showed a correlation between NSs such as DHEA, DHEA-S, and pregnenolone regarding cognitive function and psychological well-being differences between genders. In males, serum DHEA levels correlated positively with quality of life while DHEA-S and pregnenolone levels were correlated with cognitive function. On the other hand, a correlation between DHEA-S and working memory was found in females [62]. These results indicate that NSs had a relevant role in cognitive function and quality of life, with a difference between genders [63][64][65].
4. Neuroplasticity and the Role of Neurosteroids
Neuroplasticity is considered the capacity of neural networks to modify their structure and function in response to environmental and biological inputs. It is regulated by different mechanisms, but NSs seem to play an important role. NSs are found in the brain at high concentrations, and their presence after steroidogenesis suggests that there is a local synthesis [66][67]. However, to better understand the role of NSS in modulating brain function, it is noteworthy to talk about neuroplasticity in terms of neurogenesis, structural and functional plasticity [42][61]. In fact, the earliest work on neuroplasticity was conducted by Hebb, which assumed the involvement of structural and functional changes that seem to occur in excitatory (glutamate) and inhibitory (GABAergic) networks [68][69]. Structural plasticity refers to the dimension of the neural arbor, dendritic length and number or ramifications [69], while functional plasticity is considered as an increase or decrease of electrical activity in cerebral regions, called LTP and long-term depression (LTD), respectively [70]. LTP and LTD are sustained by NMDA and AMPA receptors and modulated by GABAergic neurotransmission [71]. Various studies have demonstrated that NSs have been characterized as neuroplasticity modulators, regulating neurogenesis and structural and functional plasticity [72]. Schverer et al. [72] described the effects that specific NSs had in neuroplasticity, regarding Pregnenolone (PREG), Dehydroepiandrosterone (DHEA), their sulphate derivatives, PREGS and DHEAS, progesterone (PROG), and ALLO [54]. PREG increases the functional plasticity through NMDA receptors and stimulates NMDA receptors in newborn neurons. DHEA increases functional plasticity in terms of synaptic efficacy developed LTP via NMDA receptor signaling, and increases short-term potentiation, neurogenesis and structural plasticity through an increase in spine density. ALLO potentiates both mature excitatory synapses in vitro, possibly via presynaptic GABA-receptors modulation, and neurogenesis through GABA receptor activation in neuroprogenitor cells [23][24]. It is known that steroids may act through a genomic action mediated by a specific steroid receptor, as well as through a nongenomic action mediated by certain receptors for neurotransmitters or neuromodulatory proteins [73][74][75].
Based on these assumptions, steroids are considered neuroactive steroids, they have pharmacological effects specifically on the receptor GABAA, NMDA [76] and on the sigma-1 receptor [74]. In fact, progesterone and some of its metabolites ALLO are potent positive modulators of the GABAergic function, while DHEA, PREG, and their sulfate esters are negative modulators of the GABAA receptor and positive modulators of the NMDA and sigma-1 receptor. There is growing evidence about the interactions between neuroactive steroids and the serotonergic system. DHEA and ALLO are believed to modulate the activity of the serotonergic neurons in the dorsal raphe nucleus, either through their direct action on these neurons or in combination with some serotonin receptor inhibitors [73][77]. Sexual steroids play an important role in the development, growth, maturation and differentiation of the Central (CNS) and Peripheral Nervous System (PNS) [75]. Moreover, estrogen and progesterone affect neuronal plasticity differently in males and females regarding changes in structure and function of neurons in different regions of the brain. SNs, namely 17β-estradiol (E2) and 5α-dehydrotestosterone (DHT), are synthesized in the hippocampus and provide some sex-specific circuit modifications, i.e., a different modification in the number of excitatory spine synapses [78]. In general, hippocampal neurons synthesize sex steroids de novo from cholesterol, since the brain is equipped with all the enzymes required for the synthesis of estradiol and testosterone, the end products of sex steroidogenesis. Locally, estradiol and testosterone maintain synaptic transmission and synaptic connectivity [79]. Remarkably, the NSs estradiol is effective in females, but not in males, and vice versa DHT is effective in males, but not in females [80]. In fact, the inhibition of estradiol synthesis in females and DHT in males causes synapse loss, LTP impairment, and synaptic protein downregulation [80].
References
- Fuxe, K.; Gustafsson, J.A.; Wetterberg, L. Steroid Hormone Regulation of the Brain; Section I “Steroid Hormones in the Brain: Several Mechanisms?”; Pergamon: Oxford, UK, 1981; pp. 3–14.
- Baulieu, E.E. Neurosteroids: A novel function of the brain. Psychoneuroendocrinology 1998, 23, 963–987.
- Rupprecht, R. Neuroactive steroids: Mechanisms of action and neuropsychopharmacological properties. Psychoneuroendocrinology 2003, 28, 139–168.
- Akk, G.; Covey, D.F.; Evers, A.S.; Steinbach, J.H.; Zorumski, C.F.; Mennerick, S. The influence of the membrane on neurosteroid actions at GABAA receptors. Psychoneuroendocrinology 2009, 34, S59–S66.
- Reddy, D.S.; Rogawski, M.A. Stress-induced deoxycorticosterone-derived neurosteroids modulate GABAA receptor function and seizure susceptibility. J. Neurosci. 2022, 22, 3795–3805.
- Do Rego, J.L.; Seong, J.Y.; Burel, D.; Leprince, J.; Luu-The, V.; Tsutsui, K.; Tonon, M.C.; Pelletier, G.; Vaudry, H. Neurosteroid biosynthesis: Enzymatic pathways and neuroendocrine regulation by neurotransmitters and neuropeptides. Front. Neuroendocrinol. 2009, 30, 259–301.
- Schumacher, M.; Robel, P.; Baulieu, E.E. Development and Regeneration of the Nervous System: A Role for Neurosteroids (Part 1 of 2). Dev. Neurosci. 1996, 18, 6–13.
- Melcangi, R.C.; Poletti, A.; Cavarretta, I.; Celotti, F.; Colciago, A.; Magnaghi, V.; Motta, M.; Negri-Cesi, P.; Martini, L. The 5α-reductase in the central nervous system: Expression and modes of control. J. Steroid Biochem. Mol. Biol. 1998, 65, 295–299.
- Petratos, S.; Hirst, J.J.; Mendis, S.; Anikijenko, P.; Walker, D.W. Localization of p450scc and 5α-reductase type-2 in the cerebellum of fetal and newborn sheep. Dev. Brain Res. 2000, 123, 81–86.
- Mellon, S.H.; Griffin, L.D.; Compagnone, N.A. Biosynthesis and action of neurosteroids. Brain Res. Rev. 2001, 37, 3–12.
- Reddy, D.S. Neurosteroids: Endogenous role in the human brain and therapeutic potentials. Prog. Brain Res. 2010, 186, 113–137.
- Patte-Mensah, C.; Kappes, V.; Freund-Mercier, M.J.; Tsutsui, K.; Mensah-Nyagan, A.G. Cellular distribution and bioactivity of the key steroidogenic enzyme, cytochrome P450side chain cleavage, in sensory neural pathways. J. Neurochem. 2003, 86, 1233–1246.
- Agís-Balboa, R.C.; Pinna, G.; Zhubi, A.; Maloku, E.; Veldic, M.; Costa, E.; Guidotti, A. Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proc. Natl. Acad. Sci. USA 2006, 103, 14602–14607.
- Costa, E.; Guidotti, A. Diazepam binding inhibitor (DBI): A peptide with multiple biological actions. Life Sci. 1991, 49, 325–344.
- Benarroch, E.E. Neurosteroids: Endogenous modulators of neuronal excitability and plasticity. Neurology 2007, 68, 945–947.
- Papadopoulos, V.; Baraldi, M.; Guilarte, T.R.; Knudsen, T.B.; Lacapère, J.J.; Lindemann, P.; Norenberg, M.; Nutt, D.; Weizman, A.; Zhang, M.-R.; et al. Translocator protein (18 kDa): New nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol. Sci. 2006, 27, 402–409.
- Guennoun, R.; Fiddes, R.J.; Gouezou, M.; Lombes, M.; Baulieu, E.E. A key enzyme in the biosynthesis of neurosteroids, 3β-hydroxysteroid dehydrogenase/Δ5-Δ4-isomerase (3β-HSD), is expressed in rat brain. Mol. Brain Res. 1995, 30, 287–300.
- Corpechot, C.; Young, J.; Calvel, M.; Wehrey, C.; Veltz, J.N.; Touyer, G.; Mouren, M.; Prasad, V.V.; Banner, C.; Sjövall, J.; et al. Neurosteroids: 3 alpha-hydroxy-5 alpha-pregnan-20-one and its precursors in the brain, plasma, and steroidogenic glands of male and female rats. Endocrinology 1993, 133, 1003–1009.
- Purdy, R.H.; Morrow, A.L.; Moore Jr, P.H.; Paul, S.M. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proc. Natl. Acad. Sci. USA 1991, 88, 4553–4557.
- Belelli, D.; Lambert, J.J. Neurosteroids: Endogenous regulators of the GABA(A) receptor. Nat. Rev. Neurosci. 2005, 6, 565–575.
- Guarneri, P.; Cascio, C.; Russo, D.; D’Agostino, S.; Drago, G.; Galizzi, G.; Leo, G.D.; Piccoli, F.; Guarneri, M.; Guarneri, R. Neurosteroids in the retina: Neurodegenerative and neuroprotective agents in retinal degeneration. Ann. N. Y. Acad. Sci. 2003, 1007, 117–128.
- Joëls, M. Steroid hormones and excitability in the mammalian brain. Front. Neuroendocrinol. 1997, 18, 2–48.
- Belelli, D.; Lambert, J.J.; Peters, J.A.; Gee, K.W.; Lan, N.C. Modulation of human recombinant GABAA receptors by pregnanediols. Neuropharmacology 1996, 35, 1223–1231.
- Majewska, M.D. Neurosteroids: Endogenous bimodal modulators of the GABAA receptor mechanism of action and physiological significance. Prog. Neurobiol. 1992, 38, 379–394.
- Sieghart, W. Structure, pharmacology, and function of GABAA receptor subtypes. Adv. Pharmacol. 2006, 54, 231–263.
- Puia, G.; Santi, M.; Vicini, S.; Pritchett, D.B.; Purdy, R.H.; Paul, S.M.; Seeburg, P.H.; Costa, E. Neurosteroids act on recombinant human GABAA receptors. Neuron 1990, 4, 759–765.
- Hosie, A.M.; Wilkins, M.E.; da Silva, H.M.; Smart, T.G. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature 2006, 444, 486–489.
- Mihalek, R.M.; Banerjee, P.K.; Korpi, E.R.; Quinlan, J.J.; Firestone, L.L.; Mi, Z.P.; Lagenaur, C.; Tretter, V.; Sieghart, W.; Anagnostaras, S.G.; et al. Attenuated sensitivity to neuroactive steroids in γ-aminobutyrate type A receptor delta subunit knockout mice. Proc. Natl. Acad. Sci. USA 1999, 96, 12905–12910.
- Spigelman, I.; Li, Z.; Banerjee, P.K.; Mihalek, R.M.; Homanics, G.E.; Olsen, R.W. Behavior and physiology of mice lacking the GABAA-receptor δ subunit. Epilepsia 2002, 43, 3–8.
- Stell, B.M.; Brickley, S.G.; Tang, C.Y.; Farrant, M.; Mody, I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by δ subunit-containing GABAA receptors. Proc. Natl. Acad. Sci. USA 2003, 100, 14439–14444.
- Wohlfarth, K.M.; Bianchi, M.T.; Macdonald, R.L. Enhanced neurosteroid potentiation of ternary gabaareceptors containing the δ subunit. J. Neurosci. 2002, 22, 1541–1549.
- Harrison, N.L.; Simmonds, M.A. Modulation of the GABA receptor complex by a steroid anaesthetic. Brain Res. 1984, 323, 287–292.
- Mienville, J.M.; Vicini, S. Pregnenolone sulfate antagonizes GABAA receptor-mediated currents via a reduction of channel opening frequency. Brain Res. 1989, 489, 190–194.
- Akk, G.; Bracamontes, J.; Steinbach, J.H. Pregnenolone sulfate block of GABAA receptors: Mechanism and involvement of a residue in the M2 region of the α subunit. J. Physiol. 2001, 532, 673–684.
- Majewska, M.D.; Schwartz, R.D. Pregnenolone-sulfate: An endogenous antagonist of the γ-aminobutyric acid receptor complex in brain? Brain Res. 1987, 404, 355–360.
- Park-Chung, M.; Malayev, A.; Purdy, R.H.; Gibbs, T.T.; Farb, D.H. Sulfated and unsulfated steroids modulate γ-aminobutyric acidA receptor function through distinct sites. Brain Res. 1999, 830, 72–87.
- Wu, H.; Reynolds, A.B.; Kanner, S.B.; Vines, R.R.; Parsons, J.T. Identification and characterization of a novel cytoskeleton-associated pp60src substrate. Mol. Cell. Biol. 1991, 11, 5113–5124.
- Wang, Y.; Zhang, Y.; Zhu, Z.; Zhu, S.; Li, Y.; Li, M.; Yu, B. Exploration of the correlation between the structure, hemolytic activity, and cytotoxicity of steroid saponins. Bioorg. Med. Chem. 2007, 15, 2528–2532.
- Pisu, M.G.; Serra, M. Neurosteroids and neuroactive drugs in mental disorders. Life Sci. 2004, 74, 3181–3197.
- Maurice, T.; Lockhart, B.P.; Privat, A. Amnesia induced in mice by centrally administered β-amyloid peptides involves cholinergic dysfunction. Brain Res. 1996, 706, 181–193.
- Mellon, S.H.; Griffin, L.D. Neurosteroids: Biochemistry and clinical significance. Trends Endocrinol. Metab. 2002, 13, 35–43.
- Mameli, M.; Carta, M.; Partridge, L.D.; Valenzuela, C.F. Neurosteroid-induced plasticity of immature synapses via retrograde modulation of presynaptic NMDA receptors. J. Neurosci. 2005, 25, 2285–2294.
- Kellogg, C.K. Sex differences in long-term consequences of prenatal diazepam exposure: Possible underlying mechanisms. Pharmacol. Biochem. Behav. 1999, 64, 673–680.
- Grobin, A.C.; Heenan, E.J.; Lieberman, J.A.; Morrow, A.L. Perinatal neurosteroid levels influence gabaergic interneuron localization in adult rat prefrontal cortex. J. Neurosci. 2003, 23, 1832–1839.
- Gizerian, S.S.; Morrow, A.L.; Lieberman, J.A.; Grobin, A.C. Neonatal neurosteroid administration alters parvalbumin expression and neuron number in medial dorsal thalamus of adult rats. Brain Res. 2004, 1012, 66–74.
- Muneoka, K.T.; Borella, A.; Whitaker-Azmitia, P.M. Transient expression of s-100beta immunostaining in developing thalamus and somatosensory cortex of rat. Dev. Brain Res. 2003, 142, 101–104.
- Mellon, S.H. Neurosteroid regulation of central nervous system development. Pharmacol. Ther. 2007, 116, 107–124.
- Darbra, S.; Pallarès, M. Alterations in neonatal neurosteroids affect exploration during adolescence and prepulse inhibition in adulthood. Psychoneuroendocrinology 2010, 35, 525–535.
- Kilts, C.D. The changing roles and targets for animals models of schizophrenia. Biol. Psychiatry 2001, 50, 845–855.
- Swerdlow, N.R.; Light, G.A.; Cadenhead, K.S.; Sprock, J.; Hsieh, M.H.; Braff, D.L. Startle gating deficits in a large cohort of patients with schizophrenia: Relationship to medications, symptoms, neurocognition, and level of function. Arch. Gen. Psychiatry 2006, 63, 1325–1335.
- Li, L.; Kang, Y.-X.; Ji, X.-M.; Li, Y.-K.; Li, S.-C.; Zhang, X.-J.; Cui, H.-X.; Shi, G.-M. Finasteride inhibited brain dopaminergic system and open-field behaviors in adolescent male rats. CNS Neurosci. Ther. 2018, 24, 115–125.
- Castelli, M.P.; Casti, A.; Casu, A.; Frau, R.; Bortolato, M.; Spiga, S.; Ennas, M.G. Regional distribution of 5α-reductase type 2 in the adult rat brain: An immunohistochemical analysis. Psychoneuroendocrinology 2013, 38, 281–293.
- Patchev, V.K.; Schroeder, J.; Goetz, F.; Rohde, W.; Patchev, A.V. Neurotropic action of androgens: Principles, mechanisms and novel targets. Exp. Gerontol. 2004, 39, 1651–1660.
- Hojo, Y.; Hattori, T.A.; Enami, T.; Furukawa, A.; Suzuki, K.; Ishii, H.T.; Mukai, H.; Morrison, J.; Janssen, W.; Kominami, S.; et al. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017α and P450 aromatase localized in neurons. Proc. Natl. Acad. Sci. USA 2004, 101, 865–870.
- Woolley, C.S.; Gould, E.; McEwen, B.S. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 1990, 531, 225–231.
- Gould, E.; Woolley, C.S.; Frankfurt, M.; McEwen, B.S. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J. Neurosci. 1990, 10, 1286–1291.
- Leranth, C.; Hajszan, T.; MacLusky, N.J. Androgens increase spine synapse density in the CA1 hippocampal subfield of ovariectomized female rats. J. Neurosci. 2004, 24, 495–499.
- Fester, L.; Rune, G.M. Sexual neurosteroids and synaptic plasticity in the hippocampus. Brain research 2015, 1621, 162–169.
- Leranth, C.; Prange-Kiel, J.; Frick, K.M.; Horvath, T.L. Low CA1 spine synapse density is further reduced by castration in male nonhuman primates. Cereb. Cortex 2004, 14, 503–510.
- Fester, L.; Zhou, L.; Butow, A.; Huber, C.; von Lossow, R.; Prange-Kiel, J.; Jarry, H.; Rune, G. Cholesterol-promoted synaptogenesis requires the conversion of cholesterol to estradiol in the hippocampus. Hippocampus 2009, 19, 692–705.
- Brandt, N.; Fester, L.; Rune, G.M. Neural sex steroids and hippocampal synaptic plasticity. Vitam. Horm. 2020, 114, 125–143.
- Chen, C.Y.; Wu, C.C.; Huang, Y.C.; Hung, C.F.; Wang, L.J. Gender differences in the relationships among neurosteroid serum levels, cognitive function, and quality of life. Neuropsychiatr. Dis. Treat. 2018, 14, 2389–2399.
- Barron, A.M.; Pike, C.J. Sex hormones, aging, and Alzheimer’s disease. Front. Biosci. 2012, 4, 976.
- Pike, C.J. Sex and the development of Alzheimer’s disease. J. Neurosci. Res. 2017, 95, 671–680.
- Dorostkar, M.M.; Zou, C.; Blazquez-Llorca, L.; Herms, J. Analyzing dendritic spine pathology in Alzheimer’s disease: Problems and opportunities. Acta Neuropathol. 2015, 130, 1–19.
- Compagnone, N.A.; Mellon, S.H. Neurosteroids: Biosynthesis and function of these novel neuromodulators. Front. Neuroendocrinol. 2000, 21, 1–56.
- Torres, J.M.; Ruiz, E.; Ortega, E. Effects of CRH and ACTH administration on plasma and brain neurosteroid levels. Neurochemical. Res. 2001, 26, 555–558.
- Sala, C.; Segal, M. Dendritic spines: The locus of structural and functional plasticity. Physiol. Rev. 2014, 94, 141–188.
- Linden, D.J.; Connor, J.A. Long-term synaptic depression. Annu. Rev. Neurosci. 1995, 18, 319–357.
- Wu, E.S.; Gibbs, T.T.; Farb, D.H. Pregnenolone sulfate: A positive allosteric modulator at the N-methyl-D-aspartate receptor. Mol. Pharmacol. 1991, 40, 33–36.
- Schverer, M.; Lanfumey, L.; Baulieu, E.E.; Froger, N.; Villey, I. Neurosteroids: Non-genomic pathways in neuroplasticity and involvement in neurological diseases. Pharmacol. Ther. 2018, 191, 190–206.
- Robichaud, M.; Debonnel, G. Modulation of the firing activity of female dorsal raphe nucleus serotonergic neurons by neuroactive steroids. J. Endocrinol. 2004, 182, 11–21.
- Paul, S.M.; Purdy, R.H. Neuroactive steroids. FASEB J. 1992, 6, 2311–2322.
- Griffin, L.D.; Mellon, S.H. Selective serotonin reuptake inhibitors directly alter activity of neurosteroidogenic enzymes. Proc. Natl. Acad. Sci. USA 1999, 96, 13512–13517.
- Woolley, C.S.; Gould, E.; Frankfurt, M.; McEwen, B.S. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J. Neurosci. 1990, 10, 4035–4039.
- Monnet, F.P.; Maurice, T. The sigma1 protein as a target for the non-genomic effects of neuro(active)steroids: Molecular, physiological, and behavioral aspects. J. Pharmacol. Sci. 2006, 100, 93–118.
- McEwen, B.S. Steroid hormone actions on the brain: When is the genome involved? Horm. Behav. 1994, 28, 396–405.
- Brunne, B.; Rune, G.M. Sex neurosteroidogenesis and hippocampal network maintenance. Curr. Opin. Endocr. Metab. Res. 2022, 23, 100316.
- Bernardi, F.; Genazzani, A.R. The brain: Target and source for sex steroid hormones. In Women’s Health and Menopause; Springer: Dordrecht, The Netherlands, 1999; pp. 137–143.
- Brökling, J.; Brunne, B.; Rune, G. Sex-dependent responsiveness of hippocampal neurons to sex neurosteroids: A role of Arc/Arg3.1. J. Neuroendocrinol. 2022, 34, e13090.
More
Information
Subjects:
Neurosciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
615
Revisions:
2 times
(View History)
Update Date:
30 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




