Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Fabiano Vargas Costa | -- | 2093 | 2023-05-19 00:36:48 | | | |
| 2 | Rita Xu | Meta information modification | 2093 | 2023-05-19 03:57:11 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Costa, F.V.; Kolesnikova, T.O.; Galstyan, D.S.; Ilyin, N.P.; De Abreu, M.S.; Petersen, E.V.; Demin, K.A.; Yenkoyan, K.B.; Kalueff, A.V. Zebrafish Model of Psychiatric Disorders. Encyclopedia. Available online: https://encyclopedia.pub/entry/44531 (accessed on 13 January 2026).
Costa FV, Kolesnikova TO, Galstyan DS, Ilyin NP, De Abreu MS, Petersen EV, et al. Zebrafish Model of Psychiatric Disorders. Encyclopedia. Available at: https://encyclopedia.pub/entry/44531. Accessed January 13, 2026.
Costa, Fabiano V., Tatiana O. Kolesnikova, David S. Galstyan, Nikita P. Ilyin, Murilo S. De Abreu, Elena V. Petersen, Konstantin A. Demin, Konstantin B. Yenkoyan, Allan V. Kalueff. "Zebrafish Model of Psychiatric Disorders" Encyclopedia, https://encyclopedia.pub/entry/44531 (accessed January 13, 2026).
Costa, F.V., Kolesnikova, T.O., Galstyan, D.S., Ilyin, N.P., De Abreu, M.S., Petersen, E.V., Demin, K.A., Yenkoyan, K.B., & Kalueff, A.V. (2023, May 18). Zebrafish Model of Psychiatric Disorders. In Encyclopedia. https://encyclopedia.pub/entry/44531
Costa, Fabiano V., et al. "Zebrafish Model of Psychiatric Disorders." Encyclopedia. Web. 18 May, 2023.
Copy Citation
Psychiatric disorders are highly prevalent brain pathologies that represent an urgent, unmet biomedical problem. Since reliable clinical diagnoses are essential for the treatment of psychiatric disorders, their animal models with robust, relevant behavioral and physiological endpoints become necessary. Zebrafish (Danio rerio) display well-defined, complex behaviors in major neurobehavioral domains which are evolutionarily conserved and strikingly parallel to those seen in rodents and humans.
Danio rerio
animal modelling
psychiatric disorders
1. Introduction
Psychiatric disorders are highly prevalent brain illnesses that represent a major urgent, unmet biomedical problem [1][2][3][4][5]. Their prevention and treatment involves three main challenges: to identify a genotype associated with the disorder in question, to characterize molecular pathology underlying each disorder, and to develop novel efficient therapies [6]. Unlike clinically robust neurological disorders, such as Alzheimer’s and Parkinson’s diseases, most psychiatric pathologies do not have detectable pathobiological signs (e.g., neuronal loss or protein aggregation), hence heavily relying on behavioral and cognitive phenotypes for correct diagnostics [1]. Although complex genetic bases of human psychiatric disorders and their clinical heterogeneity make it impossible to fully mimic clinical conditions using laboratory animals [2][7], such experimental models represent an increasingly important tool in translational research of various pathogenic aspects of psychiatric disorders [8][9].
Zebrafish (Danio rerio) are small freshwater teleost fish that have recently become a powerful model organism in translational neuroscience research [10]. These fish are currently widely used in major universities and research centers worldwide, bringing to neuroscience research both reliability and high throughput [10]. Multiple advanced genetic tools (e.g., CRISPR-Cas9 or transcription activator-like effector nucleases, TALENS) [11], as well as optogenetics-based [12][13] and neuroimaging methods [14], have also been successfully applied to zebrafish models. Furthermore, zebrafish display robust, well-defined, context-specific and complex behaviors in all major central nervous system (CNS) domains, which are generally evolutionarily conserved and strikingly parallel to those in rodents and humans [15].
2. Current State of Studying Zebrafish Model of Psychiatric Disorders
Modern classification of human psychiatric disorders is typically based on the International Classification of Diseases and Related Health Problems (ICD-11) and the Diagnostic and Statistical Manual of Mental Disorders (DSM-5, Figure 1). Since the global prevalence of major human psychiatric disorders reflects their relative clinical and societal importance, a major challenge for zebrafish-based CNS disease modeling is to ensure that clinical prevalence/importance of CNS disorders is adequately reflected in current trends of zebrafish research. Addressing this question, the analyses of current trends in zebrafish literature in PubMed database for specific CNS disorders (Figure 1) resulted in several considerations. Notably, drug-induced brain disorders are highly prevalent, societally and clinically important illnesses, whose occurrence rose by 45% in the last decade, making them a major global health problem [16]. Although cannabis remains by far the most commonly used/abused drug, opioids present the greatest harm to the health of users [16]. Importantly, zebrafish possess all opioid [17][18][19], cannabinoid [20], and monoaminergic systems [21][22][23] that play a key role in drug-induced psychiatric disorders.
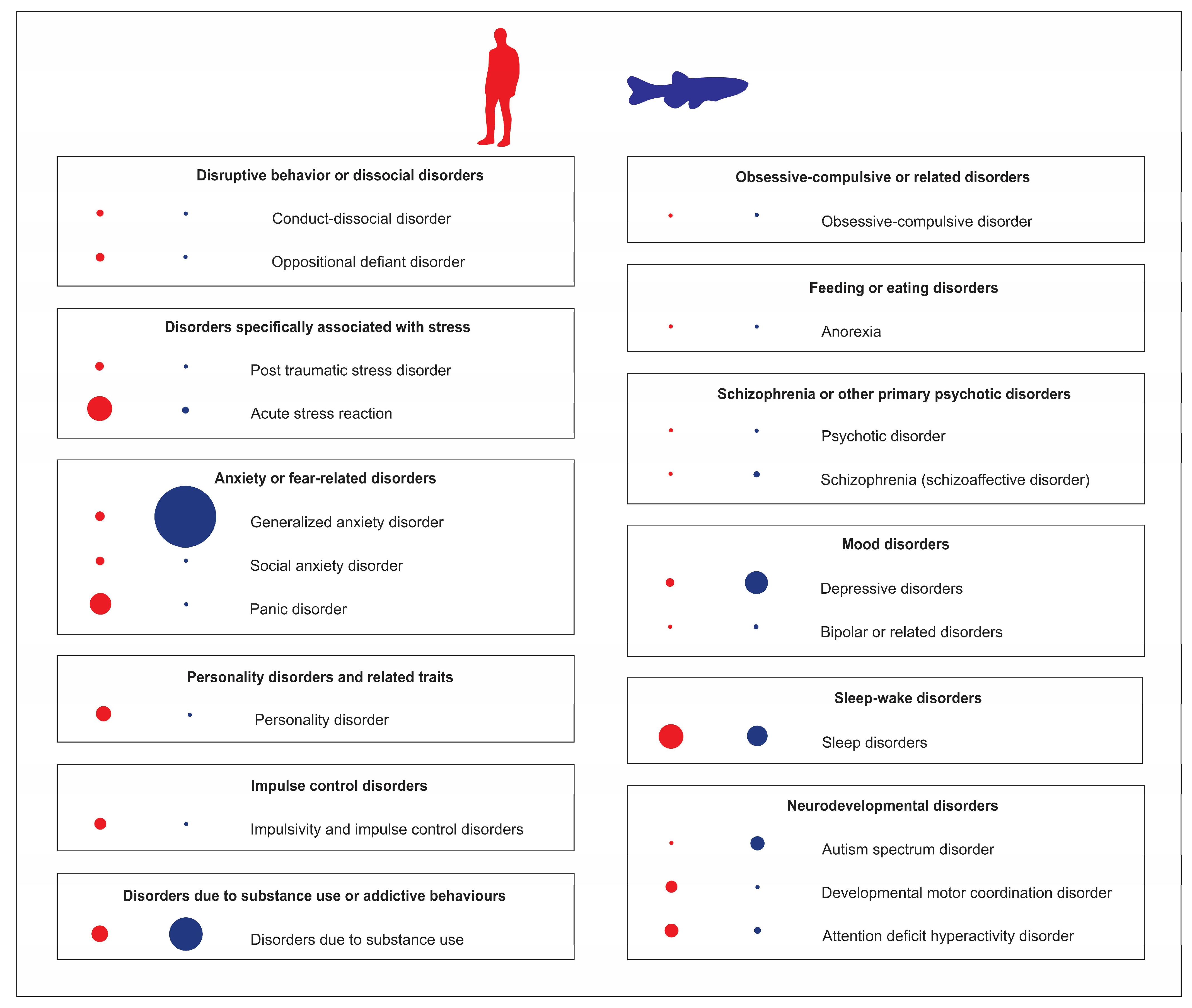
Figure 1. Analyses of Pubmed publications using zebrafish as an animal model of various human psychiatric disorders, compared to their clinical prevalence in adults. Blue dots (on the right) represent the relative number of zebrafish publications on specific psychiatric disorders, red dots (on the left) denote their relative clinical prevalence. The dot size reflects the relative frequency of each parameter.
However, as shown in Figure 1, the most studied psychiatric disorder in zebrafish models is generalized anxiety disorder, which is likely heavily overrepresented in the zebrafish literature (44%) compared to its estimated 7% global clinical prevalence. On the one hand, zebrafish are indeed a sensitive and efficient model system for studying anxiety disorders, with a set of well-described anxiety-like behaviors and easily applicable experimental protocols and assays (e.g., novel tank test; NTT, light dark box test; LDT, open field test; OFT, predator exposure test) that, like their well-established rodent counterparts, typically employ novelty-based or fear-based paradigms (see [24][25][26][27] for a comprehensive review). Paralleling behavioral endpoints, neurochemical and endocrine (e.g., cortisol) biomarkers of zebrafish anxiety are also widely used in modeling affective pathogenesis in fish [24][25][26][27]. Multiple clinically active anxiolytic and anxiogenic drugs also potently modulate anxiety-like behaviors in zebrafish, and can be reliably assessed in fish behavioral assays mentioned above [28]. Additionally, most zebrafish brain regions are well described, having major important neuroanatomical homologues for key mammalian brain regions that control behavior [29]. For example, zebrafish possess medial pallium and habenula [30][31][32], homologous to several brain structures responsible for anxiety-like behaviors in humans.
The activation of zebrafish neuroendocrine hypothalamic-pituitary-interrenal (HPI) axis, physiologically homologous to human hypothalamic-pituitary-adrenal (HPA) axis, triggers the release of cortisol [33], further strongly supporting the use of these fish for studying anxiety spectrum disorders and their pathophysiology [34].
However, there are also clear limitations in zebrafish use to study stress pathobiology. For example, since it is impossible to obtain a sufficient amount of blood without euthanizing the animal (due its small size), the long-term monitoring of stress responses from blood samples is problematic [35]. Moreover, fish live in an aquatic environment where they constantly release hormones and metabolites related to stress responses [36]. Thus, unlike terrestrial vertebrates and humans, zebrafish continuously absorb these substances, which in turn may also play a role in modulating their stress responsivity. Nevertheless, although this factor may contribute to some discrepancy in physiological and behavioral responses to stress in fish vs. humans, there are also multiple well-described and simple experimental protocols to access acute stress responses in zebrafish [34][37]. Thus, despite these environmental differences, the overall neuroendocrine similarity between zebrafish and humans, together with well-described behavioral stress protocols, collectively make zebrafish a reliable model to study stress-related brain disorders.
On the other hand, acute stress studies in zebrafish also present some discrepancies in the existing literature. For example, an analysis of acute stress reaction is currently underrepresented in zebrafish studies (3%), compared to their clinical prevalence of 15% (Figure 1). Described by ICD-11 as “development of transient emotional, somatic, cognitive, or behavioural symptoms as a result of exposure to an event or situation of an extremely threatening or horrific nature”, acute stress reaction differs from post-traumatic stress disorder (PTSD), as the former usually subsides within days after stress, whereas the latter persists for several weeks [38].
Such phenotypic variance highlights several important factors for CNS disease modelling using zebrafish. Consider, for example, a marked difference in the numbers of clinical cases of acute vs. delayed acute severe stress reactions that may correspond to underlying individual differences in stress responsivity between patients, with some subjects being more susceptible to a stress exposure (and developing longer-lasting CNS disturbances) than the others. This aspect is critical for valid CNS disease modelling, since some animals as well may not develop long-lasting deficits without genetic or environmental triggers. Furthermore, the existence of CNS pathologies that are induced by the same factor(s), but occur at different time frames, necessitates detailed phenotyping of the models at different time points. For this, zebrafish may represent a valuable model for time-dependent phenotyping by having a relatively long lifespan (~4 years) with a prolonged duration of the adult state. Such approach has already been implemented in stress studies assessing complex dynamics of behavioral and neurochemical phenotypes in zebrafish affective disorders [39].
Although sleep disorders, especially insomnia, are among the most common human psychiatric disorders (Figure 1), with global prevalence between 10 and 60%, this group is remarkably underrepresented in current zebrafish research, with only 1% of studies exploring insomnia-related behavior. Note, however, that circadian rhythm disorders are rather overrepresented in the zebrafish literature, with 10% of zebrafish studies (vs. 3% of the former global prevalence in humans) [40]. Importantly, zebrafish possess a well-described behavioral sleep state (e.g., circadian-regulated periods of reversible immobility associated with an increased arousal threshold [41][42][43] and sleep rebound in response to sleep deprivation [41][42][44]), as well as neuronal signatures of sleep [45]. Additionally, major neurocircuits responsible for the regulation of sleep–wake cycle are subcortical and evolutionarily conserved across vertebrate species, including zebrafish [41][44][46]. Thus, while some sleep disorders may be difficult or even impossible to recapitulate in zebrafish (e.g., apnea), zebrafish emerge as an important tool to investigate sleep disorders (and related psychiatric disorders), especially insomnia.
Furthermore, because many psychiatric disorders have strong genetic bases [47][48][49][50], it is logical to apply genetic modelling to recapitulate disorder-specific symptoms, and to utilize various omics-based tools to study complex molecular cascades associated with neuropsychiatric disorders. However, while many neuropsychiatric disorders are polygenic in nature [51][52], genome-wide associations studies (GWAS) often report multiple polymorphisms even within a single gene that contribute to the observed clinical phenotypes [53][54][55], further complicating genetic modelling of such conditions. Similarly, multiple transcriptomic studies show altered expression of various brain CNS genes in neuropsychiatric disorders [56][57][58][59][60][61]. Thus, it is logical to consider combining several genetic mutations to properly model specific CNS disorders of interest.
One such genetic animal model targets Alzheimer’s disease to induce a more severe experimental pathogenesis in mice that closely mimics human conditions [62]. For example, 5xFAD mice overexpress two transgenes combining five mutations—Swedish K670N/M671L, London V717I, and Florida I716V hAPP mutations with M146L and L286V hPSEN1 mutations [63], whereas 3xTg mice harbor Swedish K670N/M671L, M146L hPSEN1, and P301L hMAPT mutations [64]. However, to the best of the knowledge, there is a current lack of zebrafish studies with polygenic genetic modelling of psychiatric conditions. For example, one may consider to knockout one of the glutamate receptor (gr) copies, slc6a4a (one of serotonin transporter copies), and a key interleukin (IL), il10 gene, hence breaking proper HPI/HPA axis signaling, inducing monoamines disbalance, and increasing inflammatory response at the same time. Likewise, combining disc1 (disrupted in schizophrenia-1), nrg1 (neuregulin-1), akt1 (AKT serine/threonine kinase 1), and/or dtnbp1a/b (dysbindin-1 homologues) mutations may eventually lead to interesting models of schizophrenia-like conditions in fish. Clearly, albeit rather underdeveloped in fish, such polygenetic models are critically important and translationally relevant, as they may better reflect “true” CNS pathogenesis occurring in human psychiatric disorders.
Moreover, some translational studies may examine the molecular alterations in other (e.g., behavioral and pharmacological) animal models using omics-related tools (e.g., RNA-seq) to find evolutionally conserved biomarkers of CNS disorders that may be crucial for neuropathogenesis in both humans and zebrafish. For example, a widely used model of affective pathology in rodents and zebrafish, the chronic unpredictable stress (see further), reveals multiple transcriptomic changes in the brain that parallel deficits seen in human CNS diseases [39][65]. Specifically, chronic unpredictable stress in zebrafish induces differential expression of genes involved in the inflammation/cytokine-related signaling pathways, mitogen-activated protein kinase (MAPK) signaling, and receptor tyrosine kinases, including signal transducer and activator of transcription (stat) 1b and 4, interleukin 21 receptor (il21r), janus kinase 3 (jak3), and suppressor of cytokine signaling (socs) 1a, all long associated with clinical affective pathology and inflammation [39][65].
Furthermore, such chronic stress alters the expression of multiple endocrine and signaling receptor-related genes, further paralleling human pathology [39]. Interestingly, serpini1-/- knockout zebrafish display anxiety-like behavior, with the expression of closely related genes (e.g., socs1a and sagb) altered based on RNA-seq analysis, supporting their involvement in affective pathology [66]. At the same time, very few such molecular studies have been conducted on other psychiatric disorders (beyond anxiety spectrum) in zebrafish models, clearly necessitating further analyses.
Combining genetic, epigenetic, environmental, behavioral or drug-based experimental models to better recapitulate disorders pathogenesis, also seems timely. For instance, as already noted, only few subjects develop PTSD following a severe acute stress exposure, due to specific molecular or environmental risk factors. The gene-environment interactions (GxE) and sex-environment interactions (SxE) have recently gained an increased recognition in psychiatric disorder modeling [67][68]. GxE and other similar interactions reflect how individual genotypes influences the sensitivity to environmental stimuli that trigger CNS pathogenesis, and their use is highly beneficial for successful experimental modelling of brain disorders [69][70][71][72][73]. For example, using serotonin transporter knockout (5htt a or b) in combination with severe stress exposure may help recapitulate clinical data linking human serotonin transporter 5HTT genetic polymorphisms to affective disorders [74][75]. Likewise, combining schizophrenia-related models (e.g., disc1 knockout) with prenatal inflammatory exposure (e.g., Poly I:C or LPS) and early life stress, may also be relevant to modeling schizophrenia pathogenesis [76].
Another important factor to consider is that aberrant phenotype itself may affect the environment to which an individual is exposed, without direct effects on disorder pathogenesis per se [68]. For example, children affected by a neuropsychiatric disorder (e.g., autism or depression) may be socially isolated by their peers, further impairing their development and behaviors [77]. Taking together, such complex interplay between multiple genetic and environmental factors necessitates novel conceptual and methodological approaches that will target multiple pathogenetic factors in order to create more valid and efficient models of human psychiatric disorders. In general, current zebrafish models usually lack such integrative approaches, clearly calling for further studies in this direction.
References
- Qiu, Z.; Li, X. Non-human Primate Models for Brain Disorders—Towards Genetic Manipulations via Innovative Technology. Neurosci. Bull. 2017, 33, 247–250.
- Kalueff, A.V.; Schmidt, M.V. Novel experimental models and paradigms for neuropsychiatric disorders: Editorial. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 1355–1356.
- Stewart, A.M.; Kalueff, A.V. Developing better and more valid animal models of brain disorders. Behav. Brain Res. 2015, 276, 28–31.
- de Hert, M.; Correll, C.U.; Bobes, J.; Cetkovich-Bakmas, M.; Cohen, D.; Asai, I.; Detraux, J.; Gautam, S.; Möller, H.J.; Ndetei, D.M.; et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry 2011, 10, 52–77.
- Demyttenaere, K.; Bruffaerts, R.; Posada-Villa, J.; Gasquet, I.; Kovess, V.; Lepine, J.P.; Angermeyer, M.C.; Bernert, S.; de Girolamo, G.; Morosini, P.; et al. Prevalence, Severity, and Unmet Need for Treatment of Mental Disorders in the World Health Organization World Mental Health Surveys. J. Am. Med. Assoc. 2004, 291, 2581–2590.
- McCammon, J.M.; Sive, H. Challenges in understanding psychiatric disorders and developing therapeutics: A role for zebrafish. Dis. Model. Mech. 2015, 8, 647–656.
- Kalueff, A.V.; Ren-Patterson, R.F.; LaPorte, J.L.; Murphy, D.L. Domain interplay concept in animal models of neuropsychiatric disorders: A new strategy for high-throughput neurophenotyping research. Behav. Brain Res. 2008, 188, 243–249.
- McGonigle, P.; Ruggeri, B. Animal models of human disease: Challenges in enabling translation. Biochem. Pharmacol. 2014, 87, 162–171.
- Nestler, E.J.; Hyman, S.E. Animal models of neuropsychiatric disorders. Nat. Neurosci. 2010, 13, 1161–1169.
- Kalueff, A.V.; Stewart, A.M.; Gerlai, R. Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol. Sci. 2014, 35, 63–75.
- Curado, S.; Anderson, R.M.; Jungblut, B.; Mumm, J.; Schroeter, E.; Stainier, D.Y. Conditional targeted cell ablation in zebrafish: A new tool for regeneration studies. Dev. Dyn. 2007, 236, 1025–1035.
- Nagel, G.; Szellas, T.; Huhn, W.; Kateriya, S.; Adeishvili, N.; Berthold, P.; Ollig, D.; Hegemann, P.; Bamberg, E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl. Acad. Sci. USA 2003, 100, 13940–13945.
- Zhang, F.; Wang, L.P.; Brauner, M.; Liewald, J.F.; Kay, K.; Watzke, N.; Wood, P.G.; Bamberg, E.; Nagel, G.; Gottschalk, A.; et al. Multimodal fast optical interrogation of neural circuitry. Nature 2007, 446, 633–639.
- Higashijima, S.; Masino, M.A.; Mandel, G.; Fetcho, J.R. Imaging neuronal activity during zebrafish behavior with a genetically encoded calcium indicator. J. Neurophysiol. 2003, 90, 3986–3997.
- Kalueff, A.V.; Gebhardt, M.; Stewart, A.M.; Cachat, J.M.; Brimmer, M.; Chawla, J.S.; Craddock, C.; Kyzar, E.J.; Roth, A.; Landsman, S.; et al. Towards a comprehensive catalog of zebrafish behavior 1.0 and beyond. Zebrafish 2013, 10, 70–86.
- United Nations Office on Drugs and Crime. World Drug Report, E.21.XI.8 ed.; Boom Koninklijke Uitgevers: Boston, MA, USA, 2021; Volume 1.
- Gonzalez-Nunez, V.; Rodriguez, R.E. The zebrafish: A model to study the endogenous mechanisms of pain. ILAR J. 2009, 50, 373–386.
- Sundstrom, G.; Dreborg, S.; Larhammar, D. Concomitant duplications of opioid peptide and receptor genes before the origin of jawed vertebrates. PLoS ONE 2010, 5, e10512.
- Gonzalez-Nunez, V.; Jimenez Gonzalez, A.; Barreto-Valer, K.; Rodriguez, R.E. In vivo regulation of the mu opioid receptor: Role of the endogenous opioid agents. Mol. Med. 2013, 19, 7–17.
- Krug, R.G., 2nd; Clark, K.J. Elucidating cannabinoid biology in zebrafish (Danio rerio). Gene 2015, 570, 168–179.
- van Staden, C.; de Brouwer, G.; Botha, T.L.; Finger-Baier, K.; Brand, S.J.; Wolmarans, D. Dopaminergic and serotonergic modulation of social reward appraisal in zebrafish (Danio rerio) under circumstances of motivational conflict: Towards a screening test for anti-compulsive drug action. Behav. Brain Res. 2020, 379, 112393.
- Sim, H.R.; Choi, T.Y.; Lee, H.J.; Kang, E.Y.; Yoon, S.; Han, P.L.; Choi, S.Y.; Baik, J.H. Role of dopamine D2 receptors in plasticity of stress-induced addictive behaviours. Nat. Commun. 2013, 4, 1579.
- Diana, M. The dopamine hypothesis of drug addiction and its potential therapeutic value. Front. Psychiatry 2011, 2, 64.
- Egan, R.J.; Bergner, C.L.; Hart, P.C.; Cachat, J.M.; Canavello, P.R.; Elegante, M.F.; Elkhayat, S.I.; Bartels, B.K.; Tien, A.K.; Tien, D.H.; et al. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav. Brain Res. 2009, 205, 38–44.
- Kysil, E.V.; Meshalkina, D.A.; Frick, E.E.; Echevarria, D.J.; Rosemberg, D.B.; Maximino, C.; Lima, M.G.; Abreu, M.S.; Giacomini, A.C.; Barcellos, L.J.G.; et al. Comparative Analyses of Zebrafish Anxiety-Like Behavior Using Conflict-Based Novelty Tests. Zebrafish 2017, 14, 197–208.
- Maximino, C.; Marques de Brito, T.; Dias, C.A.; Gouveia, A., Jr.; Morato, S. Scototaxis as anxiety-like behavior in fish. Nat. Protoc. 2010, 5, 209–216.
- Stewart, A.M.; Gaikwad, S.; Kyzar, E.; Kalueff, A.V. Understanding spatio-temporal strategies of adult zebrafish exploration in the open field test. Brain Res. 2012, 1451, 44–52.
- Stewart, A.M.; Nguyen, M.; Poudel, M.K.; Warnick, J.E.; Echevarria, D.J.; Beaton, E.A.; Song, C.; Kalueff, A.V. The failure of anxiolytic therapies in early clinical trials: What needs to be done. Expert Opin. Investig. Drugs 2015, 24, 543–556.
- Porter, B.A.; Mueller, T. The zebrafish amygdaloid complex–functional ground plan, molecular delineation, and everted topology. Front. Neurosci. 2020, 14, 608.
- Amo, R.; Aizawa, H.; Takahoko, M.; Kobayashi, M.; Takahashi, R.; Aoki, T.; Okamoto, H. Identification of the zebrafish ventral habenula as a homolog of the mammalian lateral habenula. J. Neurosci. 2010, 30, 1566–1574.
- Mathuru, A.S.; Jesuthasan, S. The medial habenula as a regulator of anxiety in adult zebrafish. Front. Neural Circuits 2013, 7, 99.
- Jesuthasan, S. Fear, anxiety, and control in the zebrafish. Dev. Neurobiol. 2012, 72, 395–403.
- Wendelaar Bonga, S.E. The stress response in fish. Physiol. Rev. 1997, 77, 591–625.
- Demin, K.A.; Taranov, A.S.; Ilyin, N.P.; Lakstygal, A.M.; Volgin, A.D.; de Abreu, M.S.; Strekalova, T.; Kalueff, A.V. Understanding neurobehavioral effects of acute and chronic stress in zebrafish. Stress 2021, 24, 1–18.
- Lakstygal, A.M.; de Abreu, M.S.; Lifanov, D.A.; Wappler-Guzzetta, E.A.; Serikuly, N.; Alpsyshov, E.T.; Wang, D.; Wang, M.; Tang, Z.; Yan, D.; et al. Zebrafish models of diabetes-related CNS pathogenesis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 92, 48–58.
- Vermeirssen, E.L.; Scott, A.P. Excretion of free and conjugated steroids in rainbow trout (Oncorhynchus mykiss): Evidence for branchial excretion of the maturation-inducing steroid, 17,20 beta-dihydroxy-4-pregnen-3-one. Gen. Comp. Endocrinol. 1996, 101, 180–194.
- Borba, J.V.; Biasuz, E.; Sabadin, G.R.; Savicki, A.C.; Canzian, J.; Luchiari, A.C.; Adedara, I.A.; Rosemberg, D.B. Influence of acute and unpredictable chronic stress on spatio-temporal dynamics of exploratory activity in zebrafish with emphasis on homebase-related behaviors. Behav. Brain Res. 2022, 435, 114034.
- World Health Organization. International Classification of Diseases (ICD) Eleventh Revision; U.S. Department of Health and Human Services: Washington, DC, USA, 2021.
- Demin, K.A.; Lakstygal, A.M.; Krotova, N.A.; Masharsky, A.; Tagawa, N.; Chernysh, M.V.; Ilyin, N.P.; Taranov, A.S.; Galstyan, D.S.; Derzhavina, K.A. Understanding complex dynamics of behavioral, neurochemical and transcriptomic changes induced by prolonged chronic unpredictable stress in zebrafish. Sci. Rep. 2020, 10, 19981.
- Kim, M.J.; Lee, J.H.; Duffy, J.F. Circadian Rhythm Sleep Disorders. J. Clin. Outcomes Manag. 2013, 20, 513–528.
- Yokogawa, T.; Marin, W.; Faraco, J.; Pezeron, G.; Appelbaum, L.; Zhang, J.; Rosa, F.; Mourrain, P.; Mignot, E. Characterization of sleep in zebrafish and insomnia in hypocretin receptor mutants. PLoS Biol. 2007, 5, e277.
- Zhdanova, I.V. Sleep in zebrafish. Zebrafish 2006, 3, 215–226.
- Prober, D.A.; Rihel, J.; Onah, A.A.; Sung, R.J.; Schier, A.F. Hypocretin/orexin overexpression induces an insomnia-like phenotype in zebrafish. J. Neurosci. 2006, 26, 13400–13410.
- Appelbaum, L.; Wang, G.X.; Maro, G.S.; Mori, R.; Tovin, A.; Marin, W.; Yokogawa, T.; Kawakami, K.; Smith, S.J.; Gothilf, Y.; et al. Sleep-wake regulation and hypocretin-melatonin interaction in zebrafish. Proc. Natl. Acad. Sci. USA 2009, 106, 21942–21947.
- Leung, L.C.; Wang, G.X.; Madelaine, R.; Skariah, G.; Kawakami, K.; Deisseroth, K.; Urban, A.E.; Mourrain, P. Neural signatures of sleep in zebrafish. Nature 2019, 571, 198–204.
- Berman, J.R.; Skariah, G.; Maro, G.S.; Mignot, E.; Mourrain, P. Characterization of two melanin-concentrating hormone genes in zebrafish reveals evolutionary and physiological links with the mammalian MCH system. J. Comp. Neurol. 2009, 517, 695–710.
- Sullivan, P.F.; Kendler, K.S.; Neale, M.C. Schizophrenia as a complex trait: Evidence from a meta-analysis of twin studies. Arch. Gen. Psychiatry 2003, 60, 1187–1192.
- Bailey, A.J.; Braeutigam, S.; Jousmäki, V.; Swithenby, S.J. Abnormal activation of face processing systems at early and intermediate latency in individuals with autism spectrum disorder: A magnetoencephalographic study. Eur. J. Neurosci. 2005, 21, 2575–2585.
- Rosenberg, R.E.; Daniels, A.M.; Law, J.K.; Law, P.A.; Kaufmann, W.E. Trends in autism spectrum disorder diagnoses: 1994–2007. J. Autism Dev. Disord. 2009, 39, 1099–1111.
- Banaschewski, T.; Becker, K.; Scherag, S.; Franke, B.; Coghill, D. Molecular genetics of attention-deficit/hyperactivity disorder: An overview. Eur. Child Adolesc. Psychiatry 2010, 19, 237–257.
- Fullerton, J.M.; Nurnberger, J.I. Polygenic risk scores in psychiatry: Will they be useful for clinicians? F1000 Res. 2019, 8, F1000.
- Palk, A.C.; Dalvie, S.; De Vries, J.; Martin, A.R.; Stein, D.J. Potential use of clinical polygenic risk scores in psychiatry–ethical implications and communicating high polygenic risk. Philos. Ethics Humanit. Med. 2019, 14, 4.
- Levey, D.F.; Stein, M.B.; Wendt, F.R.; Pathak, G.A.; Zhou, H.; Aslan, M.; Quaden, R.; Harrington, K.M.; Nuñez, Y.Z.; Overstreet, C. Bi-ancestral depression GWAS in the Million Veteran Program and meta-analysis in> 1.2 million individuals highlight new therapeutic directions. Nat. Neurosci. 2021, 24, 954–963.
- Adams, M.J.; Howard, D.M.; Luciano, M.; Clarke, T.-K.; Davies, G.; Hill, W.D.; Smith, D.; Deary, I.J.; Porteous, D.J.; McIntosh, A.M. Genetic stratification of depression by neuroticism: Revisiting a diagnostic tradition. Psychol. Med. 2020, 50, 2526–2535.
- Horwitz, T.; Lam, K.; Chen, Y.; Xia, Y.; Liu, C. A decade in psychiatric GWAS research. Mol. Psychiatry 2019, 24, 378–389.
- Mehta, D.; Menke, A.; Binder, E.B. Gene expression studies in major depression. Curr. Psychiatry Rep. 2010, 12, 135–144.
- Verma, P.; Shakya, M. Transcriptomics and sequencing analysis of gene expression profiling for major depressive disorder. Indian J. Psychiatry 2021, 63, 549.
- Mahajan, G.J.; Vallender, E.J.; Garrett, M.R.; Challagundla, L.; Overholser, J.C.; Jurjus, G.; Dieter, L.; Syed, M.; Romero, D.G.; Benghuzzi, H. Altered neuro-inflammatory gene expression in hippocampus in major depressive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 82, 177–186.
- Jansen, R.; Penninx, B.; Madar, V.; Xia, K.; Milaneschi, Y.; Hottenga, J.; Hammerschlag, A.; Beekman, A.; Van Der Wee, N.; Smit, J. Gene expression in major depressive disorder. Mol. Psychiatry 2016, 21, 339–347.
- Chang, X.; Liu, Y.; Hahn, C.; Gur, R.; Sleiman, P.; Hakonarson, H. RNA-seq analysis of amygdala tissue reveals characteristic expression profiles in schizophrenia. Transl. Psychiatry 2017, 7, e1203.
- Xu, J.; Sun, J.; Chen, J.; Wang, L.; Li, A.; Helm, M.; Dubovsky, S.L.; Bacanu, S.-A.; Zhao, Z.; Chen, X. RNA-Seq analysis implicates dysregulation of the immune system in schizophrenia. BMC Genom. 2012, 13, S2.
- Myers, A.; McGonigle, P. Overview of transgenic mouse models for Alzheimer’s disease. Curr. Protoc. Neurosci. 2019, 89, e81.
- Oakley, H.; Cole, S.L.; Logan, S.; Maus, E.; Shao, P.; Craft, J.; Guillozet-Bongaarts, A.; Ohno, M.; Disterhoft, J.; Van Eldik, L. Intraneuronal β-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: Potential factors in amyloid plaque formation. J. Neurosci. 2006, 26, 10129–10140.
- Oddo, S.; Caccamo, A.; Shepherd, J.D.; Murphy, M.P.; Golde, T.E.; Kayed, R.; Metherate, R.; Mattson, M.P.; Akbari, Y.; LaFerla, F.M. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: Intracellular Aβ and synaptic dysfunction. Neuron 2003, 39, 409–421.
- Huang, V.; Butler, A.A.; Lubin, F.D. Telencephalon transcriptome analysis of chronically stressed adult zebrafish. Sci. Rep. 2019, 9, 1379.
- Han, S.; Fei, F.; Sun, S.; Zhang, D.; Dong, Q.; Wang, X.; Wang, L. Increased anxiety was found in serpini1 knockout zebrafish larval. Biochem. Biophys. Res. Commun. 2021, 534, 1013–1019.
- Genario, R.; de Abreu, M.S.; Giacomini, A.C.; Demin, K.A.; Kalueff, A.V. Sex differences in behavior and neuropharmacology of zebrafish. Eur. J. Neurosci. 2019, 52, 2586–2603.
- Jaffee, S.R.; Price, T.S. Gene–environment correlations: A review of the evidence and implications for prevention of mental illness. Mol. Psychiatry 2007, 12, 432–442.
- Baye, T.M.; Abebe, T.; Wilke, R.A. Genotype–environment interactions and their translational implications. Pers. Med. 2011, 8, 59–70.
- Duncan, L.E.; Pollastri, A.R.; Smoller, J.W. Mind the gap: Why many geneticists and psychological scientists have discrepant views about gene–environment interaction (G × E) research. Am. Psychol. 2014, 69, 249.
- Hein, R.; Beckmann, L.; Chang-Claude, J. Sample size requirements for indirect association studies of gene–environment interactions (G × E). Genet. Epidemiol. Off. Publ. Int. Genet. Epidemiol. Soc. 2008, 32, 235–245.
- Le Strat, Y.; Ramoz, N.; Gorwood, P. The role of genes involved in neuroplasticity and neurogenesis in the observation of a gene-environment interaction (GxE) in schizophrenia. Curr. Mol. Med. 2009, 9, 506–518.
- Kalueff, A.; Wheaton, M.; Murphy, D. What’s wrong with my mouse model?: Advances and strategies in animal modeling of anxiety and depression. Behav. Brain Res. 2007, 179, 1–18.
- Caspi, A.; Sugden, K.; Moffitt, T.E.; Taylor, A.; Craig, I.W.; Harrington, H.; McClay, J.; Mill, J.; Martin, J.; Braithwaite, A. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science 2003, 301, 386–389.
- Ancelin, M.-L.; Ryan, J. 5-HTTLPR× stress hypothesis: Is the debate over? Mol. Psychiatry 2018, 23, 2116–2117.
- Jaaro-Peled, H.; Ayhan, Y.; Pletnikov, M.V.; Sawa, A. Review of pathological hallmarks of schizophrenia: Comparison of genetic models with patients and nongenetic models. Schizophr. Bull. 2010, 36, 301–313.
- Homberg, J.R.; Kyzar, E.J.; Nguyen, M.; Norton, W.H.; Pittman, J.; Poudel, M.K.; Gaikwad, S.; Nakamura, S.; Koshiba, M.; Yamanouchi, H. Understanding autism and other neurodevelopmental disorders through experimental translational neurobehavioral models. Neurosci. Biobehav. Rev. 2016, 65, 292–312.
More
Information
Subjects:
Others
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
754
Revisions:
2 times
(View History)
Update Date:
19 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




