Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Audrius Menkis | -- | 4875 | 2023-05-23 13:20:28 | | | |
| 2 | Sirius Huang | Meta information modification | 4875 | 2023-05-24 04:12:32 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Matsiakh, I.; Menkis, A. Phytophthora Species on Woody Plants. Encyclopedia. Available online: https://encyclopedia.pub/entry/44715 (accessed on 07 February 2026).
Matsiakh I, Menkis A. Phytophthora Species on Woody Plants. Encyclopedia. Available at: https://encyclopedia.pub/entry/44715. Accessed February 07, 2026.
Matsiakh, Iryna, Audrius Menkis. "Phytophthora Species on Woody Plants" Encyclopedia, https://encyclopedia.pub/entry/44715 (accessed February 07, 2026).
Matsiakh, I., & Menkis, A. (2023, May 23). Phytophthora Species on Woody Plants. In Encyclopedia. https://encyclopedia.pub/entry/44715
Matsiakh, Iryna and Audrius Menkis. "Phytophthora Species on Woody Plants." Encyclopedia. Web. 23 May, 2023.
Copy Citation
The genus Phytophthora, with 326 species in 12 phylogenetic clades currently known, includes many economically important pathogens of woody plants. Different Phytophthora species often possess a hemibiotrophic or necrotrophic lifestyle, have either a broad or narrow host range, can cause a variety of disease symptoms (root rot, damping-off, bleeding stem cankers, or blight of foliage), and occur in different growing environments (nurseries, urban and agricultural areas, or forests).
forest
pathogens
oomycetes
tree diseases
woody plants
1. Introduction
The genus Phytophthora, which includes fungus-like microorganisms, also known as water molds, belongs to the family Peronosporaceae and phylum Oomycota in the Stramenopila kingdom [1][2][3][4]. Initially, the classification of Phytophthora species was based on morphological characters (e.g., sporangia, homothallism, and configuration of antheridia), showing the presence of six groups [5]. However, homology and homoplasty among different Phytophthora species showed a high plasticity of the morphological features and their often inseparability [6][7][8][9]. Since the 2000s, the number of described Phytophthora species increased by over 180 species, which was primarily due to the use of novel molecular techniques, reaching a total of 326 species distributed in 12 phylogenetic clades [10]. Consequently, the taxonomy of the genus Phytophthora shifted from the morphology-based methods towards the development of molecular markers for multilocus phylogenies [11][12][13][14][15]. For example, phylogenies of Phytophthora species were constructed using an internal transcribed spacer (ITS) region [11], four nuclear and mitochondrial genes [16], or seven nuclear markers [17]. A more recent study advanced the Phytophthora phylogeny by including more than 180 species and by creating ancestral phylogeny reconstructions on the sporangial papillation [18]. These studies allow researchers to better understand the evolution of the genus Phytophthora and to link molecular phylogenies and individual morphological and physiological traits.
2. Characteristics of Phytophthora Species Detected on Woody Plants in Sweden and Other Nordic Countries
Phytophthora × alni Clade 7a
Key woody hosts: Alnus alnobetula, A. glutinosa, A. cordata, A. incana, A. rubra, A. rubra subs. tenufolia, Castanea sativa.
Symptoms: canker, collar rot, and dieback of alders
Aggressiveness: Phytophthora disease of alder is now widespread in Europe in the riparian ecosystems where alder commonly grows. In Europe, surveys and modeling show that the risk of infection is higher in warmer, slow-moving waters, and in fine-textured soils, especially clay loams. Although the disease is usually observed along river systems, it has been found in sites far from riverbanks or other water courses, e.g., in orchard shelter belts and in new woodland plantings. This suggests that alder trees were already infected prior to planting [3]. P. × alni is an aggressive pathogen as, e.g., inoculations of mycelia culture on one-year-old seedlings of A. glutinosa and B. pendula showed the development of lesions in 89% and 67% of seedlings, respectively [19].
Occurrence: In Sweden, the first report of the P. alni complex was from nurseries and alder-planted areas in the southwest in the 1990s [20]. In 2006 and 2010, it was discovered on A. incana at the Klarälven river in the city of Karlstad. A comprehensive study on the Phytophthora alni complex in Sweden was carried out between 2013 and 2018 to investigate the pathways of introduction and the spread of the two subspecies, alni and uniformis [21][22]. Both species were associated with Phytophthora bleeding cankers on 93% of declining A. glutinosa and some A. incana trees along the riverbanks, and within sampling plots connected to river swamps or ponds (Figure 1a) [21][22]. It was considered that both P. alni subsp. alni and P. alni subsp. uniformis are invasive species that arrived in Sweden with plant material imported to forest nurseries, and that these species may further spread into natural ecosystems. P. alni subsp. uniformis was widespread throughout the country, whereas P. alni subsp. alni was only discovered in the southern and coastal part of Sweden [21][22]. P. alni subsp. alni is one of the most aggressive Phytophthora species [23], however, it is more sensitive to cold winters [24]. It was concluded that southern Sweden could be the northernmost distribution limit of P. × alni, however, there is a possible risk of its migration northwards due to climate change [22][25]. Due to the poor genetic potential of alder trees to resist P alni subsp. alni, alder decline is expected to increase in Sweden in the future [26]. In Finland, P. alni subsp. uniformis (identified as Phytophthora cf. uniformis) was found, for the first time, to cause dark stem lesions on A. glutinosa seedlings in 2015 [19]. In Denmark, P. alni subsp. uniformis (identified as Phytophthora uniformis) was isolated for the first time from symptomatic trees of A. glutinosa in 2016 [27].
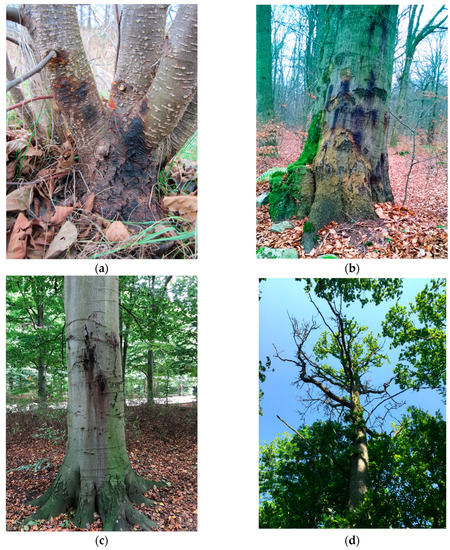
Figure 1. Phytophthora infected trees in Sweden: (a) Phytophthora alni lesions at the stem base of Alnus glutinosa (photo by Miguel Angel Redondo; retrieved from https://internt.slu.se/en/news-originals/2020/2/alders-lack-resistance-against-aggressive-type-of-pathogen/; accessed on 21 March 2023); (b) infected Fagus sylvatica with characteristic “bleeding” lesions on the stem in Kullaberg nature reserve (photo by Michelle Cleary); (c) infected Fagus sylvatica with “bleeding” lesions on the stem caused by P. plurivora and P. gonapodyides in Pildamms Park, Malmö city (photo by Mimmi Blomquist); (d) extensive crown dieback of Quesrcus robur in Visingrö oak forest caused by P. plurivora root rot infection (photo by Michelle Cleary).
Phytophthora cactorum Clade 1a
Key woody hosts: Abies sp., Acer sp., Aesculus hippocastanum, Fagus sylvatica, Juglans regia, Fraxinus excelsior, Malus domestica, Populus alba, Quercus sp., Rhododendron sp. (however, in total, at least 154 genera of vascular plants in 54 families are affected).
Symptoms: root, collar, and crown rot on many species; brown-reddish stem lesions; slow decline or rapid dieback, depending on age and location of infections; root rot of nursery plants. For example, on woody plants, such as Malus domestica, P. cactorum is causing crown and root rot; on Betula spp., it causes stem lesions; and on rhododendron, it causes root rot and dieback symptoms [28].
Aggressiveness: P. cactorum is unequivocally a serious pathogen of a wide range of plant species. Despite its broad geographic distribution and host range, P. cactorum has similar symptomatology with other species of Phytophthora. Therefore, it is difficult to ascribe specific damage to P. cactorum and evaluate the extent of its damage to forest trees. There are several reports of noticeable “outbreaks” on Fagus and Betula [29], however, it appears that there is a considerable host specificity among strains of this pathogen. Swedish isolates of P. cactorum together with P. cambivora and P. plurivora were used for inoculation of common conifer and broadleaf tree species in Sweden (Pinus sylvestris, Picea abies, Larix × eurolepis, Betula pendula, Quercus robur, Fagus sylvatica, Populus trichocarpa, and Tilia cordata) to determine their relative susceptibility to root pathogens [30]. All the tested Phytophthora species caused stem lesions of varying lengths on different host trees, except for species in the Pinaceae family, which had low susceptibility to the tested Phytophthora spp. Two-year-old bare-root seedlings of B. pendula, Q. robur, F. sylvatica, and P. sylvestris appeared to be susceptible to P. cactorum infection [30]. Inoculation trials in Finland using a Danish isolate, which was isolated from B. pendula in 2009, revealed that P. cactorum caused relatively small lesions on Rhododendron sp. and P. abies, moderate lesions on B. pendula, and no infections on Q. robur or P. sylvestris [31]. In an in vitro study using two-month-old B. pendula, roots inoculated with P. cactorum often showed dark discolorations, loss of fine roots, and decreased branching [32], even though discolorations are not a specific symptom of Phytophthora infections [33]. The symptoms of the aboveground parts included reduced height growth, lower chlorophyll fluorescence, significantly longer dark or brown discolorations in the stems, and a higher proportion of brownish and wilting leaves [32]. Furthermore, inoculation trials on three-month-old B. pendula and A. glutinosa seedlings showed that P. cactorum was able to kill 40% of the B. pendula seedlings, but caused only small lesions on 40% of A. glutinosa seedlings [34]. However, in the inoculation trials, P. cactorum was found to cause low-to-moderate symptoms on Rhododendron sp. and P. abies, and no symptoms on P. sylvestris [31]. P. cactorum was also able to cause lesions on non-wounded B. pendula seedlings [35]. In stem inoculation trials, Orlikowski et al. [36] showed that A. glutinosa, B. pendula, and Prunus padus were highly susceptible to P. cactorum. Acer saccharinum, Corylus avellana, Q. robur, Rubus caesius, Sorbus aucuparia, and Tilia cordata were moderately susceptible, while Sambucus nigra and Sorbus aucuparia were not susceptible. Interestingly, P. cactorum can be detected in B. pendula seven years after outplanting, however, at this stage, the effect of stem lesions on seedling mortality or on the number of leader shoots is limited [37]. Bunyaviruses were shown to significantly reduce hyphal growth and the production of sporangia and their size, but not the pathogenicity of P. cactorum [38].
Occurrence: In Sweden, P. cactorum was first recognized as one of the root parasites causing damping-off disease in forest nurseries in 1961 [39]. Since the beginning of the 1990s, the oak population has been declining in Sweden [40], and in 2003, P. cactorum was found to be associated with oak decline in southern Sweden [41]. Later, P. cactorum was isolated from the diseased F. sylvatica in the city of Malmö and Stora Köpinge municipality in 2016 [42]. Recently, P. cactorum was detected on F. sylvatica and Q. robur in forest nurseries (close to the rivers Säveån, Kävlingeå, and Ätran) (composing 26.3% of all detected Phytophthora spp.), in urban F. sylvatica forests (near the river Lagan) (15%), and in natural forests affecting F. sylvatica (near the river Ronnebyån), A. alba, and P. abies (near the river Ätran) (30%) [43]. In Finland, P. cactorum was found for the first time in necrotic stem lesions of B. pendula seedlings in forest nurseries in 1991 [33] and in stem lesions of A. glutinosa seedlings in 1995 [34]. It was also detected on symptomatic Rhododendron sp. seedlings during surveys that were carried out between 2004 and 2010 [31]. Irrigation water used in Finnish forest nurseries was shown to be the possible source of P. cactorum inoculum [44]. The Finnish isolates of P. cactorum were shown to have a 17.5 °C optimal growth temperature [45], i.e., much lower than the 25−30 °C reported in other studies [46][47], and that these isolates survived −5 °C temperatures on agar medium for up to 14 days [45]. The observations above suggest that Nordic isolates of P. cactorum can be better adapted to local conditions and, in the future, may pose a threat to B. pendula seedlings in forest nurseries and reforestations.
Phytophthora cambivora Clade 7a
Key woody hosts: Abies alba, Acer platanoides, Aesculus hippocastanum, Alnus glutinosa, Castanea denatata, C. crenata, C. sativa, Fagus sylvatica, Quesrcus robur, Taxus brevifolia, Platanus orientalis, Juglans regia, Malus domestica, Rhododendron sp., Pieris sp., Prunus sp., Ulmus sp.
Symptoms: canker, collar and root rot, bleeding cankers
Aggressiveness: P. cambivora is an invasive pathogen that survives and spreads in different environments. Its ability to survive as a saprotroph in the soil and to produce oospores (resting structures) increases its invasiveness. It was described as a causal agent of ink disease on chestnut trees [48][49][50]. The infection causes root destruction, which leads to leaf chlorosis and wilting in the canopies. Depending on environmental conditions, the disease may lead to a quick or to a progressive dieback of infected trees [48][51]. Inoculation tests on Abies seedlings also showed the ability of P. cambivora to infect and cause characteristic canker symptoms [52].
Occurrence: In Sweden, the first detection of P. cambivora was in association with oak health deterioration [41]. It was found together with P. cactorum in soil samples in one of ten surveyed forest stands. Later, P. cambivora was detected in soil samples collected near F. sylvatica trees with bleeding stem cankers in Bokskogen near the city of Malmö (Figure 1b) [42]. Nowadays, P. cambivora is present in nurseries, urban areas, and natural forests, and is mainly associated with F. sylvatica decline and bleeding cankers on the stems [43]. Inoculation of the stems showed that the Swedish isolate of P. cambivora is highly pathogenic to F. sylvatica, B. pendula, Tilia cordata, Q. robur, and Populus trichocarpa. P. trichocorpa is a non-native tree species in Sweden but it is important for biomass production. Therefore, the establishment of new P. trichocarpa plantations for energy should take place using clones more tolerant to Phytophthora infections [30]. In Norway, P. cambivora was detected for the first time on a 15-year-old Abies procera in 2004 [52]. The symptoms included cankers on the stems up to 1.5 m above the ground and dieback of the basal branches. There were 25% of trees that were already dead or dying. Infections of P. cambivora on F. sylvatica were observed for the first time in 2011, resulting in bleeding cankers [53]. The infected trees were in the areas of Larvik and Ås, which represent the northern limit of F. sylvatica distribution, showing that a northern location is not a limiting factor for the spread and infection by P. cambivora. The infected trees had a circumference between 40 and 310 cm, and the majority of the cankers were at the height of 0.1−2 m above the ground. Additional symptoms included crown dieback, chlorotic foliage, epicormic shoots, and cracked bark. The infection frequency in some areas around Larvik was up to 4.9% in 2012, but it was up to 9.2% in Ås in 2014 [53]. In addition to P. cambivora, P. plurivora and P. gonapodyides were detected in the water near the diseased trees in Larvik, and both species proved to be pathogenic on F. sylvativa. Today, P. cambivora can be considered an established species in different environments. To limit its spread, monitoring should take place in nurseries and on seedlings used for outplanting [54].
Phytophthora cinnamomi Clade 7a
Key woody hosts: Abies sp., Castanea sativa, Quercus sp., Chamaecyparis lawsoniana (266 genera in 90 families; commonly hardwood trees, including more than 1000 species [55]).
Symptoms: root rot, heart rot, wilt; causes ink disease of chestnut in conjunction with Phytophthora cambivora.
Aggressiveness: It is currently the most important Phytophthora pathogen of forest trees, and it is also destructive to woody ornamentals, especially rhododendrons and other Ericaceae, and orchard crops, including avocado. It is now widespread, owing to the international trade of plants, and continues to be destructive in the forests of Australia, Mediterranean countries, Mexico, and the SE United States, and is of increasing concern in the forests and wildlands of western North America. With the changing climate, P. cinnamomi is expected to expand its range and cause more damage, particularly in Europe and North America.
Occurrence: In Sweden, P. cinnamomi was detected in the rhizosphere soil of Rhododendron luteum ‘Whitethroat’ and Stewartia pseudocamellia growing in the same nursery, thereby representing the first record of this pathogen in a commercial stock of ornamental plants in the country [56].
Phytophthora citrophthora Clade 2a
Key woody hosts: Citrus sp., Aesculus hippocastanum, Buxus sp., Castanea sativa, Chamaecyparis lawsoniana, Juglans regia, Rhododendron sp. (in total, 88 genera in 51 families).
Symptoms: root rot, stem necrosis, canker, fruit rot, twig blight, seedling blight
Aggressiveness: P. citrophthora causes brown rot disease of citrus and is an economically important pathogen of citrus crops. It can also cause a dieback of rhododendron and other ornamental plants.
Occurrence: In Sweden, P. citrophthora was found in a nursery of Rhododendron catawbiense in 2018 [43]. In Norway, it was detected on Chamaecyparis lawsoniana [57].
Phytophthora cryptogea Clade 8a
Key woody hosts: Abies concolor, A. fraseri, A. procera, Chamaecyparis sp., Cupressus sp., Juglans regia, Malus domestica, Pinus mugo, P. nigra, P. contorta, Pseudotsuga menziesii, Rhododendron catawbiense, R. maximum (in total, 141 genera in 49 families).
Symptoms: damping-off, foot rot, stem rot, leaf rot, wilt.
Aggressiveness: P. cryptogea is primarily a soil-borne plant pathogen in the temperate regions, but it also exists in nature (fresh water) as a saprotroph. It is most active at temperatures between 10 °C and 20 °C [55]. It is a serious plant pathogen in many countries, causing great damage to ornamentals produced in nurseries, greenhouses, and hydroponics. P. cryptogea is an aggressive soil-borne pathogen of fir species, which are produced as Christmas trees [58][59].
Occurrence: In Sweden, P. cryptogea was detected in soil samples associated with symptomatic F. sylvatica trees in a nursery (near the river Ätran) and in an urban forest (near the river Alsterån) [43]. It was also found in soil samples in Christmas tree plantations and, together with P. megasperma, demonstrated a high aggressiveness to P. abies and A. nordmanniana trees [60].
Phytophthora gonapodyides Clade 6b
Key woody hosts: Chamaecyparis lawsoniana, Corylus avellana, Fagus sylvatica, Juglans regia, Malus domestica, Quercus sp., Rhododendron sp.
Symptoms: stem bleeding cankers, root rot
Aggressiveness: P. gonapodyides is considered as a weak parasite with saprophytic abilities usually associated with aquatic environments, such as rivers, riparian areas, and wetlands [55]. However, some isolates of P. gonapodyides can be highly virulent [36][61]. Their aggressiveness appears to be stimulated by prolonged root flooding and cool soil conditions. P. gonapodyides can hinder seed germination and cause root rot and stem lesions in Q. robur and Q. ilex [62][63].
Occurrence: In Sweden, the first report of P. gonapodyides was in 2016, when the pathogen was isolated from characteristic bleeding cankers on F. sylvatica trees growing in Pildamms Park in the city of Malmö (Figure 1c) [64]. It was suggested that recent changes in local climatic conditions, such as high summer precipitation coupled with mild winter temperatures, could favor the multicyclic spread of P. gonapodyides via zoospores and/or that the increased average age of F. sylvatica stands contributed to their higher susceptibility [64]. P. gonapodyides was also reported in a nursery (Lagan area) in association with F. sylvatica seedlings [43]. In Denmark, P. gonapodyides was recovered from rainwater ponding in an old declining F. excelsior stand [36].
Phytophthora inundata Clade 6a
Key woody hosts: Aesculus hippocastanum, Olea sp., Salix sp., Vitis sp.
Symptoms: root and collar rot of trees or shrubs in wet or flooded areas
Aggressiveness: P. inundata is responsible for wilting and the root rot of olive trees [65]. It can also act as an opportunistic, albeit aggressive root pathogen. On A. nordmanniana, it caused poorly developed roots and brown to reddish discoloration under the bark at the stem base and downwards. The foliage exhibited drought symptoms, with leaves that were pale green, yellow, or brown [57].
Occurrence: There are no reports from Sweden. In Norway, P. inundata and P. megasperma were reported from Christmas tree plantations of A. nordmanniana and A. lasiocarpa in 2004, respectively [57]. Approx. 70% of A. nordmanniana and 25% of A. lasiocarpa were symptomatic. As the site was grassland for decades with no history of Christmas tree cultivation, it was suggested that the disease followed imported transplants [57].
Phytophthora megasperma Clade 6b
Key woody hosts: Aesculus hippocastanum, Castanea sativa, Juglans regia, Prunus domestica, Pseudotsuga menziesii, Sorbus aucuparia
Symptoms: root rot, crown rot, storage rot, seedling damping-off, fruit rot, foot rot, stem canker, tuber rot, collar rot, sudden wilt, apoplexy, stunting, chlorosis. The symptoms of P. megasperma on A. lasiocarpa were pale yellow foliage and girdling at the stem bases [57].
Aggressiveness: It is primarily a root-rotting organism, causing the most serious losses on fruit and broadleaf trees. It appears to be restricted to more temperate regions of the world, however, its oospores can survive for up to 5 years, either free in the soil or in host tissue [66]. Prolonged wet conditions and heavy clay soils and soil impaction layers, which allow the maintenance of high soil water content, are often needed for the development of disease epidemics by P. megasperma [67].
Occurrence: In Sweden, P. megasperma was isolated from roots of a symptomatic P. abies seedling [60]. In Norway, P. megasperma was reported in association with A. lasiocarpa [57]. Both species P. cryptogea and P. megasperma may become problematic for Christmas tree and bough production, especially in saturated soils, which favor disease development.
Phytophthora pini Clade 2c
Key woody hosts: Pinaceae
Symptoms: root rot, canker
Aggressiveness: There is only limited information about this species. It can cause root rot and rapid mortality of olive trees [68]. Inoculations of P. abies seedlings using both wound-mycelia and zoospore suspension showed that after seven days, P. pini caused 100% disease incidence and a high frequency of severe symptoms [45].
Occurrence: In Sweden, P. pini was reported in commercial nurseries (near rivers of Säveån and Mölndalsån) in association with R. catawbiense [43]. In Finland, it was also detected on Rhododendron sp. [31].
Phytophthora plurivora Clade 2c
Key woody hosts: Acer platanoides, Aesculus hippocastanum, Alnus glutinosa, Fraxinus excelsior, Quercus robur, Quercus petrea, Tilia cordata, Fagus sylvatica, Rhododendron sp.
Symptoms: stem cankers, collar and root rot, dieback. P. plurivora can cause wilting and discoloration of current year shoots (on P. abies seedlings), bark necroses, fine root losses, and dieback on at least 45 woody host species [31][45].
Aggressiveness: P. plurivora is a highly aggressive plant pathogen, which has a worldwide distribution and a high diversity of hosts. P. plurivora is a hemibiotrophic organism that possesses the ability to infect living tissues and to continue its life cycle on dead tissues. In inoculation trials, P. plurivora was able to cause relatively large lesions or, in many cases, stem girdling on Rhododendron sp., B. pendula, A. incana, A. glutinosa, and P. abies, showing a high virulence on several woody plants and especially on P. abies as compared to other Phytophthora spp. tested [31]. The disease incidence on P. abies was also shown to be dependent on a particular isolate and inoculation method, as there were 83.3−100% disease incidence using wound inoculation with living mycelia and 0−77.8% using zoospores [45]. In P. abies shoot tissues, P. plurivora can grow both inter- and intracellularly, which is largely in the vascular tissues [45]. Pinus sylvestris and Q. robur showed no symptoms after four weeks of P. plurivora inoculation [31][69]; however, in other studies, this pathogen caused extensive bark lesions on Q. robur after a longer time [70]. In stem inoculation trials, Orlikowski et al. [36] showed that A. saccharinum, A. glutinosa, B. pendula, C. avellana, P. padus, R. caesius, and S. nigra were highly susceptible to P. plurivora, while Q. robur, S. aucuparia, and T. cordata were moderately susceptible. In addition to stem inoculations, soil infestation trials may also be needed to examine the susceptibility of fine roots of different tree species to P. plurivora and other Phytophthora spp. [31].
Occurrence: In Sweden, the earliest report of P. plurivora was from alder trees in Asslebyn (Bengtsfors locality) in Sept 2012 [43], even though the species was likely found during surveys near the city of Nyköping [21]. In 2016, P. plurivora was detected in soil samples and bleeding stem lesions of F. sylvatica in the city of Malmö (Figure 1c) [42]. It is one of the most abundantly detected Phytophthora species in natural forests and urban areas with declining and symptomatic F. sylvatica and Q. robur trees (Figure 1d) [43]. P. plurivora was also found to be highly virulent on F. sylvatica and Q. robur seedlings, causing large lesions, thus, it should be considered as a high-risk species to Swedish forests with a potential to severely destabilize the broadleaf forest ecosystems [64]. P. plurivora was also reported from Denmark and Norway as a disease agent of several deciduous tree species [27][71][72]. In Norway, P. plurivora was reported on F. sylvatica in a park in Oslo and in Ålesund [53]. Interestingly, the infection process for some F. sylvatica trees was rather fast and took only two years before the tree was dead. In Finland, surveys in 2005 on symptomatic Rhododendron sp. resulted in the detection of P. plurivora (originally identified as P. inflata) [31][69].
Phytophthora pseudosyringae Clade 3a
Key woody hosts: Quercus spp., Fagus sylvatica, Alnus glutinosa, Carpinus betulus
Symptoms: root and collar rot, stem bleeding cankers
Aggressiveness: It is an aggressive pathogen on several broadleaf tree species.
Occurrence: In Sweden, P. pseudosyringae was reported causing basal cankers and dieback on horse chestnut in June 2014 in Sankt Jörgens Park in the city of Gothenburg [73].
Phytophthora quercina Clade 3b
Key woody hosts: Quercus spp.
Symptoms: rot of fine roots, overall oak decline
Aggressiveness: P. quercina is often associated with other Phytophthora spp. [40], shows adaptation to different site conditions and soil pH, and has a high host specificity i.e., a high aggressiveness to different oak species [46][63]. P. quercina is also well-adapted to temporary dry conditions, possibly due to its particularly thick oospore walls [46]. Oaks with P. quercina or other Phytophthora spp. in their rhizosphere have ca. 50% higher probability of exhibiting severe aboveground disease symptoms than oaks without Phytophthora spp. [74]. Jönsson et al. [75] showed that Swedish isolates of P. quercina had the capacity to induce fine-root dieback of Q. robur seedlings growing in acid, N-rich but otherwise nutrient-poor forest soils (dominant in Sweden), as well as in high pH, nutrient-rich soils under the mesic water regime. Their aggressiveness, together with a high infection rate (all the seedlings were infected) showed a potential capacity of P. quercina to infect plants in acid forest soils [75]. In addition, the stress-induced susceptibility of the seedlings and/or increased aggressiveness of the pathogen in the forest soil could be factors accounting for differences of root dieback between soil types [75].
Occurrence: The decline of European oaks mainly occurs in trees older than 100 years, and in this process, trees may survive for a long time. It is only under exceptional circumstances that oaks may die in large areas [63][76]. A similar decline of oaks (in particular, Q. robur) has occurred in Sweden during the recent decades [40]. The reason for this loss was unclear until the three different Phytophthora species were recovered from 11 out of 32 oak stands in the southernmost part of the country, with P. quercina being the most frequent species [41]. However, a weak association was found between the occurrence of P. quercina and the vitality of mature oak stands [77]. Thus, the decline of oaks in southern Sweden can probably be attributed to several different site-specific factors, such as infection by P. quercina or unusual weather events, which interact with a number of biotic and abiotic factors, leading to oak decline [78]. Later, Jönsson-Belyazio and Rosengren [79] summarized that P. quercina contributes to oak decline in southern Sweden. A conceptual model for the development of Phytophthora disease in Q. robur suggested that the link between the root damage caused by Phytophthora species and overall tree vitality is in the assimilation and allocation of carbon within the plants [80]. More recently, P. quercina was also found in both urban (Mölndalsån area) and natural forests (Säveån and Lyckebyån areas), but not in forest nurseries [43].
Phytophthora ramorum Clade 8c
Key woody hosts: Abies sp., Aesculus hippocastanum, Alnus sp., Betula pendula, Fagus sylvatica, Fraxinus excelsior, Larix kaempferi, Notholithocarpus densiflorus, Pseudotsuga menziesii, Quercus sp., Rhododendron ponticum, Rhododendron sp.
Symptoms: lethal stem cankers, shoot dieback, foliage blight
Aggressiveness: P. ramorum is one of the most aggressive Phytophthora species. It is considered a highly invasive species due to its ability to spread, persist, and reproduce in new environments. The pathogen can infect plants in nurseries situated in close proximity to streams, later causing significant outbreaks on outplanted ornamentals. Spread events appear to be associated with either the movement of infected plant parts, normally from large wild infestations, or the introduction of infected plants, normally from infested ornamental nursery stock [81]. Infected nursery plants, such as Rhododendron, Camellia, and Viburnum, often contribute to long distance dispersal of the pathogen. Inoculation trials on stems showed that in addition to Rhododendron sp., P. ramorum caused necrotic lesions on A. glutinosa, A. incana, and B. pendula, while P. sylvestris and P. abies showed no disease symptoms [31][69], even though the pathogen might be able to infect individual P. abies needles [82]. Inoculation trials suggest that the damage can be substantial, as used isolates of P. ramorum were able to cause stem lesions in over 80% of B. pendula and over 30% of A. glutinosa seedlings [31].
Occurrence: In Sweden, the first report of P. ramorum was in 2002, which was found on 11 plants of Rhododendron sp. [83]. In 2017, it was detected on two plants of Rhododendron yakushimanum in a nursery in the municipality of Skurup. In 2018, P. ramorum was detected on four Rhododendron plants in a private garden in the municipality of Klippan. In Finland, P. ramorum was detected for the first time in 2004 [69]. It was found on marketed plants of Rhododendron spp., which were imported from other EU member states. The same year, P. ramorum was also discovered on Rhododendron sp. in a Finnish nursery, and detection was also successful in the following years, i.e., 2004−2010, except 2007, showing the persistent establishment of this pathogen despite the annual sanitation measures [31]. In Norway, P. ramorum was reported for the first time in 2002 [84]. It was isolated from symptomatic R. catawbiense in a nursery in Bergen. In the following years, the number of locations with P. ramorum has gradually increased (in 2004, there were 29 new locations, and in 2005, there were 43 locations), showing a broader distribution and/or rapid spread within the country. Apart from rhododendron, P. ramorum was also detected on Pieris japonica and Viburnum fragrans, and the latter was heavily infected. P. ramorum was most likely imported to Norway with symptomless plants and/or with plants that had mild symptoms, which are difficult to detect using random controls [84].
Phytophthora rosacearum Clade 6a
Key woody hosts: Malus domestica, Prunus spp. (Rosaceae)
Symptoms: pathogenic
Aggressiveness: Not clear
Occurrence: In Sweden, P. rosacearum was reported in commercial nurseries (near Kävlingeå) in association with Prunus laurocerasus [43].
Phytophthora syringae Clade 8d
Key woody hosts: Aesculus hippocastanum, Fagus sylvatica, Camelia sp., Rhododendron sp., Prunus sp. (29 genera in 14 families, including Syringa vulgaris (Oleaceae) and Rosaceae).
Symptoms: twig blight, fruit rot, root and collar rot, stem canker, wilt, leaf spot, and shoot dieback of lilac
Aggressiveness: P. syringae is known to infect nursery plants, particularly apple and pear trees. It infects plants through wounded areas and is most pathogenic during cold and wet weather conditions.
References
- Beakes, G.; Honda, T.; Thines, M. Systematics of the Stramenipila: Labyrinthulomycota, Hyphochytridiomycota, and Oomycota. In Systematics and Evolution; McLaughlin, D., Spatafora, J., Eds.; Springer: New York, NY, USA, 2014; pp. 39–97.
- Dick, M. Straminipilous Fungi: Systematics of the Peronosporomycetes Including Accounts of the Marine Straminipilous Protists, the Plasmodiophorids and Similar Organisms; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001.
- Jung, T.; Pérez-Sierra, A.; Durán, A.; Horta Jung, M.; Balci, Y.; Scanu, B. Canker and decline diseases caused by soil- and airborne Phytophthora species in forests and woodlands. Persoonia 2018, 40, 182–220.
- Thines, M.; Choi, Y.J. Evolution, diversity and taxonomy of the Peronosporaceae, with focus on the genus Peronospora. Phytopathology 2016, 106, 6–18.
- Waterhouse, G.M. Key to the species of Phytophthora de Bary. Mycol. Pap. 1963, 92, 1–22.
- Jung, T.; Stukely, M.J.C.; Hardy, G.E.S.J.; White, D.; Paap, T.; Duncan, W.A.; Burgess, T.I. Multiple new Phytophthora species from ITS Clade 6 associated with natural ecosystems in Australia: Evolutionary and ecological implications. Persoonia 2011, 26, 13–39.
- Kroon, L.P.N.M.; Brouwer, H.; De Cock, A.W.A.M.; Govers, F. The genus Phytophthora anno 2012. Phytopathology 2012, 102, 348–364.
- Yang, X.; Hong, C.X. Phytophthora virginiana sp. nov., a high-temperature tolerant species from irrigation water in Virginia. Mycotaxon 2013, 126, 167–176.
- Hong, C.X.; Gallegly, M.E.; Richardson, P.A.; Kong, P.; Moorman, G.W.; Lea-Cox, J.D.; Ross, D.S. Phytophthora hydropathica, a new pathogen identifed from irrigation water, Rhododendron catawbiense and Kalmia latifolia. Plant Pathol. 2010, 59, 913–921.
- Scott, P.; Bader, M.K.-F.; Burgess, T.; Hardy, G.; Williams, N. Global biogeography and invasion risk of the plant pathogen genus Phytophthora. Environ. Sci. Policy 2019, 101, 175–182.
- Cooke, D.E.L.; Drenth, A.; Duncan, J.M.; Wagels, G.; Brasier, C.M. A molecular phylogeny of Phytophthora and related oomycetes. Fungal Genet. Biol. 2000, 30, 17–32.
- Lara, E.; Belbahri, L. SSU rRNA reveals major trends in oomycete evolution. Fungal Divers. 2011, 49, 93–100.
- Martin, F.N.; Blair, J.E.; Coffey, M.D. A combined mitochondrial and nuclear multilocus phylogeny of the genus Phytophthora. Fungal Genet. Biol. 2014, 66, 19–32.
- Robideau, G.P.; De Cock, A.W.A.M.; Coffey, M.D.; Voglmayr, H.; Brouwer, H.; Bala, K.; Chitty, D.W.; Désaulniers, N.; Eggertson, Q.A.; Gachon, C.M.M.; et al. DNA barcoding of oomycetes with cytochrome c oxidase subunit, I and internal transcribed spacer. Mol. Ecol. Resour. 2011, 11, 1002–1011.
- Tyler, B.M.; Tripathy, S.; Zhang, X.M.; Dehal, P.; Jiang, R.H.Y.; Aerts, A.; Arredondo, F.D.; Baxter, L.; Bensasson, D.; Beynon, J.L.; et al. Phytophthora genome sequences uncover evolutionary origins and mechanisms of pathogenesis. Science 2006, 313, 1261–1266.
- Kroon, L.P.N.M.; Bakker, F.T.; Van Den Bosch, G.B.M.; Bonants, P.J.M.; Flier, W.G. Phylogenetic analysis of Phytophthora species based on mitochondrial and nuclear DNA sequences. Fungal Genet. Biol. 2004, 41, 766–782.
- Blair, J.E.; Coffey, M.D.; Park, S.-Y.; Geiser, D.M.; Kang, S. A multilocus phylogeny for Phytophthora utilizing markers derived from complete genome sequences. Fungal Genet. Biol. 2008, 45, 266–277.
- Yang, X.; Tyler, B.M.; Hong, C. An expanded phylogeny for the genus Phytophthora. IMA Fungus 2017, 8, 355–384.
- Poimala, A.; Werres, S.; Pennanen, T.; Hantula, J. First report of alder Phytophthora closely related to P. uniformis on Alnus glutinosa seedling in Finland. Plant Dis. 2018, 102, 454.
- Olsson, C.H. Diagnosis of Root-Infecting Phytophthora spp. Ph.D. Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 1999.
- Redondo, M.A.; Boberg, J.; Olsson, C.H.; Oliva, J. Winter conditions correlate with Phytophthora alni subspecies distribution in southern Sweden. Phytopathology 2015, 105, 1191–1197.
- Redonto, M.A. Invasion Biology of Forest Phytophthora Species in Sweden: Pathways, Traits, Climate and Host Adaptation. Ph.D. Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2018.
- Érsek, T.; Nagy, Z.A. Species hybrids in the genus Phytophthora with emphasis on the alder pathogen Phytophthora alni: A review. Eur. J. Plant Pathol. 2008, 122, 31–39.
- Černý, K.; Filipová, N.; Strnadová, V. Influence of low temperature and frost duration on Phytophthora Alni subsp. alni. viability. For. Syst. 2012, 21, 337–342.
- Aguayo, J.; Elegbede, F.; Husson, C.; Saintonge, F.-X.; Marçais, B. Modeling climate impact on an emerging disease, the Phytophthora alni-induced alder decline. Glob. Change Biol. 2014, 20, 3209–3221.
- Redondo, M.A.; Stenlid, J.; Oliva, J. Genetic variation explains changes in susceptibility in a naïve host against an invasive forest pathogen: The case of alder and the Phytophthora alni complex. Phytopathology 2020, 110, 517–525.
- Redondo, M.A.; Thomsen, I.T.; Oliva, J. First report of Phytophthora uniformis and P. plurivora causing stem cankers on Alnus glutinosa in Denmark. Dis. Notes 2016, 101, 512.
- Hantula, J.; Lilja, A.; Nuorteva, H.; Parikka, P.; Werres, S. Pathogenicity, morphology and genetic variation of Phytophthara cactorum from strawberry, apple, rhododendron, and silver birch. Mycol. Res. 2000, 104, 1062–1068.
- Černý, K.; Strnadová, V.; Gregorova, B.; Holub, V.; Tomsovsky, M.; Mrazkova, M.; Gabrielova, S. Phytophthora cactorum causing bleeding canker of common beech, horse chestnut, and white poplar in the Czech Republic. Plant Pathol. 2009, 58, 394.
- Cleary, M.; Blomquist, R.R.; Vetukuri, H.; Böhlenius, J.; Witzell, J. Susceptibility of common tree species in Sweden to Phytophthora cactorum, P. cambivora and P. plurivora. For. Pathol. 2017, 47, e12329.
- Rytkönen, A.; Lilja, A.; Vercauteren, A.; Sirkia, S.; Parikka, P.; Soukainen, M.; Hantula, J. Identity and potential pathogenicity of Phytophthora species found on symptomatic Rhododendron plants in a Finnish nursery. Can. J. Plant Pathol. 2012, 34, 255–267.
- Hamberg, L.; Poimala, A.; Velmala, S.; Perttunen, J.; Muilu-Makela, R.; Sievanen, R. Root discoloration and shoot symptoms in silver birch after Phytophthora infection in vitro. Plant Biol. 2021, 23, 162–171.
- Lilja, A.; Rikala, R.; Hietala, A.; Heinonen, R. Stem lesions on Betula. pendula seedlings in Finnish forest nurseries and the pathogenicity of Phytophthora cactorum. Eur. J. For. Pathol. 1996, 26, 89–96.
- Hantula, J.; Lilja, A.; Parikka, P. Genetic variation and host specificity of Phytophthora cactorum isolated in Europe. Mycol. Res. 1997, 101, 565–572.
- Lilja, A.; Karjalainen, R.; Parikka, P.; Kammiovirta, K.; Nuorteva, H. Pathogenicity and genetic variation of Phytophthora cactorum from silver birch and strawberry. Eur. J. Plant Pathol. 1998, 104, 529–535.
- Orlikowski, L.B.; Ptaszek, M.; Rodziewicz, A.; Nechwatal, J.; Thinggaard, K.; Jung, T. Phytophthora root and collar rot of mature Fraxinus excelsior in forest stands in Poland and Denmark. For. Pathol. 2011, 41, 510–519.
- Lilja, A.; Rytkonen, A.; Parikka, P.; Kokkola, M.; Hantula, J. Alien species in Finnish nurseries, Phytophthora spp. Acta Silv. Lignaria Hung. 2007, 219, 227.
- Poimala, A.; Raco, M.; Haikonen, T.; Cerny, M.; Parikka, P.; Hantula, J.; Vainio, E.J. Bunyaviruses affect growth, sporulation, and elicitin production in Phytophthora cactorum. Viruses 2022, 14, 2596.
- Molin, N.; Persson, M.; Persson, S. Root Parasites on Forest Tree Seedlings: Some Exploratory Tests of the Resistance of Germinant Seedlings and the Virulence of Some Potential Parasites; Statens Skogsforskningsinstitut: Stockholm, Sweden, 1961; Volume 49, pp. 1–16.
- Sonesson, K.; Anderson, S. Forest Condition of Beech and Oak in Southern Sweden 1999; National Board of Forestry: Jönköping, Sweden, 2001.
- Jönsson, U.; Lundberg, L.; Sonesson, K.; Jung, T. First records of soilborne Phytophthora species in Swedish oak forests. For. Pathol. 2003, 33, 175–179.
- Blomquist, M. Invasive Phytophthora Species Affecting Broadleaved Tree Species in Urban and Landscape Settings in Southern Sweden. Master’s Thesis, Swedish University of Agricultural Sciences, Alnarp, Sweden, 2016.
- Redondo, M.A.; Boberg, J.; Stenlid, J.; Oliva, J. Functional traits associated with the establishment of introduced Phytophthora spp. in Swedish forests. J. Appl. Ecol. 2018, 55, 1538–1552.
- Rytkönen, A.; Lilja, A.; Petaisto, R.L.; Hantula, J. Irrigation water and Phytophthora cactorum in a forest nursery. Scand. J. Res. 2008, 23, 404–411.
- Rytkönen, A.; Lilja, A.; Werres, S.; Sirkia, S.; Hantula, J. Infectivity, survival and pathology of Finnish strains of Phytophthora plurivora and Ph. pini in Norway spruce. Scand. J. Res. 2013, 28, 307–318.
- Jung, T.; Cooke, D.E.L.; Blaschke, H.; Duncan, J.M.; Oßwald, W. Phytophthora quercina sp. nov., causing root rot of European oaks. Mycol. Res. 1999, 103, 785–798.
- Rea, A.J.; Burgess, T.I.; Hardy, G.E.S.J.; Stukely, M.J.C.; Jung, T. Two novel and potentially endemic species of Phytophthora associated with episodic dieback of Kwongan vegetation in the south-west of Western Australia. Plant Pathol. 2011, 60, 1055–1068.
- Vannini, A.; Vettraino, A.M. Ink disease in chestnuts: Impact on the European chestnut. For. Snow Landsc. Res. 2001, 76, 345–350.
- Puccinelli, M. Giornale di Agricoltura; Camera del Commercio e dell’Agricoltura: Lucca, Italy, 1859.
- Vettraino, A.M.; Morel, O.; Perlerou, C.; Robin, C.; Diamandis, S.; Vannini, A. Occurrence and distribution of Phytophthora species in European chestnut stands, and their association with ink disease and crown decline. Eur. J. Plant Pathol. 2005, 111, 169–180.
- Vettraino, A.M.; Barzanti, G.P.; Bianco, M.C.; Ragazzi, A.; Capretti, P.; Paoletti, E.; Luisi, N.; Anselmi, N.; Vannini, A. Occurrence of Phytophthora species in oak stands in Italy and their association with declining oak trees. For. Pathol. 2002, 32, 19–28.
- Talgø, V.; Herrero, M.; Toppe, B.; Klemsdal, S.; Stensvand, A. First report of root rot and stem canker caused by Phytophthora cambivora on Noble Fir (Abies procera) for bough production in Norway. Plant Dis. 2006, 90, 682.
- Telfer, K.H.; Brurberg, M.B.; Herrero, M.-L.; Stensvand, A.; Talgø, V. Phytophthora cambivora found on beech in Norway. For. Pathol. 2015, 45, 415–425.
- Jung, T.; Vettraino, A.M.; Cech, T.L.; Vannini, A. The impact of invasive Phytophthora species on European forests. In Phytophthora: A Global Perspective; Lamour, K., Ed.; CABI: Wallingford, UK, 2013; pp. 146–158.
- Erwin, D.C.; Ribeiro, O.K. Phytophthora Diseases Worldwide; APS Press: St. Paul, MN, USA, 1996; p. 562.
- Cleary, M.; Blomquist, M.; Marchand, M.; Witzell, J. Oomycetes in rhizosphere soil of ornamental plants from retail nurseries in Southern Sweden. For. Pathol. 2021, 51, e12673.
- Talgø, V.; Herrero, M.L.; Toppe, B.; Klemsdal, S.S.; Stensvand, A. Phytophthora root rot and stem canker found on Nordmann and Subalpine fir in Norwegian Christmas tree plantations. Plant Health Prog. 2007, 8, 29.
- Shafizadeh, S.; Kavanagh, J.A. Pathogenicity of Phytophthora species and Pythium undulatum isolated from Abies procera Christmas trees in Ireland. For. Pathol. 2005, 35, 444–450.
- Chastagner, G.A.; Benson, D.M. The Christmas tree: Traditions, production, and diseases. Plant Health Prog. 2000, 1, 15.
- Pettersson, M.; Frampton, J.; Rönnberg, J.; Bente Brurberg, M.; Talgø, V. Presence of Phytophthora species in Swedish Christmas tree plantations. Eur. J. Plant Pathol. 2019, 153, 1221–1236.
- Belisario, A.; Luongo, L.; Vitale, S.; Galli, M.; Haegi, A. Phytophthora gonapodyides causes decline and death of English (Persian) walnut (Juglans regia) in Italy. Plant Dis. 2016, 100, 2537.
- Corcobado, T.; Cubera, E.; Pérez-Sierra, A.; Jung, T.; Solla, A. First report of Phytophthora gonapodyides involved in the decline of Quercus ilex in xeric conditions in Spain. New Dis. Rep. 2010, 22, 33.
- Jung, T.; Blaschke, H.; Neumann, P. Isolation, identification and pathogenicity of Phytophthora species from declining oak stands. Eur. J. For. Pathol. 1996, 26, 253–272.
- Cleary, M.; Blomquist, M.; Ghasemkhani, M.; Witzell, J. First report of Phytophthora gonapodyides causing stem canker on European beech (Fagus sylvatica) in southern Sweden. Plant Dis. 2016, 100, 2174.
- Kurbetli, I.; Karaca, G.; Aydoğd, M.; Sülü, G. Phytophthora species causing root and collar rot of pomegranate in Turkey. Eur. J. Plant Pathol. 2020, 157, 485–496.
- Mircetich, S.M.; Matheron, M.E. Phytophthora root and crown rot of cherry trees. Phytopathology 1976, 66, 549–558.
- Robertson, G.I.; Dance, H.M. The association of Phytophthora megasperma with crown rot of apple trees. N. Z. J. Agric. Res. 1971, 14, 509–551.
- Linaldeddu, B.T.; Bregant, C.; Montecchio, L.; Favaron, F.; Sella, L. First report of Phytophthora acerina, P. pini, and P. plurivora causing root rot and sudden death of olive trees in Italy. Plant Dis. 2020, 104, 996.
- Lilja, A.; Rytkonen, A.; Kokkola, M.; Parikka, P.; Hantula, J. First report of Phytophthora ramorum and P. inflata in ornamental rhododendrons in Finland. Plant Dis. 2007, 91, 1055.
- Jung, T.; Blaschke, H. Phytophthora root rot in declining forest trees. Phyton Ann. Rei Bot. 1995, 36, 95–102.
- Thinggaard, K. Phytophthora–en ny og alvorlig trussel mod de danske skove. Skoven 2009, 11, 478–481. (In Danish)
- Talgø, V.; Herrero, M.; Toppe, B.; Brurberg, M.B.; Thurston, R.; Stensvand, A. Phytophthora plurivora, ny skadegjerar på tre i Norge. Bioforsk Fokus 2009, 5, 238–239. (In Norwegian)
- Redondo, M.A.; Boberg, J.; Stenlid, J.; Oliva, J. First report of Phytophthora pseudosyringae causing basal cankers on horse chestnut in Sweden. Plant Dis. 2016, 100, 1024–1025.
- Jung, T.; Blaschke, H.; Osswald, W. Involvement of soilborne Phytophthora species in Central European oak decline and the effect of site factors on the disease. Plant Pathol. 2000, 49, 706–718.
- Jönsson, U.; Jung, T.; Rosengren, U.; Nihlgård, B.; Sonesson, K. Pathogenicity of Swedish isolates of Phytophthora quercina to Quercus robur in two different soil types. New Phytol. 2003, 158, 355–364.
- Delatour, C. Les dépérissements de chênes en Europe. Rev. For. Fr. 1983, 35, 265–282.
- Jönsson, U. Phytophthora species and oak decline–can a weak competitor cause significant root damage in a nonsterilized acidic forest soil? New Phytol. 2004, 162, 211–222.
- Jönsson, U.; Jung, T.; Sonesson, K.; Rosengren, U. Relationships between health of Quercus robur, occurrence of Phytophthora species and site conditions in southern Sweden. Plant Pathol. 2005, 54, 502–511.
- Jönsson-Belyazio, U.; Rosengren, U. Can Phytophthora quercina have a negative impact on mature pedunculate oaks under field conditions? Ann. Sci. 2006, 63, 661–672.
- Jönsson, U. A conceptual model for the development of Phytophthora disease in Quercus robur. New Phytol. 2006, 171, 55–68.
- Croucher, P.J.P.; Mascheretti, S.; Garbelotto, M. Combining field epidemiological information and genetic data to comprehensively reconstruct the invasion history and the microevolution of the sudden oak death agent Phytophthora ramorum (Stramenopila: Oomycetes) in California. Biol. Invasions 2013, 15, 2281–2297.
- Denman, S.; Kirk, S.A.; Brasier, C.M.; Webber, J.F. In vitro leaf inoculation studies as an indication of tree foliage susceptibility to Phytophthora ramorum in the UK. Plant Pathol. 2005, 54, 512–521.
- EPPO Reporting Service (2003/020). First Report of Phytophthora ramorum in Sweden. Available online: https://gd.eppo.int/reporting/article-2006 (accessed on 24 April 2023).
- Herrero, M.L.; Toppe, B.; Klemsdal, S.S.; Stensvand, A. First report of Phytophthora ramorum in ornamental plants in Norway. Plant Dis. 2006, 90, 1458.
More
Information
Subjects:
Anatomy & Morphology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
773
Revisions:
2 times
(View History)
Update Date:
24 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




