1. Carbon-Based Nanomaterials
Carbon nanotubes (CNTs) are a special group of nanomaterials since they present a unique geometry, as well as novel electrical and mechanical properties, which make CNTs a superconducting substrate, valuable for sensor development
[1]. CNTs are hollow cylinders formed by rolling carbon atoms linked in hexagonal shapes with diameters lower than 100 nm. CNTs, namely single-walled carbon nanotubes (SWCNTs) and multi-walled carbon nanotubes (MWCNTs), have been used to implement electrochemical procedures for the evaluation of different antidepressants, in various samples
[2][3][4][5][6][7][8].
The small nanosize and the unique optical, thermal, electrical, catalytic, magnetic, and mechanical properties of nanomaterials make them potentially useful as sensor modifiers. Nevertheless, it is difficult to control the nanomaterial based-film thickness and porosity, and its assemblage at the electrode surface needs careful supervision to achieve the desired electrode reproducibility. Amitriptyline (AMT) is a widely used tricyclic antidepressant, being the second antidepressant of its group to be approved for MDD treatment in 1961
[5][6]. AMT’s lower electroactivity at conventional electrodes paved the way for the development of a CNT-modified electrode to evaluate this antidepressant in pharmaceutical formulations.
Using a rational mixture of CNTs and carbon paste, the electrochemical behavior of AMT presented a well-defined irreversible oxidation peak at +1.35 V vs. Ag/AgCl. Duarte et al. found that the use of sulfuric acid as an electrolyte improved AMT oxidation, possibly because of the oxidation of the alkylamine nitrogen atoms with one electron transfer and the formation of radical cations. Voltammetric techniques such as DPV, SWV, and CV were used for AMT determination achieving a LOD of 1.55 µmolL
−1. This nano sensor was successfully applied to six different commercial pharmaceutical formulations (tablets) of this medication
[6].
Glassy carbon electrodes (GCE) are widely used as a conductive substrate in electrochemistry since they are inexpensive, present a wide potential range, and are chemically stable. In carbon-based surfaces, such as GCE, graphite and carbon paste, it is possible to assemble different modifiers in a simple way.
Using a functionalized MWCNTs modified GCE (MWCNTs/GCE), Jat and collaborators studied the electrochemical behavior of the tricyclic antidepressant clomipramine (CMP). Similarly, to AMT, CMP also presents low redox activity. Applying this CME, it was found that CMP generated an irreversible oxidation peak at +1.120 V vs. Ag/AgCl in pH 5.5. This CME exhibited a considerable enhancement in voltammetry response, presenting a LOD of 13.2 ngmL
−1 (41.9 nmolL
−1). Employing voltammetric techniques, namely differential pulse anodic adsorptive stripping voltammetry (DP-AAdSV), this proposed electrochemical procedure was successfully used to quantify CMP in spiked human serum and urine samples
[7]. When compared to the CNTs used as modifier, the use of MWCNTs as electrode surface modifier boosted the sensor’s sensitivity by lowering the LOD by 37-fold
[6][8].
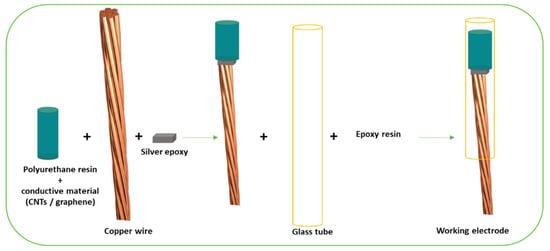
Baccarin and colleagues developed a CME (
Figure 1) for escitalopram (EST) determination, a widely used SSRI, approved for MDD and other disorders like anxiety and obsessive compulsive disorder (OCD)
[9][10]. Based on CV responses, the EST scanning towards positive potential revealed an irreversible oxidation process at +0.80 V (vs. SCE) when pH = 8.0. The reverse potential scans showed no indication of any reduction processes, suggesting the irreversible behavior of EST. The observed cathodic reaction of EST was attributed to the transference of two electrons in the terminal tertiary amine group. Under the optimal experimental conditions, this CME presented a linear dynamic ranges between 1.5 to 12 µmolL
−1 and a LOD of 0.45 µmolL
−1 with applicability to determine EST in urine and cerebrospinal fluid samples
[11].
Figure 1. Generic scheme of composite electrode fabrication, adapted with permission from
[11].
An electrochemical sensor for the detection of imipramine (IMP) was developed by Neto et al. The strategy followed by Neto and his team consisted of the use of a composite composed by ferrocene carboxylic acid (FCA), β-cyclodextrin (CD), and oxidized MWCNTs as modifiers on a GCE surface. The reason to use such composites was to combine the unique electronic properties of the MWCNTs, the electrochemical properties of FCA, and the ability of CD to form a complex with FCA at the electrode’s surface. Using this FCA-CD/MWCNTs-modified electrode, it was possible to analyze IMP at a very low potential (+0.02 V at pH 7.0). CV and DPV techniques were implemented, and linear ranges of 10–350 µmolL
−1 and 0.1–10 µmolL
−1 were obtained, respectively, enabling the detection of the antidepressant in pharmaceutical tablets and urine samples
[12].
Sertraline (SRT) is one of the most widely prescribed SSRIs, marketed initially for the treatment of MDD, but is currently approved for the management of panic disorder, OCD, and post-traumatic stress disorder (PTSD)
[13]. Atty et al. reported on the modification of a carbon paste rod with MWCNTs and cesium in a sodium dodecyl sulfate (SDS) medium (MWCNTs/CsCPE/SDS), capable of simultaneously detecting SRT and paracetamol in biological samples. Using CV techniques, the researchers studied the electrochemical behavior of SRT and paracetamol. An irreversible oxidation peak at +0.418 V vs. Ag/AgCl was registered for paracetamol, while an irreversible peak at +1.018 V vs. Ag/AgCl was recorded for SRT, resulting in a good separation between the two drugs and well-defined peaks, with a difference of peak potential of 0.6 V. SWV procedures were also applied to determine a LOD and limit of quantification (LOQ) for sertraline of 9.2 and 31 nmolL
−1, respectively. The developed CME was able to detect the antidepressant and paracetamol in spiked plasma
[14].
Trimipramine (TMP) is classified as a classical first-generation tricyclic antidepressant, with a good range of therapeutic effects in depressed patients with insomnia
[15]. To enhance the sensitivity and selectivity in compound detection, different types of extraction have been applied prior to the application of detection methods. Ensafi and collaborators investigated the combination of a microporous membrane-based liquid–liquid–liquid microextraction (MM-LLLME) technique and a MWCNT-CPE to improve the selectivity and sensitivity of TMP detection. In this sense, an MM-LLLME technique, based on a polyethylene membrane saturated with isoamyl benzoate, was used to extract, purify and preconcentrate TMP from aqueous samples, thereby enhancing the sensitivity and selectivity of the CME. Utilizing this system, TMP presented an oxidation peak at +1.0 V. DPV was selected as the detection technique, estimating a limit of detection of 2.0 nmolL−1. The applicability of the newly developed method was successfully verified in real plasma and urine samples, with recovery rates around 100%
[16].
Venlafaxine (VEN) is an SNRI, approved by FDA in 1993 and widely used nowadays with good efficacy
[17]. The electrocatalytic behavior of VEN was studied with CM/GCE. The chemical electrode modification was performed by using a gel containing MWCNTs and ionic liquid (IL), 1-butyl-3-methylimidazolium hexafluorophate (BMIMPF) in phosphate buffer at pH 6.8. A well-defined irreversible oxidation peak was observed at +0.780 V vs. Ag/AgCl. The electrode reaction was controlled by a diffusion process, and the redox reaction involved two electrons transferring and two protons’ participation. After the experiment conditions optimization, a linear range of 2.0 µmolL
−1–2.0 mmolL
−1 and a LOD of 1.69 µmolL
−1 was determined. In the end, the proposed methodology was applied to commercial VEN capsules, with no significant differences between the proposed method and the reported conventional techniques
[18]. The sensitivity of this CME was reduced when compared to the sensors produced in
[8][14][16], which had a LOD 1000 times greater. Graphene is a carbon-based nanomaterial composed of a single carbon layer. In graphene, every carbon atom is linked to three adjacent carbon atoms ready to form chemical bonds. Additionally, graphene has four valence electrons. The fourth electron is free to move, enabling the graphene to conduct electricity. Graphene also has excellent semiconductor, electroactive, and transparent characteristics, with unique chemical and electrical properties that make it ideal for the development of sensor technologies
[19]. In fact, graphene’s excellent ability to conduct electric charges establishes it as an excellent tool in the specific field of electrochemical biosensors
[20]. Combining graphene and other electroactive materials is one of the approaches involving carbon-based materials
[21][22][23].
Toledo et al. studied IMP oxidation mechanisms through electrochemical techniques, using a graphite-polyurethane composite electrode. CV allowed the establishment of the antidepressant’s voltammetric behavior, while also optimizing experimental conditions, such as pH. On the other hand, after this optimization, SWV was used to achieve a calibration curve with an IMP LOD of 4.60 nmolL
−1. In addition, the developed method was tested in commercial pharmaceutical tablets with great success
[21].
Paroxetine (PRX) is a SSRI, effective in both short-term and long-term management of depression and with efficacy in other co-morbid mental illnesses
[24]. A study by Oghli and colleagues explores the fabrication of an electrochemical sensor based on a modified pencil graphite electrode (PGE). Both graphene oxide and phosphotungstic acid (GO/PWA) were used as modifiers to enhance sensitivity and catalysis of the PRX oxidation. The presence of these compounds was successfully verified through X-ray diffraction (XRD), and the modified electrode was studied by scanning electron microscopy (SEM), confirming the incorporation of GO/PWA into the PGE. Cyclic voltammograms were performed for the modified and unmodified electrodes. The CV showed an irreversible oxidation process for PRX at +1.0 V on the modified PGE.
This CME sensor exhibited a linear current response from 8.0 nmolL
−1 to 1.0 µmolL
−1 and a low LOD 0.9 nmolL
−1. In addition, the influence of possible interferents was also examined, revealing the occurrence of PRX determination in the presence of probable diverse species and excipients in the pharmaceutical media. Applicability of the sensor was successfully proven by measurements in pharmaceutical tablets, urine and blood serum samples, with recovery percentages around 100% and an accurate determination of low concentrations of PRX
[22].
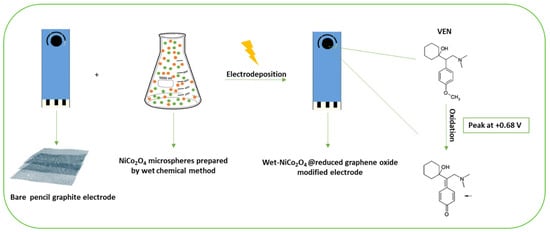
On the other hand, a study performed by Ali et al. described an additional electrochemical sensor for VEN based on the electrodeposition of nickel-cobalt oxide (NiCo
2O
4) microspheres (MSs), attached to a graphene oxide electrode (
Figure 2). Two different deposition methods were carried out for the binary metallic oxide microstructures supported on a reduced graphene oxide (rGO) modified graphite electrode: Wet chemical and in situ-electrical deposited methods. The deposition methods of NiCo
2O
4 MSs were found to affect the electrochemical behavior of the modified electrodes towards the oxidation of VEN. Using the wet-NiCo
2O
4@rGO modified electrode, an oxidation peak was observed at +0.68 V and the peak intensity was four-times higher than in a bare PGE. Therefore, it is possible to conclude that the wet-NiCo
2O
4@rGO modified electrode showed the highest sensitivity in terms of the VEN oxidation peak current intensity. These results confirm the significance of the modification step’s, suitability, and sensitivity of the fabricated sensor for trace analysis of VEN in different matrices. After the characterization of the wet-NiCo
2O
4@rGO modified electrode and optimization of experimental parameters (pH, supporting electrolyte, etc.), SWV methods were applied to determine analytical parameters and a LOD of 3.4 nmolL
−1 was obtained for the fabricated sensor. Furthermore, the developed method was applied to human serum samples and pharmaceutical tablets
[23].
Figure 2. Scheme of the fabrication of a reduced graphene oxide electrochemical sensor modified by NiCo
2O
4 microspheres for antidepressant venlafaxine, adapted with permission from
[23].
2. Metal-Based Nanoparticles
Metal and metal-organic based nanoparticles (MNPs) have many advantageous properties that make MNPs useful in the transducer component of (bio)sensors. Noble metals, such as gold, silver, platinum, rhodium, and palladium, or other metals, like nickel, copper, and bismuth NPs, have been the most popular and extensively studied. Although these noble metals are chemically inert in their macroscale form, they display unique physiochemical features at the nanoscale
[25][26][27][28][29].
Due to the MNPs small size and large surface-to-volume ratio, metal NPs possess unique electrical, optical, magnetic, and catalytic properties, which makes them a promising candidate for the development of electrochemical sensors. Moreover, some metal NPs, namely gold NPs, also present biological compatibility, high binding affinity, and enhanced target selectivity
[30]. Nonetheless, some of these MNPs are very expensive and toxic.
Titanium dioxide (TiO
2) NPs are being used as electrode surface modifiers because of their high surface area, versatility, optical transparency, good biocompatibility, and relatively good conductivity. Kalanur et al. reported on a voltammetric sensor based on titanium dioxide TiO
2-NPs, incorporated in a CPE matrix for the determination of an antidepressant used in the treatment of functional intestinal disorder: Buzepide methiodide (BZP). BZP is an organic iodide salt of buzepide and a cholinergic antagonist. A variety of electrochemical studies were carried out on BZP. Indeed, the electrochemical behavior of BZP at TiO
2NPs-CPE indicated a quasireversible peak with an oxidation peak potential of +0.649 V and a corresponding reduction peak potential at +0.459 V. One irreversible oxidation peak of +0.945 V was also observed. TiO
2NPs, when compared to an unmodified CPE, significantly increased the oxidation peak current of BZP, enhancing the electrochemical behavior of this analyte. After all optimized conditions were met, a linear peak current, among other characteristics, was achieved in the 50 nmolL−1−50 µmolL
−1 range, detected through DPV. In addition, the most significant features of the sensor were its ability to detect BZP in the presence of a variety of interferents (ascorbic acid, glucose, sucrose, dextrose, acacia powder, starch, and talc) and its successful application on urine and human blood serum samples, with an average recovery percentages ranging from 95.8–98.8% and 89.6–97.2%, respectively
[31].
A study by Tajik et al. described a nanosensor for SRT determination based on a screen-printed electrode (SPE) modified by ZnFe
2O
4NPs, since these have lower toxicity and higher reference daily intake values than other NPs-based materials
[32]. After their synthesis, a ZnFe
2O
4NPs solution was added to the working electrode of the SPEs. SRT electrochemical behavior on the electrodes was then evaluated, and pH value of the sertraline concentration was optimized to 7.0. The CV scan indicated that at ZnFe
2O
4-SPE, SRT presented an oxidative peak at +840 mV
[33], which is 178 mV less positive when compared to the CNT/CsMCPE/SDS sensor
[14]. Chronoamperometric measurements of the drug at the ZnFe
2O/SPE revealed a linear concentration range between 0.07–300 µmolL
−1 and a LOD of 0.02 μM. Furthermore, application of the sensor to pharmaceuticals and human urine samples revealed acceptable recovery rates comparable to spectrophotometric methods
[33]. When compared to the CNT-sensor
[6] or MWCNTs-sensor
[8][14] the ZnFe
2O/SPE presented increased LOD (1000 fold higher).
Sultan and colleagues reported a novel electrochemical sensor, able to determine the antidepressant VEN, modified by a stable bimetallic catalyst: palladium cobalt/aluminum oxide (Co-Pd@Al
2O
3). The modified electrode was characterized electrochemically by CV. Square wave anodic stripping voltammetric techniques (SWAV) were applied, and an electro-oxidation response of the drug was obtained at +0.65V vs. Ag/AgCl. Using the same technique, a linear range of 1.95 nmolL
−1–0.5 µmolL
−1 and a LOD of 1.86 pmolL
−1 was obtained. Additionally, the sensor was successfully applied to aqueous and serum samples, with recovery rates around 100%
[34]. The utilization of a bimetallic catalyst composed of palladium cobalt/aluminum oxide increased the sensitivity of the sensor by lowering the LOD to 1.8 pmolL
−1 when compared with the carbon-based nanomaterials where LOD are at nmolL
−1 levels.
In order to enhance electrochemical responses even further, recent approaches involve combining both CNTs and metallic nanoparticle modification techniques
[35][36]. Using MWCNTs and mercury-modified NPs (HgNPs), Madrakian et al. developed a modified glassy carbon electrode (HgNPs/MWCNTs/GCE) capable of detecting fluvoxamine (FLV) in body fluids.
The doubly modified electrodes revealed, by CV techniques, intensity values of cathodic peak currents for FLV 2 to 3-fold higher than the GCE modified by either only MWCNTs or mercury nanoparticles. In fact, with a bare GCE, FLV did not present any electrochemical behavior. However, at HgNPs/GCE, MWCNTs/GCE, and HgNPs/MWCNTs/GCE, FLV presented electrocatalytic activity, via a reduction process. At the HgNPs/GCE, MWCNTs/GCE, and HgNPs/MWCNT/GCE reduction peaks appeared at approximately −0.87, −0.82, and −0.76 V vs. Ag/AgCl, respectively, and at the oxidation step, no oxidation peak was observed. After optimization of the experimental conditions, DPV techniques were able to determine a LOD of 0.01 µmolL
−1. The novel sensor was applied to human urine samples and pharmaceutical tablets, with recovery rates of 96%, high sensitivity, and reproducibility
[35].
Shoja et al. reported the development of a modified GCE with gold NPs (AuNPs), enriched with MWCNTs and an electropolymerized nanostructured levodopa film (NiLD/AuNPs/MWCNTs/GCE) for the detection of SRT. After studying the electrochemical behavior of the CME electrode and SRT through CV and DPV techniques determining a linear range of 0.05–5.50 mmolL
−1 for the compound was used. Furthermore, the sensor was successfully applied to human serum samples
[36].
3. Molecularly Imprinted Polymers
In the last years, the integration of molecularly imprinted polymers (MIPs) into different sensing devices to detect measurable signal after achieving selective molecular binding has been widely studied as an alternative to natural biological elements. MIPs are one of the most promising tools for the design and construction of synthetic biomimetic recognition systems, since they are analogs of natural antibody-antigen systems
[37].
This has allowed the development of several MIP based sensors for detection of a wide range of analytes with applications in different matrices. Among several transducers (optical, mass), the combination of MIPs with electrochemical transduction has attracted attention due to their simplicity, ease of preparation, versatility, and ease of miniaturization.
MIPs are typically easy to prepare, reusable, cheap, and can be prepared to selectively recognize the target molecule of interest. They are resistant to different changes in chemical and thermal conditions, which is an advantage when compared to natural receptors.
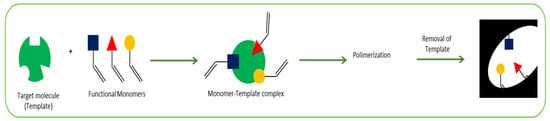
The most common procedure for MIP synthesis is chemical polymerization. Initially, the target molecule is mixed with functional monomers in the presence of a cross-linking agent and a porogenic solvent. Then, a polymerization reaction occurs, triggered by an initiator (thermal or photo initiated), and a polymer with the target molecule incorporated within it is obtained. The last step is the removal of the imprinted molecules from the polymer matrix, normally using solvent extraction, producing specific binding sites which are complementary in size, shape, and functionality to the target analyte (
Figure 3). This allows the obtention of a highly selective polymer. A control designated non-imprinted polymer (NIP), under the same conditions but without the presence of the analyte, is normally prepared to validate the selectivity
[38][39].
Figure 3. Summary of the MIPs preparation procedure.
The key factor in MIP preparation is the monomer choice since its selectivity is strongly dependent on the integration between the monomer and the template. The stronger the integration in the pre-polymerization solution, the better the recognition sites are obtained. Typically, monomer selection is achieved by trial and error. However, recently computer modeling studies have proven to be an excellent solution to aid in the selection of the monomer, reducing the amount of experiments and reagents
[40][41][42]. Two types of interaction between template and functional monomer can be used, namely covalent and non-covalent approaches. Covalent imprinting is based on the use of reversible reactions, allowing a strong interaction and the obtention of highly specific binding sites. Still, the number of reactions available is limited and the approach leads to slow binding and the removal of templates. Therefore, non-covalent imprinting, where integrations are based on ionic interactions, van der Waals forces, π-π interactions, and hydrogen bonding, is the most used approach. With this, the removal and rebinding of the analyte are simpler and faster
[38].
MIP preparation can be obtained with different procedures and imprinting techniques. Free-radical polymerization is the most common reaction utilized in conjugation with different techniques such as bulk, emulsion, suspension, seed, and precipitation polymerization. The choice of the imprinting technique is related with the type of particles to be obtained and the type of application to be used. In the preparation of electrochemical sensors, a special type of preparation, electropolmerizing has been widely used and proved to be an excellent choice for this kind of sensor. It is based on the use of a monomer that can be polymerized by the passage of current. It is a very simple and fast method. The polymerization occurs in situ on the working electrode’s surface. It can reduce the number of reagents used, and polymers with different conductivities can be obtained. The film thickness can be easily controlled by changing the current, which traduces into good reproducibility. Another strategy to modify nanoparticles is to synthesize MIPs and combine them with NPs or CNTs.
Fluoxetine (FLU) is an SSRI that increases the concentration of 5-hydroxytryptamine(5-HT) in the brain areas without affecting other neurotransmitter receptors
[43]. Alizadeh and colleagues described a highly promising graphene electrochemical sensing platform, molecularly imprinted polymer-modified CPE (graphene MIP-modified CPE) for the determination of FLU. The synthesis of the MIP was conducted through the precipitation polymerization method. Moreover, methacrylic acid and vinyl benzene (VB) were used as functional monomers. Graphene augmenting characteristics were evaluated through the incorporation of the material in the nano-MIP-modified CPE by two different methods. While in the first method, graphene was mixed with graphite and nano-MIP (nano-MIP/G1-CP), the second involved the addition of the compound and nano-MIP to dimethyl formamide (nano-MIP/G2-CP). Nano-MIP/G2-CP showed the most potential in terms of optimal DPV results. Selectivity of the proposed sensor was evaluated through the comparison with other drugs (FLU, trifluoperazine, clozapine, oxazepam, and salbutamol), with DPV values significantly higher for FLU. The sensor was successfully used for the detection of FLU in both plasma and pharmaceutical detection assays, with recovery percentages ranging from 91.5 to 110%
[44].
A paper by Khosrokhavar et al. reported a screen-printed carbon electrode (SPCE) altered by MIP nanoparticles and embedded with graphene suspension. The researchers evaluated the electrochemical performance of 3 different types of electrodes: the bare SPCE, SPCEs embedded with graphene (Gr-SPCE), and SPCEs modified by both the MIP and graphene (MIP/Gr-SPCE). K
3[Fe(CN)
6] reduction to K
4[Fe (CN)
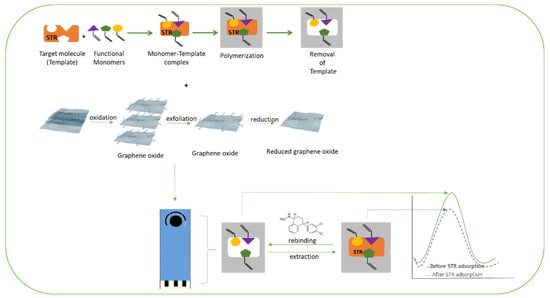
6] was considered as the redox couple used in the DPV and CV methods. MIP/Gr-SPCEs exhibited greater intensity in voltammetric signals when compared to the other electrodes. The MIP/Gr-SPCEs also showed a much higher response to SRT, which suggests that the specific imprinting sites for SRT in the MIP are a major factor in potentiating the sensor’s capabilities (
Figure 4). A linear response between 5.0 nmolL
−1–0.75 µmolL
−1 was determined, and the novel sensor was successfully applied to pharmaceuticals and human serum samples, revealing its ability to detect the drug in a variety of complex samples
[45].
Figure 4. Schematic representation of the modification of a screen-printed carbon electrode by MIP and graphene oxide nanosheets for determination of sertraline, adapted with permission from
[45].
A CPE, combined with MWCNT’s and MIP selective for TMP, was fabricated and reported by Akhoundian et al. The MIPs were synthesized via precipitation polymerization method. A mixture of MWCNT and graphite was added to the TMP-MIP, and the resulting compound was added to the electrodes. Conditions such as the pH of the TMP solution, extraction solution volume and extraction time were all optimized. The sensor revealed a linear response range of 0.1–25 nmolL−1, very high sensitivity (2131 µA µmolL−1), and good selectivity, when compared with other molecules (nortriptyline, IMP, and AMT), confirmed through application on blood and serum samples
[46].
Trazodone (TZD) is an antidepressant drug that belongs to the class of serotonin antagonists and reuptake inhibitors. Isabel et al. described a disposable voltammetric MIP based sensor for TZD determination, constructed on a commercial SPCE. The sensor was obtained by surface imprinting through electropolymerization using CV and 4-aminobenzoic acid (4-ABA) as a functional monomer. The electrochemical polymerization was studied and optimized. With this approach, the preparation is easy, fast, and green (it uses a small number of reagents). The detection was performed by measuring the DPV signal of the TZD oxidation. An imprinting factor of 71 was reported, and the sensor showed a good selectivity. A linear range of 5–80 µmolL
−1 and an LOD of 1.6 µmolL
−1 were reported. The application of the sensor was demonstrated using tap water and human serum samples
[38].
Citalopram (CTL) is largely used around de world. It is one of the most important antidepressants of the SSRIs class. Patricia et al. reported selective MIP based electrochemical sensor for CTL determination. In situ polymerization on the surface of the working electrode of an SPCE was acquired through the electropolymerization of 3-amino-4 hydroxybenzoic acid (AHBA) in the presence of CTL. Computational studies, namely molecular dynamics (MD) simulations, were executed to study the polymerization solution and optimize the polymerization conditions to obtain the most efficient MIP formulation. The obtained sensor was characterized by CV and EIS techniques. The sensor showed excellent selectivity in the presence of analog structures and an imprinting factor of 22. The oxidation of CTL was measured by SWV in the 0.1 to 1.25 µmolL
−1 range with a LOD of 0.162 µmolL
−1. The validation of the sensor was tested in spiked river water samples
[39].
Another sensitive and ultra-selective electrochemical sensor for CTL determination was reported by Aminikhah et al. A GCE was firstly modified with hollow nickel nanospheres (hNiNS)/activated MWCNTs@graphene oxide nanoribbons (AMWCNTs@GONRs) composite. This allowed to enhance the electrocatalytic response of CTL, improving the sensitivity. Then in situ electropolymerization was performed using pyrrole as the functional monomer. Several methods were used to characterize the constructed sensor, namely SEM, transmission electron microscopy (TEM), XRD, CV, and EIS. The DPV response to CTL showed two linear dynamic ranges from 0.5 to 10 µmolL
−1 and 10 to 190 µmolL
−1 with a LOD of 0.042 µmolL
−1 and proved to show excellent selectivity towards several compounds. It was successfully applied to measure CTL in urine, serum, and tablet samples
[47].