Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Yang, M.; Vanderwert, E.; Kimchi, E.T.; Staveley-O’carroll, K.F.; Li, G. Natural Killer Cells in Liver Fibrosis. Encyclopedia. Available online: https://encyclopedia.pub/entry/44443 (accessed on 07 February 2026).
Yang M, Vanderwert E, Kimchi ET, Staveley-O’carroll KF, Li G. Natural Killer Cells in Liver Fibrosis. Encyclopedia. Available at: https://encyclopedia.pub/entry/44443. Accessed February 07, 2026.
Yang, Ming, Ethan Vanderwert, Eric T. Kimchi, Kevin F. Staveley-O’carroll, Guangfu Li. "Natural Killer Cells in Liver Fibrosis" Encyclopedia, https://encyclopedia.pub/entry/44443 (accessed February 07, 2026).
Yang, M., Vanderwert, E., Kimchi, E.T., Staveley-O’carroll, K.F., & Li, G. (2023, May 17). Natural Killer Cells in Liver Fibrosis. In Encyclopedia. https://encyclopedia.pub/entry/44443
Yang, Ming, et al. "Natural Killer Cells in Liver Fibrosis." Encyclopedia. Web. 17 May, 2023.
Copy Citation
Natural killer (NK) cells are a key component of innate immunity and have miscellaneous roles in liver health and disease. Accumulating evidence shows that NK cells play dual roles in the development and progression of liver fibrosis, including profibrotic and anti-fibrotic functions.
liver fibrosis
hepatic stellate cells
natural killer cells
1. Introduction
Liver fibrosis is associated with the progression of various chronic liver diseases, such as hepatitis viral infection and alcoholic or non-alcoholic steatohepatitis (ASH or NASH). It is characterized by the abnormal accumulation of extracellular matrix proteins (ECM, e.g., collagen and integrin) and impaired ECM degradation (e.g., metalloproteinases) [1]. Hepatic stellate cells (HSCs), the vitamin A-storing cells in the healthy liver, can be activated and differentiated to ECM-producing myofibroblasts in chronic liver diseases, leading to liver fibrosis [2][3]. Without effective treatments, liver fibrosis can progress to cirrhosis, causing liver cancer or failure. Unfortunately, there still lacks approved treatments for liver fibrosis. Current strategies for liver fibrosis treatment mainly are limited to the protection and prevention of liver injury and fibrosis-causing factors. These strategies include treatments of anti-etiology (e.g., anti-viral treatment and reduction in alcohol consumption), anti-inflammation, antioxidant stress, inhibition of cell apoptosis, genetic and epigenetic modification, inhibition of HSC activation and proliferation, and promotion of ECM degradation [4][5][6]. Thus, exploration of new treatment options for liver fibrosis is urgently needed.
In the liver, both innate and adaptive immune cells play a pivotal role in the development of liver fibrosis and its associated liver diseases [7], such as alcoholic liver disease (ALD) [8][9], non-alcoholic fatty liver disease (NAFLD) [10][11], and liver cancer [12][13]. Natural killer (NK) cells are an essential component of innate immunity. In humans, NK cells comprise approximately 5–15% of circulating lymphocytes [14]. About half of the intrahepatic lymphocytes (50%) are NK cells, and they modulate the intrahepatic immune response in both physiological and pathological conditions [15]. In laboratory inbred mice, NK cells constitute about 2–5% of lymphocytes in spleens and bone marrows, and the number of NK cells doubles in wild-type mice [16]. In mouse livers, NK cells comprise about 2–5% of non-parenchymal cells (NPCs), and the frequency can be increased to around 10% in NASH livers of mice treated with high-fat and high-sugar diets [17][18].
NK cells have miscellaneous roles in liver health and disease [19][20]. For example, NK cells can be recruited by C-X-C motif chemokine ligand 9 (CXCL9), produced in activated CD103+DCs (dendritic cells), into the tumor microenvironment to express granzyme B, interferon-gamma (IFN-γ), and tumor necrosis factor-alpha (TNF-α) to kill HCC cells [21]. NK cells also have anti-viral infection capabilities. A study showed that there was a negative correlation between the deoxyribonucleic acid (DNA) titer of hepatitis B viruses and the frequency of CD56brightNK cells in CHB patients with chronic hepatitis B (CHB) and peginterferon alpha-2a treatment [22]. In addition, the expression of interferon alpha receptor 2 (IFNAR2) and NK cell p46-related protein (NKp46) in NK cells was remarkably increased in patients with functional cure compared to that in CHB patients without functional cure. Furthermore, NK cells are implicated in the pathogenesis of liver fibrosis. The frequency of peripheral blood NK cells (CD3−CD16+CD56+) was increased in patients with alcoholic liver fibrosis (ALF) compared to that in patients without ALF. NK cell frequency was negatively correlated with total T cell frequency but positively associated with the percentage of CD3−CD8+ cells [23]. In the murine NASH model, DX5+ (anti-CD49b antibody) NKp46+NK cells can prevent liver fibrosis progression by suppressing M2-like macrophage polarization and the expression of profibrotic genes (e.g., Tgfb1 encoding transforming growth factor-beta 1) [24].
2. The Phenotypes of NK Cells in the Liver
In the liver, NK cells can be divided into two main populations: transient conventional NK (cNK) cells and liver resident NK (lr-NK) cells [15]. Mouse lr-NK cells are CD49a+DX5−NK cells, while their cNK cells are CD49a−DX5+NK cells [25]. The phenotype of “memory-like” NK (ml-NK) cells in mice is the same as the phenotype of lr-NK cells or CD49a+DX5−NK cells. Human cNK cells can be subdivided into CD56dimCD16+NK cells and CD56brightCD16−NK cells. In the human liver, CD56dimNK cells and CD56brightNK cells have similar frequencies [26]. Human liver CD56brightlr-NK cells are CCR5+CXCR6+CD69+NK cells, whereas CD56dimcNK cells lack expression of CD69, CCR5, and CXCR6 [27]. Human liver ml-NK cells are CXCR6+CD94/NKG2C+ (killer cell lectin-like receptor C2) NK cells [27]. The maturation of NK cells can be defined for four populations by characterizing the expression of CD11b and CD27 (Figure 1A), including CD11b−CD27−NK cells, CD11b−CD27+NK cells, CD11b+CD27+NK cells, and CD11b+CD27−NK cells [28][29]. CD11b−CD27−NK cells are immature NK cells with differentiation potential, both CD11b−CD27+NK cells and CD11b+CD27+NK cells have the best ability to secrete cytokines, and CD11b+CD27−NK cells display high cytolytic function [28]. Human NK cells can also be classified into three functional subsets (Figure 1B): tolerant NK cells (CD56brightCD27−CD11b−), regulatory NK cells (CD56brightCD27+CD11b+/−), and cytotoxic NK cells (CD56dimCD27−CD11b+) [30].
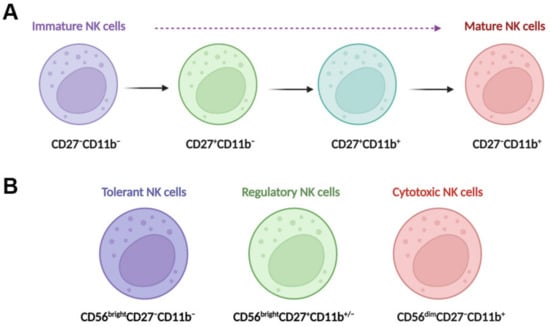
Figure 1. NK cell maturation and functional subsets. (A) Maturing NK cells can be divided into four populations by characterizing the expression of CD11b and CD27; these populations include CD11b−CD27−NK cells, CD11b−CD27+NK cells, CD11b+CD27+NK cells, and CD11b+CD27−NK cells. CD11b−CD27−NK cells are immature NK cells with differentiation potential, both CD11b−CD27+NK cells and CD11b+CD27+NK cells have the best ability to secrete cytokines, and CD11b+CD27−NK cells display high cytolytic function. (B) Human NK cells can also be classified into three functional subsets, tolerant NK cells (CD56brightCD27−CD11b−), regulatory NK cells (CD56brightCD27+CD11b+/−), and cytotoxic NK cells (CD56dimCD27−CD11b+).
Change in NK phenotypes happens in liver disease. For example, peripheral activated CD56brightNK cells in patients with hepatitis B virus (HBV)-related decompensated liver cirrhosis (HBV-DLC) have been shown to have a phenotype with an increased expression of natural killer group 2, member D (NKG2D), perforin, and granzyme A/B and a decreased expression of inhibitory receptor CD158b1/2 compared NK cells from healthy controls, showing an immune activation status [31]. In contrast, the circulating CD56dimNK cells express low levels of CD107a and perforin with an impaired cytolytic capacity [31].
Single-cell RNA-sequencing (scRNA-seq) data illustrate that there are several subtypes of NK cells in chronic liver disease, which display multiple roles based on the different gene expressions. For example, scRNA-seq data analysis showed that the subpopulation of NK cells expressing SELL, a gene encoding the cell surface adhesion molecule L-selectin, was proliferated in alcohol-induced cirrhotic livers, whereas the subtype of NK cells expressing XCL2 (X-C motif chemokine ligand 2) was enriched in the healthy livers [32]. The heterogeneity of peripheral NK cells has also been shown in the development of NASH from simple steatosis with a decreased expression of NK cell activation marker natural cytotoxicity receptor 3 (NCR3, also known as NKp30) in NASH [33]. In addition, several intracellular signaling mediators were less activated, including in the phosphorylation of extracellular signal-regulated protein kinases (pERK) and the phosphorylation of signal transducer and activator of transcription (pSTATs) 1, 2, 3, and 5 [33].
3. The Profibrotic Function of NK Cells
NK cells isolated from patients with chronic hepatitis C virus (HCV) infection and liver fibrosis (METAVIR fibrosis scores: F1 and F4) compared to NK cells isolated from healthy donors showed a significant reduction in the expression of NK activation markers CD107a (lysosomal-associated membrane protein 1, LAMP-1) and INF-γ [34]. In addition, these NK cells from HCV patients significantly promoted the proliferation of co-cultured human HSC cell line LX-2 cells, especially for the subpopulation that expressed intermediate intensity of α-smooth muscle actin (α-SMA) with small sizes according to the forward scatter profile in flow cytometry gating [34]. In addition, hepatic NKp44+NK cells from patients with HCV infection were positively correlated with liver fibrosis and viral load, producing TNF-α to promote liver injury [35]. In patients with HBV infection, transforming growth factor-beta (TGF-β) produced by activated HSCs can suppress the anti-fibrotic effect of NK cells by suppressing NK cell degranulation and IFN-γ production [36]. In patients with severe alcoholic hepatitis (SAH), NK cells can induce lysis of endothelial progenitor cells via the CX3CR1/fractalkine axis to promote inflammation and SAH progression [37]. Overall, in different liver disease conditions, NK cells have been demonstrated to be positively associated with the progression of liver fibrosis.
Recent studies showed that NK cells have long-term graft survival in patients with liver transplantation [38]. The recurrence of HCV infection in patients with liver transplantation can cause graft cirrhosis. The mismatch between the killer cell immunoglobulin-like receptor, two Ig domains, and long cytoplasmic tail 3 (KIR2DL3) and the ligands of human leukocyte antigen (HLA) class I antigens induces the progression of hepatitis to liver fibrosis [39]. Thus, inhibition of NK cell-mediated HSC activation in liver injury can prevent liver fibrosis.
4. The Anti-Fibrotic Function of NK Cells
NK cells have also been shown to display anti-fibrotic effects in the liver. For example, CD27+CD11b+ NK cells can display cytotoxicity against activated HSCs via the interactions of integrin alpha-4 (Itga4, also known as CD49d)–vascular cell adhesion molecule 1 (VCAM1), which can be suppressed by depletion of prostaglandin E receptor 3 (EP3) [40]. Mechanistically, activation of EP3 can increase the nuclear translocation of phosphorylated Spi-C transcription factor (Spic) by regulating protein kinase C (PKC) to upregulate Itga4 expression in NK cells. The binding of NK cells and activated HSC cells is mediated by the interaction of Itga4 expressed by NK cells with VCAM1 expressed by activated HSCs [40], as shown in Figure 2. NK cells activated by natural product curcumin induced senescence of LX-2 cells and promoted fibrotic cell clearance by stimulating granule exocytosis [41]. The increased expression of NKG2D ligand major histocompatibility complex class I chain-related gene A (MICA) and UL16-binding protein 2 (ULBP2) on senescent LX-2 cells activate this process [41]. MICA is a polymorphic gene, and its alleles have been demonstrated to be associated with the histologic features of NASH such as liver inflammation and fibrosis [42]. However, the anti-fibrotic effect of NK cells can be suppressed by activated HSCs via producing TGF-β [43]. Therefore, understanding the molecular mechanisms that mediate the interaction of NK cells with activated HSCs or myofibroblasts can improve NK cell-mediated anti-fibrotic effects.
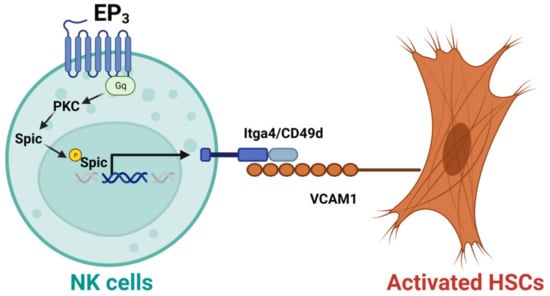
Figure 2. Activation of EP3 can improve the cytotoxicity of CD27+CD11b+ NK cells against activated hepatic stellate cells (HSCs). Mechanistically, activation of prostaglandin E receptor 3 (EP3) can increase the nuclear translocation of phosphorylated Spi-C transcription factor (Spic) by regulating protein kinase C (PKC) to upregulate integrin alpha-4 (Itga4, or CD49d) in NK cells. The binding of NK cells with activated HSCs is mediated by the interaction of Itga4 with vascular cell adhesion molecule 1 (VCAM1).
In addition, the anti-fibrotic effects of NK cells, as well as HSC activation, can be regulated by platelets. Platelets contain proteins and growth factors required for liver regeneration and repair [44], playing important roles in chronic liver diseases, including liver fibrosis [45][46]. For example, the platelet count can be used as a simple, non-invasive index to evaluate the degree of liver fibrosis in patients with CHB [47]. In addition, platelet-derived growth factor-β (PDGF-B) can activate HSCs to promote liver fibrosis [48]. PDGF-D not only enhances human NK cell effector functions by binding to the NKp44 receptor, it regulates IL-15-induced human NK cell survival by binding to PDGF receptor-beta [49]. Platelets also play an important role in NASH pathogenesis by promoting inflammatory cell accumulation, steatosis, and liver injury [50]. However, its function on NK cells remains to be further explored.
References
- Zhao, X.; Chen, J.; Sun, H.; Zhang, Y.; Zou, D. New insights into fibrosis from the ECM degradation perspective: The macrophage-MMP-ECM interaction. Cell Biosci. 2022, 12, 117.
- Kong, M.; Dong, W.; Kang, A.; Kuai, Y.; Xu, T.; Fan, Z.; Shi, L.; Sun, D.; Lu, Y.; Li, Z.; et al. Regulatory role and translational potential of CCL11 in liver fibrosis. Hepatology, 2023; Online ahead of print.
- Knorr, J.; Kaufmann, B.; Inzaugarat, M.E.; Holtmann, T.M.; Geisler, L.; Hundertmark, J.; Kohlhepp, M.S.; Boosheri, L.M.; Chilin-Fuentes, D.R.; Birmingham, A.; et al. Interleukin-18 signaling promotes activation of hepatic stellate cells in mouse liver fibrosis. Hepatology, 2022; Online ahead of print.
- Tan, Z.; Sun, H.; Xue, T.; Gan, C.; Liu, H.; Xie, Y.; Yao, Y.; Ye, T. Liver Fibrosis: Therapeutic Targets and Advances in Drug Therapy. Front. Cell. Dev. Biol. 2021, 9, 730176.
- Shen, M.; Li, Y.; Wang, Y.; Shao, J.; Zhang, F.; Yin, G.; Chen, A.; Zhang, Z.; Zheng, S. N(6)-methyladenosine modification regulates ferroptosis through autophagy signaling pathway in hepatic stellate cells. Redox. Biol. 2021, 47, 102151.
- Koyama, Y.; Xu, J.; Liu, X.; Brenner, D.A. New Developments on the Treatment of Liver Fibrosis. Dig. Dis. 2016, 34, 589–596.
- Huby, T.; Gautier, E.L. Immune cell-mediated features of non-alcoholic steatohepatitis. Nat. Rev. Immunol. 2022, 22, 429–443.
- Cheng, C.; Zhang, Q.; Li, Y.; Jiang, J.; Xie, L.; Shen, H.; Wu, D.; Zhang, H.; Zhang, H.; Wang, X.; et al. Interplay Between Liver Type 1 Innate Lymphoid Cells and NK Cells Drives the Development of Alcoholic Steatohepatitis. Cell. Mol. Gastroenterol. Hepatol. 2022, 15, 261–274.
- Zhang, F.; Little, A.; Zhang, H. Chronic alcohol consumption inhibits peripheral NK cell development and maturation by decreasing the availability of IL-15. J. Leukoc. Biol. 2017, 101, 1015–1027.
- Stiglund, N.; Strand, K.; Cornillet, M.; Stål, P.; Thorell, A.; Zimmer, C.L.; Näslund, E.; Karlgren, S.; Nilsson, H.; Mellgren, G.; et al. Retained NK Cell Phenotype and Functionality in Non-alcoholic Fatty Liver Disease. Front. Immunol. 2019, 10, 1255.
- Sakamoto, Y.; Yoshio, S.; Doi, H.; Mori, T.; Matsuda, M.; Kawai, H.; Shimagaki, T.; Yoshikawa, S.; Aoki, Y.; Osawa, Y.; et al. Increased Frequency of Dysfunctional Siglec-7(−)CD57(+)PD-1(+) Natural Killer Cells in Patients With Non-alcoholic Fatty Liver Disease. Front. Immunol. 2021, 12, 603133.
- Zhang, P.F.; Gao, C.; Huang, X.Y.; Lu, J.C.; Guo, X.J.; Shi, G.M.; Cai, J.B.; Ke, A.W. Cancer cell-derived exosomal circUHRF1 induces natural killer cell exhaustion and may cause resistance to anti-PD1 therapy in hepatocellular carcinoma. Mol. Cancer 2020, 19, 110.
- Park, D.J.; Sung, P.S.; Kim, J.H.; Lee, G.W.; Jang, J.W.; Jung, E.S.; Bae, S.H.; Choi, J.Y.; Yoon, S.K. EpCAM-high liver cancer stem cells resist natural killer cell-mediated cytotoxicity by upregulating CEACAM1. J. Immunother. Cancer 2020, 8, e000301.
- Freud, A.G.; Mundy-Bosse, B.L.; Yu, J.; Caligiuri, M.A. The Broad Spectrum of Human Natural Killer Cell Diversity. Immunity 2017, 47, 820–833.
- Mikulak, J.; Bruni, E.; Oriolo, F.; Di Vito, C.; Mavilio, D. Hepatic Natural Killer Cells: Organ-Specific Sentinels of Liver Immune Homeostasis and Physiopathology. Front. Immunol. 2019, 10, 946.
- Abel, A.M.; Yang, C.; Thakar, M.S.; Malarkannan, S. Natural Killer Cells: Development, Maturation, and Clinical Utilization. Front. Immunol. 2018, 9, 1869.
- Yang, M.; Kimchi, E.T.; Staveley-O’Carroll, K.F.; Li, G. Astaxanthin Prevents Diet-Induced NASH Progression by Shaping Intrahepatic Immunity. Int. J. Mol. Sci. 2021, 22, 11037.
- Yang, M.; Qi, X.; Li, N.; Kaifi, J.T.; Chen, S.; Wheeler, A.A.; Kimchi, E.T.; Ericsson, A.C.; Scott Rector, R.; Staveley-O’Carroll, K.F.; et al. Western diet contributes to the pathogenesis of non-alcoholic steatohepatitis in male mice via remodeling gut microbiota and increasing production of 2-oleoylglycerol. Nat. Commun. 2023, 14, 228.
- Yu, L.; Sun, L.; Liu, X.; Wang, X.; Yan, H.; Pu, Q.; Xie, Y.; Jiang, Y.; Du, J.; Yang, Z. The imbalance between NKG2A and NKG2D expression is involved in NK cell immunosuppression and tumor progression of patients with hepatitis B virus-related hepatocellular carcinoma. Hepatol. Res. 2023, 53, 417–431.
- Li, H.J.; Yang, N.; Mu, X.; Tang, L.; Wang, S.S.; Zhou, C.B.; Yuan, J.H.; Wang, H.Y.; Yu, Y.Y.; Li, J.; et al. Reduction of natural killer cells is associated with poor outcomes in patients with hepatitis B virus-related acute-on-chronic liver failure. Hepatol. Int. 2022, 16, 1398–1411.
- Wang, S.; Wu, Q.; Chen, T.; Su, R.; Pan, C.; Qian, J.; Huang, H.; Yin, S.; Xie, H.; Zhou, L.; et al. Blocking CD47 promotes antitumour immunity through CD103(+) dendritic cell-NK cell axis in murine hepatocellular carcinoma model. J. Hepatol. 2022, 77, 467–478.
- Cao, W.; Lu, H.; Zhang, L.; Wang, S.; Deng, W.; Jiang, T.; Lin, Y.; Yang, L.; Bi, X.; Lu, Y.; et al. Functional molecular expression of nature killer cells correlated to HBsAg clearance in HBeAg-positive chronic hepatitis B patients during PEG-IFN α-2a therapy. Front. Immunol. 2022, 13, 1067362.
- Zuluaga, P.; Teniente-Serra, A.; Fuster, D.; Quirant-Sánchez, B.; Hernandez-Rubio, A.; Martínez-Cáceres, E.; Muga, R. Increased Natural Killer Cells Are Associated with Alcohol Liver Fibrosis and with T Cell and Cytotoxic Subpopulations Change. J. Clin. Med. 2022, 11, 305.
- Tosello-Trampont, A.C.; Krueger, P.; Narayanan, S.; Landes, S.G.; Leitinger, N.; Hahn, Y.S. NKp46(+) natural killer cells attenuate metabolism-induced hepatic fibrosis by regulating macrophage activation in mice. Hepatology 2016, 63, 799–812.
- Peng, H.; Jiang, X.; Chen, Y.; Sojka, D.K.; Wei, H.; Gao, X.; Sun, R.; Yokoyama, W.M.; Tian, Z. Liver-resident NK cells confer adaptive immunity in skin-contact inflammation. J. Clin. Investig. 2013, 123, 1444–1456.
- Highton, A.J.; Schuster, I.S.; Degli-Esposti, M.A.; Altfeld, M. The role of natural killer cells in liver inflammation. Semin. Immunopathol. 2021, 43, 519–533.
- Hudspeth, K.; Donadon, M.; Cimino, M.; Pontarini, E.; Tentorio, P.; Preti, M.; Hong, M.; Bertoletti, A.; Bicciato, S.; Invernizzi, P.; et al. Human liver-resident CD56(bright)/CD16(neg) NK cells are retained within hepatic sinusoids via the engagement of CCR5 and CXCR6 pathways. J. Autoimmun. 2016, 66, 40–50.
- Fu, B.; Wang, F.; Sun, R.; Ling, B.; Tian, Z.; Wei, H. CD11b and CD27 reflect distinct population and functional specialization in human natural killer cells. Immunology 2011, 133, 350–359.
- Chiossone, L.; Chaix, J.; Fuseri, N.; Roth, C.; Vivier, E.; Walzer, T. Maturation of mouse NK cells is a 4-stage developmental program. Blood 2009, 113, 5488–5496.
- Fu, B.; Tian, Z.; Wei, H. Subsets of human natural killer cells and their regulatory effects. Immunology 2014, 141, 483–489.
- Jiang, Y.; Chen, Y.; Chen, L.; Yao, W.; Guan, J.; Liu, X.; Wei, X.; Lin, X. Impaired circulating CD56(dim) NK cells are associated with decompensation of HBV-related cirrhosis. Hum. Immunol. 2020, 81, 32–40.
- Ren, A.; He, W.; Rao, J.; Ye, D.; Cheng, P.; Jian, Q.; Fu, Z.; Zhang, X.; Deng, R.; Gao, Y.; et al. Dysregulation of innate cell types in the hepatic immune microenvironment of alcoholic liver cirrhosis. Front. Immunol. 2023, 14, 1034356.
- Waller, K.J.; Saihi, H.; Li, W.; Brindley, J.H.; De Jong, A.; Syn, W.K.; Bessant, C.; Alazawi, W. Single-cell phenotypes of peripheral blood immune cells in early and late stages of non-alcoholic fatty liver disease. Clin. Mol. Hepatol. 2023, 29, 417–432.
- Amer, J.; Salhab, A.; Doron, S.; Morali, G.; Safadi, R. A novel flow cytometry tool for fibrosis scoring through hepatic stellate cell differentiation. Cytometry A 2018, 93, 427–435.
- Nel, I.; Lucar, O.; Petitdemange, C.; Béziat, V.; Lapalus, M.; Bédossa, P.; Debré, P.; Asselah, T.; Marcellin, P.; Vieillard, V. Accumulation of Intrahepatic TNF-α-Producing NKp44+ NK Cells Correlates With Liver Fibrosis and Viral Load in Chronic HCV Infection. Medicine 2016, 95, e3678.
- Shi, J.; Zhao, J.; Zhang, X.; Cheng, Y.; Hu, J.; Li, Y.; Zhao, X.; Shang, Q.; Sun, Y.; Tu, B.; et al. Activated hepatic stellate cells impair NK cell anti-fibrosis capacity through a TGF-β-dependent emperipolesis in HBV cirrhotic patients. Sci. Rep. 2017, 7, 44544.
- Sehgal, R.; Kaur, S.; Shasthry, S.M.; Agrawal, T.; Dwivedi, V.; Seth, D.; Ramakrishna, G.; Sarin, S.K.; Trehanpati, N. Natural Killer Cells Contribute to Pathogenesis of Severe Alcoholic Hepatitis by Inducing Lysis of Endothelial Progenitor Cells. Alcohol Clin. Exp. Res. 2020, 44, 78–86.
- Legaz, I.; Bolarín, J.M.; Campillo, J.A.; Moya-Quiles, M.R.; Miras, M.; Muro, M.; Minguela, A.; Álvarez-López, M.R. Killer Cell Immunoglobulin-like Receptors (KIR) and Human Leucocyte Antigen C (HLA-C) Increase the Risk of Long-Term Chronic Liver Graft Rejection. Int. J. Mol. Sci. 2022, 23, 2155.
- de Arias, A.E.; Haworth, S.E.; Belli, L.S.; Burra, P.; Pinzello, G.; Vangeli, M.; Minola, E.; Guido, M.; Boccagni, P.; De Feo, T.M.; et al. Killer cell immunoglobulin-like receptor genotype and killer cell immunoglobulin-like receptor-human leukocyte antigen C ligand compatibility affect the severity of hepatitis C virus recurrence after liver transplantation. Liver Transpl. 2009, 15, 390–399.
- Tao, X.; Zhang, R.; Du, R.; Yu, T.; Yang, H.; Li, J.; Wang, Y.; Liu, Q.; Zuo, S.; Wang, X.; et al. EP3 enhances adhesion and cytotoxicity of NK cells toward hepatic stellate cells in a murine liver fibrosis model. J. Exp. Med. 2022, 219, e20212414.
- Jin, H.; Jia, Y.; Yao, Z.; Huang, J.; Hao, M.; Yao, S.; Lian, N.; Zhang, F.; Zhang, C.; Chen, X.; et al. Hepatic stellate cell interferes with NK cell regulation of fibrogenesis via curcumin induced senescence of hepatic stellate cell. Cell Signal. 2017, 33, 79–85.
- Karrar, A.; Rajput, B.; Hariharan, S.; Abdelatif, D.; Houry, M.; Moosvi, A.; Ali, I.; Tan, D.; Noor, S.; Esmaeili, D.; et al. Major Histocompatibility Complex Class I-Related Chain a Alleles and Histology of Nonalcoholic Fatty Liver Disease. Hepatol. Commun. 2021, 5, 63–73.
- Jeong, W.I.; Park, O.; Suh, Y.G.; Byun, J.S.; Park, S.Y.; Choi, E.; Kim, J.K.; Ko, H.; Wang, H.; Miller, A.M.; et al. Suppression of innate immunity (natural killer cell/interferon-γ) in the advanced stages of liver fibrosis in mice. Hepatology 2011, 53, 1342–1351.
- Kurokawa, T.; Ohkohchi, N. Platelets in liver disease, cancer and regeneration. World J. Gastroenterol. 2017, 23, 3228–3239.
- Czajka, P.; Przybyłkowski, A.; Nowak, A.; Postula, M.; Wolska, M.; Mirowska-Guzel, D.; Czlonkowska, A.; Eyileten, C. Antiplatelet drugs and liver fibrosis. Platelets 2022, 33, 219–228.
- Lisman, T.; Luyendyk, J.P. Platelets as Modulators of Liver Diseases. Semin. Thromb. Hemost. 2018, 44, 114–125.
- Zhong, L.K.; Zhang, G.; Luo, S.Y.; Yin, W.; Song, H.Y. The value of platelet count in evaluating the degree of liver fibrosis in patients with chronic hepatitis B. J. Clin. Lab. Anal. 2020, 34, e23270.
- Yoshida, S.; Ikenaga, N.; Liu, S.B.; Peng, Z.W.; Chung, J.; Sverdlov, D.Y.; Miyamoto, M.; Kim, Y.O.; Ogawa, S.; Arch, R.H.; et al. Extrahepatic platelet-derived growth factor-β, delivered by platelets, promotes activation of hepatic stellate cells and biliary fibrosis in mice. Gastroenterology 2014, 147, 1378–1392.
- Ma, S.; Tang, T.; Wu, X.; Mansour, A.G.; Lu, T.; Zhang, J.; Wang, L.S.; Caligiuri, M.A.; Yu, J. PDGF-D-PDGFRβ signaling enhances IL-15-mediated human natural killer cell survival. Proc. Natl. Acad. Sci. USA 2022, 119, e2114134119.
- Malehmir, M.; Pfister, D.; Gallage, S.; Szydlowska, M.; Inverso, D.; Kotsiliti, E.; Leone, V.; Peiseler, M.; Surewaard, B.G.J.; Rath, D.; et al. Platelet GPIbα is a mediator and potential interventional target for NASH and subsequent liver cancer. Nat. Med. 2019, 25, 641–655.
More
Information
Subjects:
Immunology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
674
Revisions:
2 times
(View History)
Update Date:
18 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




