Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Vadims Nefjodovs | -- | 3083 | 2023-05-22 14:35:52 | | | |
| 2 | Peter Tang | Meta information modification | 3083 | 2023-05-23 03:59:36 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Nefjodovs, V.; Andze, L.; Andzs, M.; Filipova, I.; Tupciauskas, R.; Vecbiskena, L.; Kapickis, M. Wood as Possible Renewable Material for Bone Implants. Encyclopedia. Available online: https://encyclopedia.pub/entry/44668 (accessed on 01 March 2026).
Nefjodovs V, Andze L, Andzs M, Filipova I, Tupciauskas R, Vecbiskena L, et al. Wood as Possible Renewable Material for Bone Implants. Encyclopedia. Available at: https://encyclopedia.pub/entry/44668. Accessed March 01, 2026.
Nefjodovs, Vadims, Laura Andze, Martins Andzs, Inese Filipova, Ramunas Tupciauskas, Linda Vecbiskena, Martins Kapickis. "Wood as Possible Renewable Material for Bone Implants" Encyclopedia, https://encyclopedia.pub/entry/44668 (accessed March 01, 2026).
Nefjodovs, V., Andze, L., Andzs, M., Filipova, I., Tupciauskas, R., Vecbiskena, L., & Kapickis, M. (2023, May 22). Wood as Possible Renewable Material for Bone Implants. In Encyclopedia. https://encyclopedia.pub/entry/44668
Nefjodovs, Vadims, et al. "Wood as Possible Renewable Material for Bone Implants." Encyclopedia. Web. 22 May, 2023.
Copy Citation
Bone fractures and bone defects affect millions of people every year. Metal implants for bone fracture fixation and autologous bone for defect reconstruction are used extensively in treatment of these pathologies. Simultaneously, alternative, sustainable, and biocompatible materials are being researched to improve existing practice. Wood as a biomaterial for bone repair has been considered.
wood implants
bone repair
biocomposites
osteosynthesis
1. Introduction
Bone fractures first drew prehistoric humans’ attention up to 46 thousand years ago during the Early Upper Paleolithic age, the period from which the first healed bone fractures were found by archeologists [1]. After thousands of years of using traction and immobilization as the only treatment for bone fractures, the first true external fixation was applied only 120 years ago. That was developed by a Belgian surgeon Albin Lambotte. Lambotte who also introduced the term “osteosynthesis”—fixation of bone by using mechanical devices [2]. At the beginning of the 20th century, with the development of antiseptics, anesthesiology, and bone imaging possibilities, the modern principles of the internal fixation of fractures were developed. The first material for osteosynthesis implants was nickel-coated steel, developed in the 19th century [3][4]. Other metals such as silver [5], aluminium, and brass [6] have been used to produce different bone implants. Nevertheless, these materials were found not to be fully suitable due to inadequate mechanical properties and corrosion. The first successful material was stainless steel, later joined by titanium and cobalt-chromium alloys [7]. Although the problem with obvious and quick corrosion was resolved, there are still a few debatable issues. First, the density of a metal alloys is up to three times higher than cancellous bone [8][9]. Thus, aseptic loosening of the metallic implants is considered a possible complication within 15 years after surgery [10]. Second, bio-corrosion of stainless steel [11] and titanium [12] alloys is being investigated as well. Demand for non-metallic implant materials is growing, not only because of bone damage over time due to the loosening and biocorrosion of implant material, but also because of the increased use of modern medical diagnostic systems, e.g., nuclear magnetic resonance [13]. Metal implants cause significant artifacts in computer tomography and magnetic resonance images. The lower image quality of artifacts cause blurring. In the last decades, numerous studies have been published about reducing the effects of artifacts. However, the issue is still present in everyday clinical practice [14][15].
Aside from bone fractures, bone defects are a common issue in orthopaedic and reconstructive surgery. Bone defects can be caused by severe injuries, congenital anomalies, and tissue resection due to oncological masses. Although bone has great capabilities for rejuvenation, the healing of large defects is challenging. Treatment with bone xenografts (grafts from animals) from dogs and goats for cranioplasty was first described more than 500 years ago by Ottoman empire surgeon Ibrahim Bin Abdullah [16]. Nowadays, bone defect reconstruction still relies mostly on autologous (from the patient), allogeneic (from another human donor), and xenogeneic (animal-derived) bone grafts. For very extensive defects, vascularized bone flaps are harvested from the patient. Harvesting bone tissues from the patient adds additional surgical sites, with possible complications. Using allogeneic and xenogeneic grafts posts immunological challenges, as well as logistical and ethical issues [17]. In attempts to improve bone defect reconstruction, different biomaterials have been widely investigated—calcium phosphates [18][19], bioactive glass [20], collagen [21], silk fibroin [22], etc.—for potential use in clinical practise [23].
2. Similarities between Wood and Bone
Humanity has known about wood as a biomaterial since the Stone Age, with wood has played a major role in humanity’s greatest achievements—from discovering fire to creating transport. Wood is an anisotropic natural material usually obtained from the trunk of a tree. It can be defined as a heterogeneous composite that consists mainly of natural polymers such as cellulose (40–50%), hemicelluloses (15–25%), and lignin (15–30%) [24][25]. Tree cross-sections can be distinguished into three components—the bark, cambium, and wood parts—xylem. The bark consists of a cork layer on the outside and a phloem layer on the inside. The cambium, located between the bark and the xylem, consists of living cells that form the new xylem and phloem layers. Xylem has two wood parts—sapwood and heartwood. The sapwood consists of dead cells and a small number of living parenchymal cells. It acts as food storage, as a water and nutrients transporter, and as mechanical support for the tree. The heartwood consists entirely of dead cells and provides only a support function for the tree [26].
Similar to wood, bone is also an anisotropic heterogeneous composite material, as it consists of about 60% inorganic material (calcium and phosphate in a form of natural or calcium-deficient hydroxyapatite), 30% organic material (collagen) and 10% water [27][28][29]. Similar to wood, which fulfills the function of support in a tree, the main function of bone is to support the static and movement functions of the body. Bone also acts as storage for minerals such as calcium and phosphate, as well as in the maintenance of homeostasis [30]. Three parts of bone can be observed in the cross-section—cortical bone, also called dense or compact bone, trabecular bone, also called spongy or cancellous bone, and bone marrow cavity. Bone tissue contains three main cell types: osteoblasts, osteoclasts, and osteocytes. Osteoblasts are responsible for bone formation, while osteoclasts are cells that resorb bone. Bone homeostasis is maintained by the connection between bone formation and bone resorption (bone turnover). Osteocytes are cells that are found in fully formed bone and make up most of the bone [31]. Similarities between wood and bone have been observed and were described as early as the invention of the microscopic magnification itself. The pioneer of microscopy, Antonie van Leeuwenhoek, described the analogy between the osteoid bone structure and the fibre structure of wood [32]. Since then, several authors have continued researching similarities between wood and bone, revealing the hierarchical macroscopic and microscopic structures of both, as well as functional similarities such as biomechanical characteristics, remodeling, and liquid transportation abilities [33][34][35][36][37]. Figure 1 shows the structural similarity between cortical bone and wood at the micro and nano level.
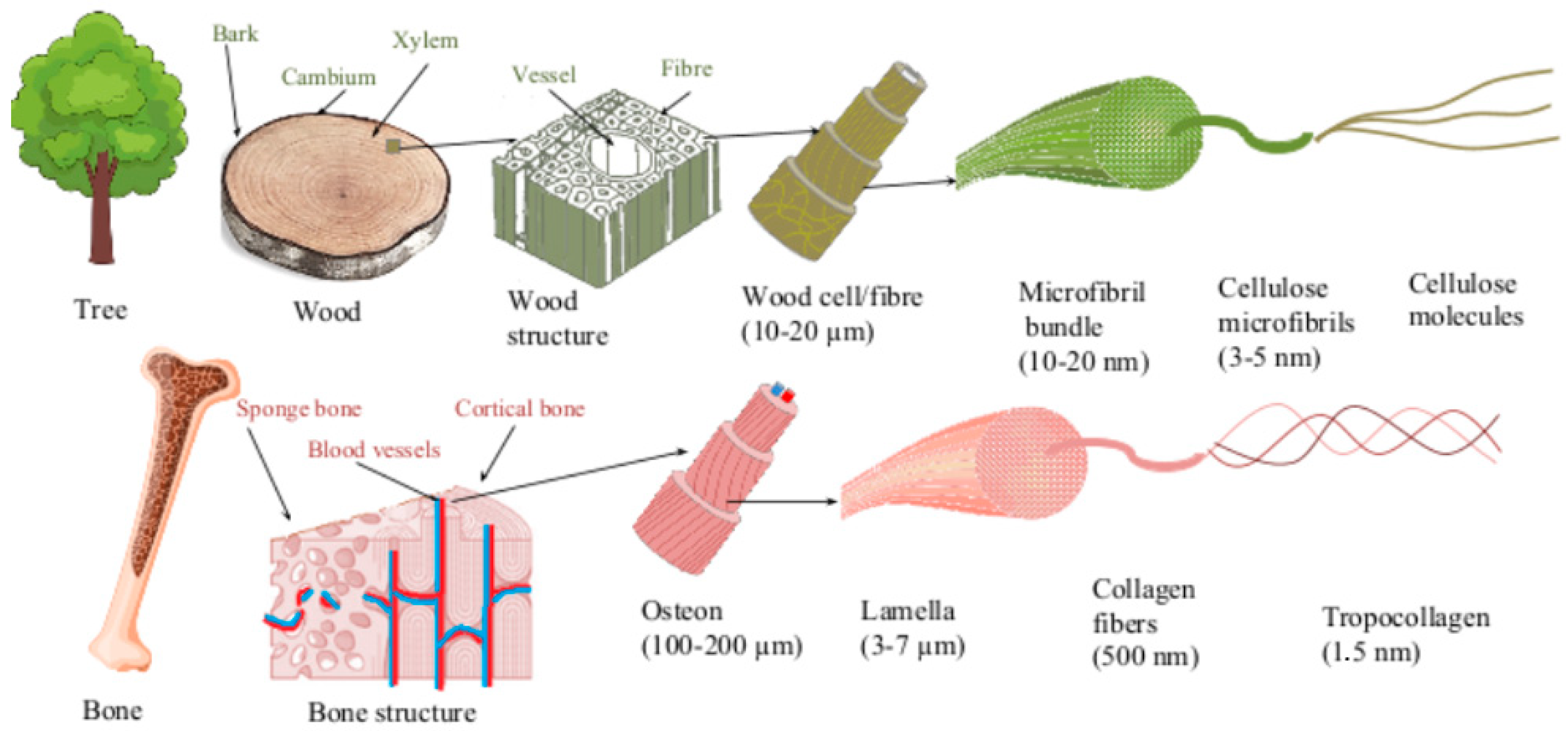
Figure 1. Schematical structure of bone and wood in macro, micro and nano scale.
The strength of both cortical bone and wood is ensured by its structural construction. The cortical bone base is formed by osteons, while wood consists of wood cells. Both the osteon and the tree cell are oriented in the direction of the long axis of the bone and wood, respectively, and are composed of several concentric layers of parallel fibers or fibrils. Each layer is oriented in different directions, thus providing mechanical strength. In bone, the layers are formed of collagen fibers, while wood cells are composed of bundles of cellulose micro fibrils. Collagen fiber consists of collagen fibrils constructed from triple helix collagen molecules and mineral nanocrystals, while cellulose microfibril is constructed from amorphous and crystalline parts of parallel cellulose molecules [27][38][39]. Based on similarities between wood and bone, wood has been used as a testing model for orthopedic implants [40]. Despite the structural similarity between wood and bone, solid wood has not been amply considered as a possible biomaterial for bone implants. Only in recent decades has wood been studied by a few authors as a possible implant material.
3. Wood Species for Bone Implants
Individual species of trees have been studied as a source of biomaterial. Most of the published studies have main purpose of creating biomaterials for bone defect substitution and rarely for creating wood-based orthopedic implants.
3.1. Birch
One of the first studies regarding wood as a possible implant material was performed by Kristen, Bösch et al. using birch wood [41]. Birch is one of the most widespread and economically important species of deciduous trees in Europe and Scandinavia. Silver birch (Betula pendula) and European white birch (Betula pubescens) are among the most common birch species found in most of Europe, up to Central Siberia. Betula pubescens, which is the northernmost tree species, is more common in the Northern and Eastern parts of Europe. Betula pendula is more common in the southern regions of Europe, such as the Iberian Peninsula, southern Italy, and Greece [42]. Birch wood has an average density of 600–650 kg/m3 and a high Jank hardness of 4000–5000 N, and contains little extractive material, which makes this wood well suited for bone implants [43][44]. The early studies were conducted in vivo using rabbits. Birch implants were pre-treated with ethanol and placed transcortically into rabbit tibias. Evaluation was done after 3, 5, 14, and 32 weeks. Although the tissues produced a foreign body reaction, a new bone formed around the wood implants. Additionally, bone ingrowth into the implant’s pores was recorded [45]. Similar ethanol pre-treated birch implants were implanted into a rabbit’s soft tissues. After controls within 2, 6, 12, and 30 weeks, it was concluded that ethanol pre-treatment was not sufficient to prevent a foreign body reaction [41]. Years later, Rekola, Aho et al. published a novel wood pre-treatment method—preheating of birch implants at different temperatures—140 °C, 200 °C, and 220 °C for 2 h. The implants were placed into the drilled cavities of rabbit femurs and observed after 4, 8 and 20 weeks detecting the bone ingrowth. Preheated birch implants showed better osteoconductivity compared to untreated implants. However, when applying the highest temperature of 220 °C, the biomechanical characteristics of the implants were decreased [46]. In vitro studies were performed by immersing the birch implants into simulated body fluid (SBF) for 63 days at 37 °C. It was documented that immersion in the SBF significantly decreases the biomechanical properties of the untreated implants, while heat pre-treated implants preserve these properties [47][48].
3.2. Ash
Ash is a tree of the olive family that is widespread in Europe, Asia, Canada, and North America. As a hardwood with a low content of extractive substances, a high density of 600–680 kg/m3, strength, and flexibility, it is suitable for bone implant materials [49]. The in vivo study with ash implants was conducted simultaneously with early birch studies. Ash specimens were ethanol-pretreated and fixed in rabbit calcaneus bones with Achilles tendons reattached and analyzed after 5 and 14 weeks. The ethanol pre-treatment of ash resulted not only in bone ingrowth, but the tendons’ tissues grew into the wood pores as well, along with moderate foreign body reaction [50].
3.3. Lime, Willow, and Fir
Spruce wood is widespread in Scandinavia, Northern Eastern Europe, North America, Canada, and Japan, and is one of the most economically important coniferous wood species [51]. The white willow (Salix alba) is the most well-known of the willows, widely distributed throughout Europe except for the most northern regions. The northern part of Europe, where willow is common, includes the British Isles, the Netherlands, and the Baltic coast (Latvia and Lithuania). Willow is also found in Mediterranean regions, as well as in North Africa (Morocco and Algeria) [52]. Lime trees are common in Eastern North America (Tilia americana) and Europe (Tilia Europen; hybrid wood). All named wood species have a low density—400–450 kg/m3 for fir and willow, 450–550 kg/m3 for lime wood. These wood species, together with birch and ash, were used to fix fractures in rabbit femurs by Horsky, Huraj, Paukovic. Implants were untreated before implanting in vivo. Birch, ash, and fir were well tolerated, while lime and willow caused acute inflammatory reaction, indicating that differences in wood species meant that not all species would be suitable for bone implants [53]. Although all the mentioned wood species contain a high extractive content, they differ in their composition. Fir extract contains the most lignans [54], while willow extracts contain a large amount of salicylic compounds, flavonoids, and tannins. These substances are bioactive compounds characterized by antipyretic, analgesic, anti-inflammatory, antirheumatic, and anticoagulant properties. As with all bioactive substances, they can be toxic at certain levels [55][56].
3.4. Juniper
Juniper is the world’s most widespread and northernmost coniferous tree. It is common both in Europe and Asia, as well as in North America and Japan. Juniper can be found both in the farthest North areas of Scandinavia and in the mountain areas of the warmer regions of Southern Europe. The density of juniper is 450–600 kg/m3 [57]. Juniper has long been studied for its antibacterial properties, but not for use in bone implants. The essential oils in juniper wood can also be toxic at high dosages; therefore, pre-treatment is required [58]. A unique in vivo study considered juniper wood for potential orthopedic hardware. Hip prostheses were crafted and pre-treated in boiling water for 10 min. The proximal part of rabbit femurs were resected and hemiarthroplasty with the juniper prostheses was performed. Rabbits were allowed to bear weight with no restrictions. Histological analysis was done after 3, 6, 18, and 36 months. No foreign body reaction was documented in any specimens. Initial bone ingrowth was detected after 6 months. After 3 years, wood implants were fully integrated with bone tissues (Figure 2). Essential oils from juniper were tested for their capacity to induce a toxic response in rats and was demonstrated to be well tolerated, especially when released slowly [59]. Almost 20 years later, preliminary studies have been carried out for the possible development of bone implants from partially delignified and compressed solid juniper wood, thus improving the mechanical properties of the implant. A compressed wood density of 1170 kg/m3 was achieved (100% increase compared to natural juniper wood). The modulus of rupture was increased by 85%, reaching 174 MPa, and the modulus of elasticity by 620%, reaching 12,500 MPa [60].
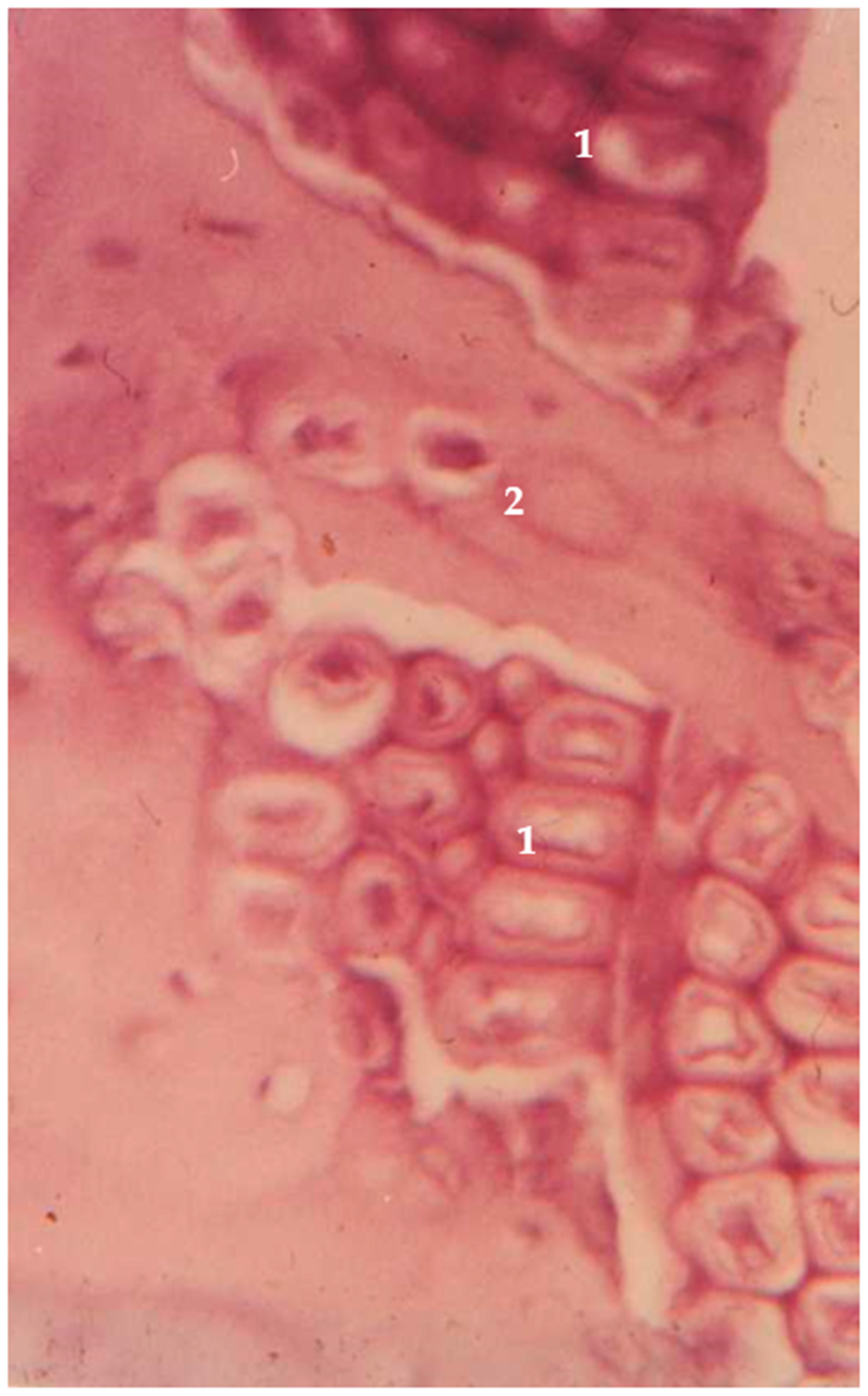
Figure 2. Juniper implant in the in vivo model. 1—juniper implant; 2—bone tissue ingrowth.
3.5. Carbonized Wood
Another trial for the development of bone implants has been proposed by pre-treating wood at high temperatures to create a charcoal-type material. In one of the earliest studies, wood from clematis was carbonized at 850 °C for 5 h. Samples were implanted in vivo into rabbit bone, whose tissue was able to grow into the carbonized wood [61]. A similar in vivo study was performed with bamboo charcoal. The results showed that charcoal bamboo as a bone substitute has good biocompatibility and osteoconductivity [62]. Although pure carbonized wood had good biocompatibility and osteoconductivity, the complete loss of its mechanical properties made it an impractical material. Years later, the mechanical properties of pure carbonized wood were improved by an impregnation with silicon carbide (SiC) to produce a biomaterial called ecoceramics. In the preparation process, natural wood was pyrolyzed at 1000 °C using argon gas; the natural wood lost around 75% of its weight and 60% of its volume as a result of the treatment. The remaining scaffold was infiltrated with melted Si at 1550 °C. Si reacts with carbon in pyrolyzed wood to form SiC. Different wood species have been used to produce wood-based ecoceramics, for example, maple [63], eucalyptus [64], mango [65], oak [66], beech [67], pine [68], and others [69]. The technique preserved the porous structure of the wood while adding the rigidity of SiC. It is also a light-weight material, with density around 1100–2300 kg/cm3, depending on the selected wood [65][66][67]. In addition, ecoceramics have great heat and electric resistance [70][71]. Due to various properties, ecoceramics have attracted more interest of researchers in civil [72], aeronautical [73] and electronic [74] engineering, and only a small number of studies consider ecoceramics as a material for medical applications. One in vivo study has been done with SiC scaffolds that were implanted in sheep metatarsal bones. Histological analysis was performed after 4, 8, 12, and 48 weeks. Analysis revealed good scaffold-to-bone adhesion, and new bone ingrowth inside the scaffolds was documented as well [75]. Since then, few authors have proposed combining wooden scaffolds with other biomaterials. In one study, SiC scaffolds derived from beech, eucalyptus, and sapele were combined with bioactive glass. An in vitro study with MG-63 osteoblasts showed good cellular attachment to both coated and uncoated SiC scaffolds. Additionally, the osteoblasts proliferated equally in standardized environments and on the surface of bioactive-glass-coated SiC scaffolds [76]. In another study, carbonized wood scaffolds derived from cane and pine [77][78] or rattan [79] were combined with hydroxyapatite (HA). The obtained samples showed the preserved porous structure and improved mechanical properties; the compressive strength reached 0.4 MPa and the tensile modulus increased 2–3 times [77][78][79].
3.6. Cellulose-Based Scaffold
Few authors have considered wood as a base for cellulose-based scaffolds. To create such scaffolds, more extensive wood processing is required. Firstly, wood is processed into cellulose. Cellulose is a natural linear cell polysaccharide consisting of glucose (C6H10O5)n. (Figure 3). It is the main component of cell wall in green plants and algae, and bacteria produce cellulose to form a biofilm as well. While the purest natural form of cellulose is cotton, where cellulose comprises about 90% of cotton’s mass, wood is made of around 57% cellulose and remains the main source for producing cellulose [80]. As a natural raw material, cellulose has been used for fabrics and papers for hundreds of years, but only 185 years ago, in 1838, the chemical structure of cellulose was discovered and described by French chemist Anselme Payen [81]. Since then, production of cellulose from wood stock is performed by chemically dissolving unwanted components such as lignin, short-chained polymers, etc. Cellulose is widely used for its porous structure and insolubility in water and organic substances in medical filters [82], pharmacy [83], and wound dressings [84]. In the last few decades, cellulose has also attracted researchers’ attention as a potential biomaterial for medical applications, similar to using wood as an implant material. In the 1960s, implantation of cellulose sponges was used to study tissue inflammation and granulation formation shortly after implantation [85]. Years later, in the 1990s, researchers began to investigate the long-term effects of cellulose implantation. Märtson, Viljanto et al. used industrial soft cellulose sponges derived from eucalyptus, birch, or oak. Cellulose sponges were tested in vivo in rat soft tissue, and histological examinations were performed consecutively after 1–60 weeks. Histological evaluation revealed that the inflammatory response of the surrounding tissues subsided after 4–6 weeks and revealed good connective tissue ingrowth into the cellulose sponges. The researchers also detected a slow resorption and degradation of the pure cellulose sponges [86][87]. In addition, the biocompatibility of cellulose sponges with bone tissue was investigated in vivo; cellulose sponges were tested into the femoral bone cavity of rats. Bone ingrowth into cellulose sponges was recorded after 4–6 weeks [88]. Later researchers started combining cellulose fibers with other biomaterials. An in vitro study was performed with chondrocytes from the bovine knee joint. Cellulose scaffolds were exposed to saturated calcium hydroxide (Ca(OH)2) solution, then immersed in supersaturated simulated body fluid (SBF). Thus, a calcium phosphate coating was created. Although the cellulose and calcium phosphate scaffolds caused an acidic reaction in solution and the pH had to be adjusted with calcium hydroxide [89], better cellular adhesion was detected compared to untreated cellulose scaffolds. In another study, cellulose fibers were impregnated with hydroxyapatite particles. Tomilla, Ekholm et al. published two studies on cellulose coating with hydroxyapatite derived from bioactive glass. Bioactive glass S53P4 (Abmin Technologies Ltd., Turku, Finland) was dissolved in SBF, and cellulose sponges were immersed in the SBF solution at 37 °C for 24 h. After 24 h of immersion in SBF, calcium hydroxyapatite was formed on the surface of the scaffold.
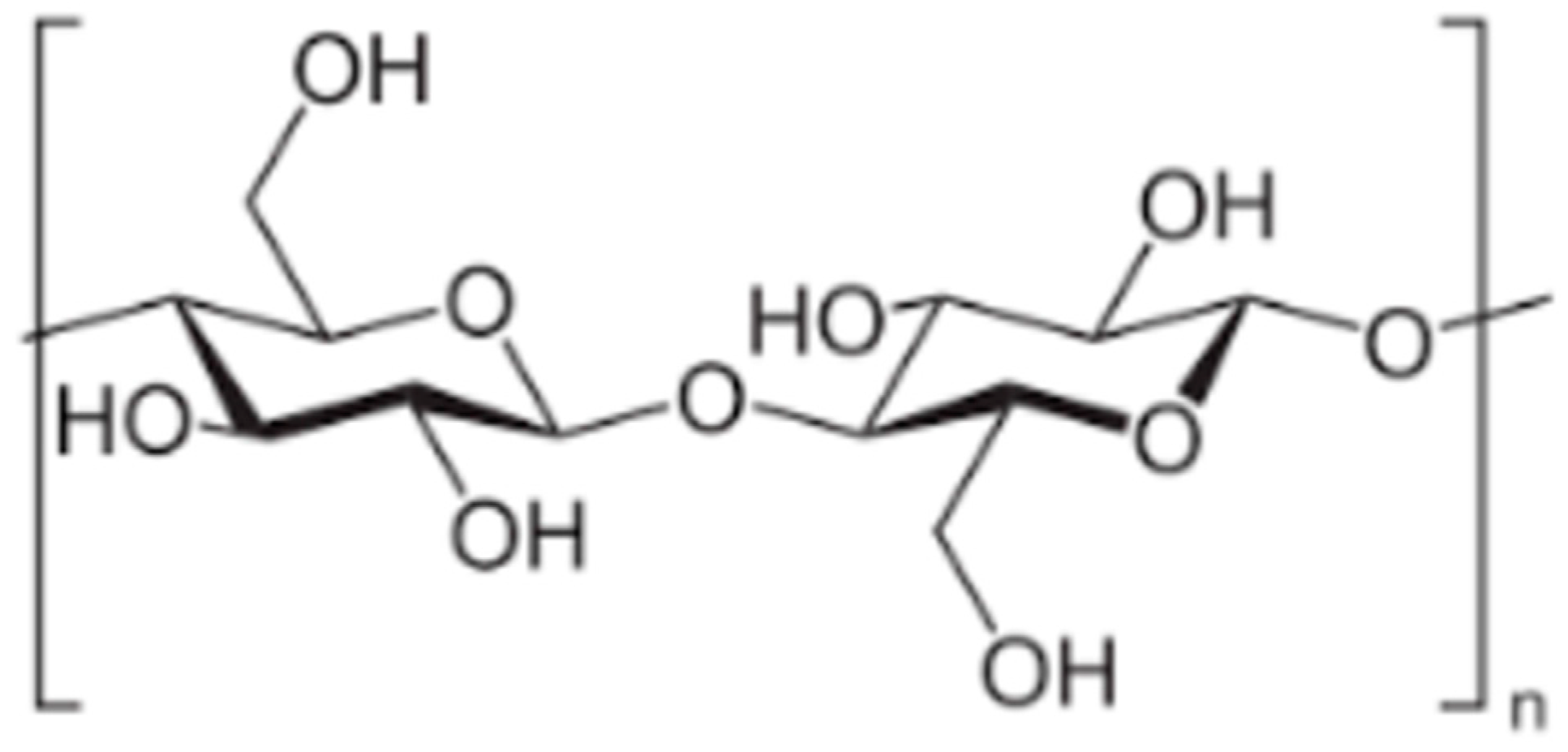
Figure 3. Cellulose molecule.
References
- Borgel, S.; Latimer, B.; McDermott, Y.; Sarig, R.; Pokhojaev, A.; Abulafia, T.; Goder-Goldberger, M.; Barzilai, O.; May, H. Early Upper Paleolithic human foot bones from Manot Cave, Israel. J. Hum. Evol. 2021, 160, 102668.
- Afshar, A.; Steensma, D.P.; Kyle, R.A. Albin Lambotte: Pioneer of Osteosynthesis (Bone Fixation). Mayo Clin. Proc. 2021, 96, 2012–2013.
- Roberts, T.T.; Prummer, C.M.; Papaliodis, D.N.; Uhl, R.L.; Wagner, T.A. History of the orthopedic screw. Orthopedics 2013, 36, 12–14.
- Fairbank, J. The Evolution of Orthopaedic Surgery, by Leslie Klenerman. Spine 2002, 27, 2298.
- Steinbach, L.W., IV. On the Use of Fixation Plates in the Treatment of Fractures of the Leg. Ann. Surg. 1900, 31, 436–442.
- Lambotte, A. Chirurgie Operatoire des Fractures; Masson: Issy-les-Moulineaux, France, 1913.
- Venable, C.S.; Stuck, W.G. The Internal Fixation of Fractures; Thomas: New York, NY, USA, 1947.
- Zioupos, P.; Currey, J.D. Changes in the stiffness, strength, and toughness of human cortical bone with age. Bone 1998, 22, 57–66.
- Wu, J.J.; Shyr, H.S.; Chao, E.Y.; Kelly, P.J. Comparison of osteotomy healing under external fixation devices with different stiffness characteristics. J. Bone Jt. Surg. Am. 1984, 66, 1258–1264.
- Sansone, V.; Pagani, D.; Melato, M. The effects on bone cells of metal ions released from orthopaedic implants. A review. Clin. Cases Miner. Bone Metab. 2013, 10, 34–40.
- Cadosch, D.; Chan, E.; Gautschi, O.P.; Simmen, H.P.; Filgueira, L. Bio-corrosion of stainless steel by osteoclasts—In Vitro evidence. J. Orthop. Res. 2009, 27, 841–846.
- Cadosch, D.; Al-Mushaiqri, M.S.; Gautschi, O.P.; Meagher, J.; Simmen, H.P.; Filgueira, L. Biocorrosion and uptake of titanium by human osteoclasts. J. Biomed. Mater. Res. A 2010, 95, 1004–1010.
- Rekola, J.; Aho, A.J.; Gunn, J.; Matinlinna, J.; Hirvonen, J.; Viitaniemi, P.; Vallittu, P.K. The effect of heat treatment of wood on osteoconductivity. Acta Biomater. 2009, 5, 1596–1604.
- Zhang, X.; Wang, J.; Xing, L. Metal artifact reduction in x-ray computed tomography (CT) by constrained optimization. Med. Phys. 2011, 38, 701–711.
- Hargreaves, B.A.; Worters, P.W.; Pauly, K.B.; Pauly, J.M.; Koch, K.M.; Gold, G.E. Metal-induced artifacts in MRI. AJR Am. J. Roentgenol. 2011, 197, 547–555.
- Aciduman, A.; Belen, D. The earliest document regarding the history of cranioplasty from the Ottoman era. Surg. Neurol. 2007, 68, 349–352.
- Kiernan, C.; Knuth, C.; Farrell, E. Chapter 6—Endochondral Ossification: Recapitulating Bone Development for Bone Defect Repair. In Developmental Biology and Musculoskeletal Tissue Engineering; Stoddart, M.J., Craft, A.M., Pattappa, G., Gardner, O.F.W., Eds.; Academic Press: Boston, MA, USA, 2018; pp. 125–148.
- Sasaki, G.; Watanabe, Y.; Miyamoto, W.; Yasui, Y.; Morimoto, S.; Kawano, H. Induced membrane technique using beta-tricalcium phosphate for reconstruction of femoral and tibial segmental bone loss due to infection: Technical tips and preliminary clinical results. Int. Orthop. 2018, 42, 17–24.
- Yan, L.; Jiang, D.M. Study of bone-like hydroxyapatite/polyamino acid composite materials for their biological properties and effects on the reconstruction of long bone defects. Drug. Des. Devel. Ther. 2015, 9, 6497–6508.
- Roffi, A.; Krishnakumar, G.S.; Gostynska, N.; Kon, E.; Candrian, C.; Filardo, G. The Role of Three-Dimensional Scaffolds in Treating Long Bone Defects: Evidence from Preclinical and Clinical Literature-A Systematic Review. Biomed. Res. Int. 2017, 2017, 8074178.
- Nguyen, B.B.; Moriarty, R.A.; Kamalitdinov, T.; Etheridge, J.M.; Fisher, J.P. Collagen hydrogel scaffold promotes mesenchymal stem cell and endothelial cell coculture for bone tissue engineering. J. Biomed. Mater. Res. A 2017, 105, 1123–1131.
- Ruan, S.Q.; Deng, J.; Yan, L.; Huang, W.L. Composite scaffolds loaded with bone mesenchymal stem cells promote the repair of radial bone defects in rabbit model. Biomed. Pharmacother. 2018, 97, 600–606.
- Zhang, M.; Matinlinna, J.P.; Tsoi, J.K.H.; Liu, W.; Cui, X.; Lu, W.W.; Pan, H. Recent developments in biomaterials for long-bone segmental defect reconstruction: A narrative overview. J. Orthop. Transl. 2020, 22, 26–33.
- Anil, A.; Ali Serdar, V. Wood-Reinforced Polymer Composites. In Wood in Civil Engineering; Giovanna, C., Ed.; IntechOpen: Rijeka, Croatia, 2017.
- Zhao, S.; Zhao, J.X.; Han, G.Z. Advances in the study of mechanical properties and constitutive law in the field of wood research. IOP Conf. Ser. Mater. Sci. Eng. 2016, 137, 012036.
- Wiedenhoeft, A.; Miller, R. 2 Structure and Function of Wood. In Handbook of Wood Chemistry and Wood Composites; CRC Press: Boca Raton, Fl, USA, 2005.
- Rosa, N.; Moura, M.F.S.F.; Olhero, S.; Simoes, R.; Magalhães, F.D.; Marques, A.T.; Ferreira, J.P.S.; Reis, A.R.; Carvalho, M.; Parente, M. Bone: An Outstanding Composite Material. Appl. Sci. 2022, 12, 3381.
- Vaz, M.F.; Canhão, H.; Fonseca, J.O. Bone: A Composite Natural Material; BioMed Central: London, UK, 2011.
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 4.
- Sansalone, V.; Naili, S.; Bousson, V.; Bergot, C.; Peyrin, F.; Zarka, J.; Laredo, J.D.; Haïat, G. Determination of the heterogeneous anisotropic elastic properties of human femoral bone: From nanoscopic to organ scale. J. Biomech. 2010, 43, 1857–1863.
- Su, N.; Yang, J.; Xie, Y.; Du, X.; Chen, H.; Zhou, H.; Chen, L. Bone function, dysfunction and its role in diseases including critical illness. Int. J. Biol. Sci. 2019, 15, 776–787.
- Van Leeuwenhoeck, A. Several observations on the texture of bone in animals compared with that of wood: On the bark of. trees: On the little scales found in the cuticula. Philos. Trans. R. Soc. Lond. 1693, 17, 838–843.
- Jeronimidis, G. Chapter 1—Structure-Property Relationships in Biological Materials. In Pergamon Materials Series; Elices, M., Ed.; JSTOR: Pergamon, Turkey, 2000; Volume 4, pp. 3–16.
- Spatz, H.C.; Köhler, L.L.; Niklas, K. Mechanical behaviour of plant tissues: Composite materials or structures? J. Exp. Biol. 2000, 202, 3269–3272.
- Fratzl, P.; Weinkamer, R. Nature’s hierarchical materials. Prog. Mater. Sci. 2007, 52, 1263–1334.
- Weinkamer, R.; Fratzl, P. Mechanical adaptation of biological materials—The examples of bone and wood. Mater. Sci. Eng. C 2011, 31, 1164–1173.
- Stanzl-Tschegg, S.E. Wood as a bioinspiring material. Mater. Sci. Eng. C 2011, 31, 1174–1183.
- McKittrick, J.; Chen, P.Y.; Tombolato, L.; Novitskaya, E.; Trim, M.; Hirata, G.; Olevsky, E.A.; Horstemeyer, M. Energy absorbent natural materials and bioinspired design strategies: A review. Mater. Sci. Eng. C 2010, 30, 331–342.
- Yi, K.; Ruffini, A.; Srivastava, S.; Srivastava, R. Bio-Inspired Technology Edited by Ruby Srivastava. SRIVASTAVA, Ruby. Introductory Chapter: DNA as Nanowires. In Bio-Inspired Technology; IntechOpen: Rijeka, Croatia, 2019.
- Murdoch, A.H.; Mathias, K.J.; Shepherd, D.E. Investigation into the material properties of beech wood and cortical bone. Biomed. Mater. Eng. 2004, 14, 1–4.
- Kristen, H.; Bosch, P.; Bednar, H.; Plenk, H., Jr. The effects of dynamic loading on intracalcaneal wood implants and on the tissues surrounding them. Arch. Orthop. Trauma Surg. 1979, 93, 287–292.
- Beck, P.; Caudullo, G.; de Rigo, D.; Tinner, W. Betula pendula, Betula pubescens and Other Birches in Europe: Distribution, Habitat, Usage and Threats; Publication Office of the European Union: Luxembourg, 2016.
- Rastogi, S.; Pandey, M.M.; Rawat, A.K.S. Medicinal plants of the genus Betula—Traditional uses and a phytochemical–pharmacological review. J. Ethnopharmacol. 2015, 159, 62–83.
- Laskowska, A. The Influence of Process Parameters on the Density Profile and Hardness of Surface-densified Birch Wood (Betula pendula Roth). Bioresources 2017, 12, 6011–6023.
- Kristen, H.; Bosch, P.; Bednar, H.; Plenk, H., Jr. Biocompatibility of wood in bone tissue (author’s transl). Arch. Orthop. Unf. 1977, 89, 1–14.
- Aho, A.J.; Rekola, J.; Matinlinna, J.; Gunn, J.; Tirri, T.; Viitaniemi, P.; Vallittu, P. Natural composite of wood as replacement material for ostechondral bone defects. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 83, 64–71.
- Rekola, J.; Lassila, L.V.; Hirvonen, J.; Lahdenpera, M.; Grenman, R.; Aho, A.J.; Vallittu, P.K. Effects of heat treatment of wood on hydroxylapatite type mineral precipitation and biomechanical properties in vitro. J. Mater. Sci. Mater. Med. 2010, 21, 2345–2354.
- Rekola, J.; Lassila, L.V.; Nganga, S.; Yla-Soininmaki, A.; Fleming, G.J.; Grenman, R.; Aho, A.J.; Vallittu, P.K. Effect of heat treatment of wood on the morphology, surface roughness and penetration of simulated and human blood. Biomed. Mater. Eng. 2014, 24, 1595–1607.
- Etiegni, L.; Campbell, A. Physical and chemical characteristics of wood ash. Bioresour. Technol. 1991, 37, 173–178.
- Bosch, P.; Kristen, H.; Braun, F.; Kovac, W. Reaction of connective tissue and striated muscle tissue to implanted ashwood (author’s transl). Wien. Med. Wochenschr. 1979, 129, 419–423.
- Yildiz, S.; Gezer, E.D.; Yildiz, U.C. Mechanical and chemical behavior of spruce wood modified by heat. Build. Environ. 2006, 41, 1762–1766.
- Hanley, S.J.; Karp, A. Genetic strategies for dissecting complex traits in biomass willows (Salix spp.). Tree Physiol. 2014, 34, 1167–1180.
- Horsky, I.; Huraj, E.; Paukovic, J. Utilization of wood in the manufacture of orthopedic implants. Acta Chir. Orthop. Traumatol. Cech. 1987, 54, 3–13.
- Füchtner, S.; Brock-Nannestad, T.; Smeds, A.; Fredriksson, M.; Pilgård, A.; Thygesen, L.G. Hydrophobic and Hydrophilic Extractives in Norway Spruce and Kurile Larch and Their Role in Brown-Rot Degradation. Front. Plant Sci. 2020, 11, 855.
- Warmiński, K.; Stolarski, M.J.; Gil, Ł.; Krzyżaniak, M. Willow bark and wood as a source of bioactive compounds and bioenergy feedstock. Ind. Crops Prod. 2021, 171, 113976.
- Tyśkiewicz, K.; Konkol, M.; Kowalski, R.; Rój, E.; Warmiński, K.; Krzyżaniak, M.; Gil, Ł.; Stolarski, M. Characterization of bioactive compounds in the biomass of black locust, poplar and willow. Trees 2019, 33, 1235–1263.
- Enescu, C.; Durrant, T.; Caudullo, G.; de Rigo, D. Juniperus Communis in Europe: Distribution, Habitat, Usage and Threats; Publication Office of the European Union: Luxembourg, 2016.
- Semerdjieva, I.; Zheljazkov, V.D.; Radoukova, T.; Dincheva, I.; Piperkova, N.; Maneva, V.; Astatkie, T.; Kačániová, M. Biological Activity of Essential Oils of Four Juniper Species and Their Potential as Biopesticides. Molecules 2021, 26, 6358.
- Gross, K.A.; Ezerietis, E. Juniper wood as a possible implant material. J. Biomed. Mater. Res. A 2003, 64, 672–683.
- Andze, L.; Andzs, M.; Skute, M.; Nefjodov, V.; Kapickis, M.; Tupciauskas, R. Preliminary Study of Chemically Pretreated Densification of Juniper Wood for Use in Bone Implants. Mater. Sci. Forum 2022, 1071, 101–108.
- Colville, J.B.P.; Hoikka, V.; Vainio, K. Wood anatomy and the use of carbonised wood as a matrix for bone regeneration in animals. Int. Assoc. Wood Anat. 1979, 12, 3–6.
- Kosuwon, W.; Laupattarakasem, W.; Saengnipanthkul, S.; Mahaisavariya, B.; Therapongpakdee, S. Charcoal bamboo as a bone substitute: An animal study. J. Med. Assoc. Thai. 1994, 77, 496–500.
- Gordic, M.; Bucevac, D.; Ružić, J.; Gavrilovic, S.; Hercigonja, R.; Stankovic, M.; Matovic, B. Biomimetic synthesis and properties of cellular SiC. Ceram. Int. 2014, 40, 3699–3705.
- Byrne, C.E.; Nagle, D.C. Carbonization of wood for advanced materials applications. Carbon 1997, 35, 259–266.
- Greil, P.; Lifka, T.; Kaindl, A. Biomorphic Cellular Silicon Carbide Ceramics from Wood: I. Processing and Microstructure. J. Eur. Ceram. Soc. 1998, 18, 1961–1973.
- Polozov, I.; Razumov, N.; Masaylo, D.; Silin, A.; Lebedeva, Y.; Popovich, A. Fabrication of Silicon Carbide Fiber-Reinforced Silicon Carbide Matrix Composites Using Binder Jetting Additive Manufacturing from Irregularly-Shaped and Spherical Powders. Materials 2020, 13, 1766.
- Varela-Feria, F.M.; Fernández, J.; Ramirez de Arellano Lopez, A.; Singh, M. Low Density Biomorphic Silicon Carbide: Microstructure and Mechanical Properties. J. Eur. Ceram. Soc. 2002, 22, 2719–2725.
- Sieber, H.; Vogli, E.; Greil, P. Biomorphic SiC-Ceramic Manufactured by Gas-Phase Infiltration of Pine Wood; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; Volume 22, pp. 109–116.
- Singh, M.; Salem, J.A. Mechanical properties and microstructure of biomorphic silicon carbide ceramics fabricated from wood precursors. J. Eur. Ceram. Soc. 2002, 22, 2709–2717.
- Singh, M. Environment conscious ceramics (Ecoceramics). Ceram. Eng. Sci. Proc. 2000, 21, 39–44.
- Parfen’eva, L.S.; Orlova, T.S.; Kartenko, N.F.; Sharenkova, N.V.; Smirnov, B.I.; Smirnov, I.A.; Misiorek, H.; Jezowski, A.; Varela-Feria, F.M.; Martinez-Fernandez, J.; et al. Thermal conductivity of the SiC/Si biomorphic composite, a new cellular ecoceramic. Phys. Solid State 2005, 47, 1216–1220.
- Kardashev, B.K.; Burenkov, Y.A.; Smirnov, B.I.; de Arellano-Lopez, A.R.; Martinez-Fernandez, J.; Varela-Feria, F.M. Elasticity and inelasticity of biomorphic silicon carbide ceramics. Phys. Solid State 2004, 46, 1873–1877.
- Naslain, R.R. SiC-Matrix Composites: Nonbrittle Ceramics for Thermo-Structural Application. Int. J. Appl. Ceram. Technol. 2005, 2, 75–84.
- Orlova, T.; Smirnov, B.; de Arellano-Lopez, A.; Martinez Fernandez, J.; Sepulveda, R. Anisotropy of electric resistivity of Sapele-based biomorphic SiC/Si composites. Phys. Solid State 2005, 47, 220–223.
- Filardo, G.; Kon, E.; Tampieri, A.; Cabezas-Rodriguez, R.; Di Martino, A.; Fini, M.; Giavaresi, G.; Lelli, M.; Martinez-Fernandez, J.; Martini, L.; et al. New bio-ceramization processes applied to vegetable hierarchical structures for bone regeneration: An experimental model in sheep. Tissue Eng. Part A 2014, 20, 763–773.
- de Carlos, A.; Borrajo, J.P.; Serra, J.; Gonzalez, P.; Leon, B. Behaviour of MG-63 osteoblast-like cells on wood-based biomorphic SiC ceramics coated with bioactive glass. J. Mater. Sci. Mater. Med 2006, 17, 523–529.
- Qian, J.; Kang, Y.; Zhang, W.; Li, Z. Fabrication, chemical composition change and phase evolution of biomorphic hydroxyapatite. J. Mater. Sci. Mater. Med. 2008, 19, 3373–3383.
- Tampieri, A.; Sprio, S.; Ruffini, A.; Celotti, G.; Lesci, G.; Roveri, N. From wood to bone: Multi-step process to convert wood hierarchical structures into biomimetic hydroxyapatite scaffolds for bone tissue engineering. J. Mater. Chem. 2009, 19, 4973–4980.
- Finardi, U.; Sprio, S. Human bone regeneration from wood: A novel hierarchically organised nanomaterial. Int. J. Healthc. Technol. Manag. 2012, 13, 171–183.
- Bayer, E.A.; Chanzy, H.; Lamed, R.; Shoham, Y. Cellulose, cellulases and cellulosomes. Curr. Opin. Struct. Biol. 1998, 8, 548–557.
- Marchessault, R.H. Cellulose Structure Modification and Hydrolysis; Raymond, A., Roger, Y., Rowell, M., Eds.; Wiley-Interscience: New York, NY, USA, 1986, p. 379. J. Polym. Sci. Part C Polym. Lett. 1987, 25, 139–140.
- Layton, M.; Roper, D. 12—Investigation of the Hereditary Haemolytic Anaemias: Membrane and Enzyme Abnormalities. In Dacie and Lewis Practical Haematology, 12th ed.; Bain, B.J., Bates, I., Laffan, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 228–253.
- Di Giuseppe, E. Analogue Materials in Experimental Tectonics. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2018.
- Gupta, B.S. 1—Manufacture, types and properties of biotextiles for medical applications. In Biotextiles as Medical Implants; King, M.W., Gupta, B.S., Guidoin, R., Eds.; Woodhead Publishing: Cambridge, UK, 2013; pp. 3–47.
- Viljanto, J. A Cellstick Device for Wound Healing Research. In Wound Healing and Skin Physiology; Springer: Berlin/Heidelberg, Germany, 1995; pp. 513–522.
- Martson, M.; Viljanto, J.; Hurme, T.; Laippala, P.; Saukko, P. Is cellulose sponge degradable or stable as implantation material? An in vivo subcutaneous study in the rat. Biomaterials 1999, 20, 1989–1995.
- Martson, M.; Viljanto, J.; Laippala, P.; Saukko, P. Connective tissue formation in subcutaneous cellulose sponge implants in the rat. The effect of the size and cellulose content of the implant. Eur. Surg. Res. 1998, 30, 419–425.
- Martson, M.; Viljanto, J.; Hurme, T.; Saukko, P. Biocompatibility of cellulose sponge with bone. Eur. Surg. Res. 1998, 30, 426–432.
- Muller, F.A.; Muller, L.; Hofmann, I.; Greil, P.; Wenzel, M.M.; Staudenmaier, R. Cellulose-based scaffold materials for cartilage tissue engineering. Biomaterials 2006, 27, 3955–3963.
More
Information
Subjects:
Medicine, Research & Experimental
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
23 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





