Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Krishna R Reddy | -- | 3918 | 2023-05-17 06:22:46 | | | |
| 2 | Jessie Wu | Meta information modification | 3918 | 2023-05-17 07:12:01 | | | | |
| 3 | Jessie Wu | Meta information modification | 3918 | 2023-05-18 02:06:28 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Reddy, K.R.; Kandou, V.; Havrelock, R.; El-Khattabi, A.R.; Cordova, T.; Wilson, M.D.; Nelson, B.; Trujillo, C. Water Reuse Treatment Technologies. Encyclopedia. Available online: https://encyclopedia.pub/entry/44411 (accessed on 07 February 2026).
Reddy KR, Kandou V, Havrelock R, El-Khattabi AR, Cordova T, Wilson MD, et al. Water Reuse Treatment Technologies. Encyclopedia. Available at: https://encyclopedia.pub/entry/44411. Accessed February 07, 2026.
Reddy, Krishna R., Valeria Kandou, Rachel Havrelock, Ahmed Rachid El-Khattabi, Teresa Cordova, Matthew D. Wilson, Braeden Nelson, Citlalli Trujillo. "Water Reuse Treatment Technologies" Encyclopedia, https://encyclopedia.pub/entry/44411 (accessed February 07, 2026).
Reddy, K.R., Kandou, V., Havrelock, R., El-Khattabi, A.R., Cordova, T., Wilson, M.D., Nelson, B., & Trujillo, C. (2023, May 17). Water Reuse Treatment Technologies. In Encyclopedia. https://encyclopedia.pub/entry/44411
Reddy, Krishna R., et al. "Water Reuse Treatment Technologies." Encyclopedia. Web. 17 May, 2023.
Copy Citation
Treating water for water reuse typically involves treating wastewater in several steps consisting of preliminary treatment, primary treatment, and secondary treatment. Tertiary treatment and advanced treatment may be needed for water reuse purposes.
water reuse
Water recycling
Wastewater treatment
Sustainability
1. Introduction
Most residential and industrial activities generate wastewater containing harmful pollutants [1]. Before this wastewater can be safely and sustainably reused, it must undergo treatment to remove these pollutants to an appropriate degree. This plain fact is important to consider since water reuse is increasingly being recognized as a sustainable solution for global water management issues. By addressing the issue of harmful pollutants in wastewater, we can ensure that it can be effectively treated and safely reused. Ensuring water quality is an essential aspect of water reuse, as the suitability of the water for a given purpose can depend on its quality. The challenge rests in implementing water reuse technologies that are cost-effective, robust, and safe for human health and the environment [2].
The goal of water reuse treatment is to produce water that meets the quality of the intended use and is safe for public health and the environment. Producing water viable for particular uses while maintaining safety standards is known as a “Fit-for-Purpose” model that can be customized to a particular purpose. In determining quality thresholds, treatment goals (e.g., salt reduction for irrigation or industrial reuse) are specifically tailored to end user needs, safe for the public and the environment while being cost-effective. This is a frequently used strategy in developing various solutions for water reuse [3].
During preliminary treatment, large objects that may damage the treatment process are removed. In primary treatment, some suspended solids and organic matters are removed from wastewater. The removal process is done by sedimentation of floating and settleable matter. In secondary treatment, most of the organic matter is removed using biological and chemical processes. Additionally, tertiary treatment and advanced treatment may be added to the system train for water reuse purposes. In tertiary treatment, disinfection and nutrient removal occurs, and the remaining suspended solids are removed using granular medium filtration or micro screens. Remaining suspended solids and other constituents that are not removed by secondary treatment are then removed by a combination of unit operations and processes in advanced treatment [4].
Wastewater treatment systems can use a variety of different technologies to treat effluent for water reuse. Table 1a,b provide an overview of the various technologies and their applications [5][6]. The various technologies fit under one or more of the following five categories:
Table 1. (a) Unit operations and process used for the removal of different constituents in water reuse applications. (b) Treatment technologies and capabilities.
| (a) | ||||||||||||||
| Constituent Class | Secondary Treatment | Secondary with nutrient removal | Depth filtration | Surface filtration | Microfiltration | Ultrafiltration | Dissolved Air Flotation | Nano filtration | Reverse Osmosis | Electro dialysis | Carbon adsorption | Ion exchange | Advanced Oxidation | Disinfection |
| Suspended Solids | ✓ | - | ✓ | ✓ | ✓ | ✓ | ✓ | - | - | - | - | - | - | - |
| Colloidal Solids | - | - | - | - | ✓ | ✓ | ✓ | - | - | ✓ | - | - | - | - |
| Particulate Organic Matter | - | - | - | ✓ | ✓ | ✓ | ✓ | ✓ | - | - | - | - | ✓ | - |
| Dissolved Organic Matter | ✓ | ✓ | - | - | - | - | - | ✓ | ✓ | - | ✓ | - | ✓ | ✓ |
| Nitrogen | - | ✓ | - | - | - | - | - | - | ✓ | - | - | ✓ | - | - |
| Phosphorous | - | ✓ | - | - | - | - | - | - | ✓ | - | - | - | - | - |
| Trace Constituents | - | - | - | - | - | - | - | ✓ | ✓ | - | ✓ | ✓ | ✓ | - |
| Total Dissolved Solids | - | - | - | - | - | - | - | ✓ | ✓ | ✓ | - | ✓ | - | - |
| Bacteria | - | - | ✓ | ✓ | ✓ | ✓ | - | ✓ | ✓ | - | - | - | ✓ | ✓ |
| Protozoan Cysts and Oocysts | - | - | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | - | - | - | ✓ | ✓ |
| Viruses | - | - | - | - | - | ✓ | ✓ | ✓ | ✓ | - | - | - | ✓ | ✓ |
| (b) | ||||||||||||||
| Overall Treatment Objective | Unit Processes | TOC | TSS | TDS | Trace Chemical Constituents | Pathogens 3 | ||||||||
| Removal of Suspended Solids | Media Filtration, Microfiltration and ultrafiltration | Partial removal | High removal | None | None | High removal 3 | ||||||||
| Reducing the Concentration of Dissolved Chemicals | NF/RO | 90% removal | High removal | High removal | High removal 1 | High removal | ||||||||
| ED/EDR | None | None | High removal | None | None | |||||||||
| PAC | High removal | None | None | Partial removal | None | |||||||||
| GAC | 40–60% removal | High removal | None | 40–60% removal | Partial removal | |||||||||
| Ion exchange | None | None | High removal | Partial removal | None | |||||||||
| Biofiltration | High removal, High degradation 2 | High removal | None | High degradation 2 | Partial removal | |||||||||
| Ozone | None | None | None | High degradation * | High degradation | |||||||||
| Disinfection and Removal of Trace Organic Compounds | UV | None | None | None | Partial degradation * | High degradation | ||||||||
| Free Chlorine | None | None | None | Partial degradation * | High degradation | |||||||||
| Chloramines 4 | None | None | None | None | Partial degradation * | |||||||||
| PAA 5 | None | None | None | Partial degradation * | High degradation | |||||||||
| Pasteurization 5 | None | None | None | Partial degradation | High degradation | |||||||||
| Ozone | None | None | None | High degradation * | High degradation | |||||||||
| Chlorine dioxide | None | None | None | Partial degradation * | High degradation | |||||||||
| Advanced oxidation processes (UV/H2O2, O3/H2O2, UV/Cl2) | None | None | None | High degradation * | High degradation | |||||||||
Notes: * Contact time and concentration dependencies. 1 Some chemical constituents may have Reverse Osmosis (RO) removal efficiencies less than 90%, such as NDMA, 1,4-dioxane, and flame retardants. Additionally, Reverse Osmosis (RO) likely has greater removal efficiency than Nanofiltration (NF). 2 BAC is effective at removing trace chemical constituents, but BAC will result in higher TOC levels than RO. 3 MF and UF membranes can remove bacteria and protozoa. MF is not considered an effective barrier against viruses, while UF can remove viruses to a certain extent. 4 Extended chloramine contact times are required for virus inactivation, but no Giardia or Cryptosporidium inactivation should be anticipated with chloramine disinfection. 5 Currently used only in wastewater treatment.
-
Removal of suspended solids;
-
Reducing dissolved chemical concentrations;
-
Removal or disinfection of trace organic compounds;
-
Stabilization;
-
Aesthetics (taste, odor, color correction).
In instances where stringent effluent disposal standards apply, implementing water reuse may require upgrading technologies used at wastewater treatment plants (WWTP) to incorporate tertiary treatment technologies to treat contaminants that remain in the effluent [5][6]. Typical WWTPs use coagulation, flocculation, and sedimentation to remove suspended particles, while medium filtration and micro/ultrafiltration can improve effluent quality by enhancing the removal of solids and microorganisms. Media filtration uses gravity or pressure differentials to pass water through porous mediums, removing solids via adsorption and separation by size. Micro/ultrafiltration use a porous polymer film acting as a selective barrier and operate under size exclusion [6].
Reverse osmosis, electrodialysis, electrodialysis reversal, nanofiltration, granulated activated carbon, ion exchange, and biologically active filtration can be used to degrade dissolved compounds. Typically, a membrane is used to separate dissolved chemical elements such as road salts or pesticides from wastewater influents [6].
Disinfection and removal of trace organic compounds come after the removal of dissolved chemicals to eliminate pathogens in wastewater. This is accomplished through UV, free chlorine/chloramines, peracetic acid, pasteurization, chlorine dioxide, and advanced oxidation processes. These methods neutralize microorganisms through inactivation processes but are dependent on contact time, pH, and temperature [6].
Certain approaches for reducing corrosion, such as reverse osmosis and nanofiltration, must be followed by stabilization. Mineralization may involve decarbonation, or addition of sodium hydroxide, lime, calcium chloride, or mixing. The desired Langelier Saturation Index (LSI) should be close to zero, and thus should produce a final product that will not corrode metal pipelines or concrete tanks [6].
Though aesthetics may appear unimportant, public opinion has a significant impact on the feasibility of wastewater recycling. Therefore, some qualities, such as flavor, odor, and color, must be treated prior to the distribution of water to public systems or agricultural systems. Activated carbon, UV, and chlorination are efficient ways of treating taste and odor. All aesthetic issues are adequately remedied with the help of ozone and biologically activated carbon [6].
Table 2 lists all treatment technologies from various case studies that were collected for this research. As the need for higher water quality increases, the degree of treatment increases. For instance, a more complex treatment process is required when the intended use of the recycled water is for indirect potable reuse (IPR) or direct potable reuse (DPR).
Table 2. Treatment technologies used existing water reuse projects.
| Category | Tertiary Treatment Process | Reuse Purposes | Location | |
|---|---|---|---|---|
| Pre-Treatment-Filtration | Disinfection | |||
| Urban Reuse | Flocculation Media Filtration |
Chlorination | Non-potable irrigation (residential, commercial, industrial) | El Segundo, CA, SUA |
| Agriculture | Flocculation Multi-media Filters |
Chlorination | Raw-eaten vegetables and fruits | Monterey One, CA, USA |
| None (Membrane Bioreactor effluent) | Ultraviolet | Vineyards | American Canyon, CA, USA | |
| Coagulation Flocculation Cloth Media Filter |
Ultraviolet | Raw-eaten fruits | Pajaro Valley, CA, USA | |
| Industrial | Microfiltration | Reverse Osmosis (Single Pass) Decarbonation |
Industrial—Boiler Feed (BF) water | El Segundo, CA, USA |
| Microfiltration | Reverse Osmosis (Single Pass) Ozone Decarbonation |
Industrial—Low-Pressured Boiler Feed | El Segundo, CA, USA | |
| Microfiltration | Reverse Osmosis (Double Pass) Ozone Decarbonation |
High-Pressure Boiler Feed | El Segundo, CA, USA | |
| Sand Filter | Addition of corrosion inhibitors, sodium hypochlorite, acid, and antifoaming agents (at power plant) | Cooling towers | Denver, CO, USA | |
| Media Filtration | Oxidized Coagulation Disinfected (UV or Chlorine) |
Pulp and paper (newspaper) | Los Angeles, CA, USA | |
| Gravity Filter | Chlorination | Textile (carpet dyeing) | Santa Fe, CA, USA | |
| Granular Coal | Ultraviolet | Geyser recharge for electricity | Santa Rosa, CA, USA | |
| Lime Softening Filtration |
Chlorination | Cooling towers | Baltimore, MD, USA | |
| Environmental | Automatic Backwash with Sand Media | Chlorination | Wetlands | Orlando, FL, USA |
| Indirect Potable Reuse | Microfiltration | Reverse Osmosis UV with Hydrogen Peroxide Lime Treatment |
Groundwater recharge | Orange County, CA, USA |
| Lime Clarification Media Filtration |
Granulated Activated Carbon Ion Exchange Chlorination |
Fairfax, VA, USA | ||
| Media Filtration | Reverse Osmosis Ultraviolet with Advanced Oxidation Process Chlorination |
Groundwater recharge via riverbank filtration | Arapahoe County, CO, USA | |
| Potable Reuse | Flocculation Biologically Active Carbon Filtration Microfiltration Ozonation Granular Activated Carbon |
Ultraviolet Chlorination |
Drinking Water (preliminary approval) |
Castle Rock, CO, USA |
| Granular Activated Carbon Filtration | Reverse Osmosis Ultraviolet with Advanced Oxidation Process |
Drinking Water (Undergoing regulatory approval) |
El Paso, TX, USA | |
| Combination | Granular Coal | Ultraviolet | Farmlands Vineyards Public urban landscaping |
Santa Rosa, CA, USA |
| None | UV | Agricultural Irrigation (Vineyards) Landscape Irrigation (excludes golf courses) Industrial use Other—Construction site dust control Other—In-plant use at City WRF |
American Canyon, CA, USA | |
| Microfiltration | Chlorine/Dechlorination Reverse Osmosis Ultraviolet |
Irrigation Industrial Streamflow Augmentation (future direction) Groundwater Recharge (future direction) |
Santa Clara, CA, USA | |
Crini and Lichtfouse (2019) gave an outline of various wastewater treatment processes and analyzed the pros and cons associated with each, considering factors such as cost, effectiveness, practicality, reliability, environmental impact, sludge production, operational complexity, pre-treatment needs, and the potential for generating hazardous byproducts (Table 3 and Table 4) [7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27]. Based on the variability of choices, advantages, and disadvantages of wastewater treatment processes and technologies, engineers, stakeholders, and people partaking in water reuse projects can select the most appropriate treatment method and technologies to achieve the desired water quality (Table 3).
Table 3. Advantages and disadvantages of various water treatment processes.
| Process | Advantages | Disadvantages |
| Advanced oxidation processes (AOP) Photolysis Heterogeneous and homogeneous photocatalytic reactions non-catalytic wet air oxidation (WAO) Catalytic wet air oxidation (CWAO) Supercritical water gasification |
|
|
| Adsorption/filtration Commercial activated carbons (CAC) Commercial activated alumina (CAA) Sand Mixed materials Silica gel |
|
|
| Biological methods Bioreactors Biological activated sludge (BAS) Microbiological treatments Enzymatic decomposition Lagoon |
|
|
| Coagulation/flocculation |
|
|
| Dialysis Electrodialysis (ED) Electro-electrodialysis (EED) Emulsion liquid membranes (ELM) Supported liquid membranes Membrane filtration Microfiltration (MF) Ultrafiltration (UF) Nanofiltration (NF) Reverse osmosis |
|
|
| Ion exchange Chelating resins Selective resins Microporous resins Polymeric adsorbents Polymer-based hybrid adsorbents |
|
|
2. Non-Potable Reuse Treatment Technologies
The most widely implemented and accepted water reuse practice is non-potable water reuse. It has been successfully implemented in many states in the US, particularly California, Texas, Arizona, and Florida. Due to the variety of non-potable water reuse, treatment goals and processes are based on specified non-potable reuse, and the requirements/guidelines to ensure the protection of public health. Water quality goals for industrial reuse are often site-specific and different from water reuse for irrigation. To achieve industrial water quality standards for cooling and boiler water applications, nutrient (e.g., nitrogen, phosphorus) and ion (e.g., chloride, hardness) removal may be necessary. Typically, tertiary treatment and disinfection are needed for agricultural and crop irrigation reuse. Several filtration technologies may be used to remove suspended particles and pathogens, including granular media filters, moving bed sand filters, cloth filters, and membrane filters. The state of California, California Title 22, maintains a list of permitted filtering methods for non-potable reuse applications. This list is helpful for those designing tertiary filtration [2].
Figure 1 provides some examples of agricultural water reuse and their treatment technologies. Treatment requirements vary depending on the intended use, though water quality restrictions for chloride, TDS, ammonia, TSS, and bacteria are regularly considered for scaling and corrosion in boiler feed and cooling towers. Industrial end-user-specified water quality standards might also alter treatment strategies. Depending on the needs of the system, no extra treatment beyond the tertiary non-potable treatment system may be required, or an independent advanced system may be required to produce higher water quality. If an advanced treatment system is required, it is normally installed by the industrial user at the point of use [2].
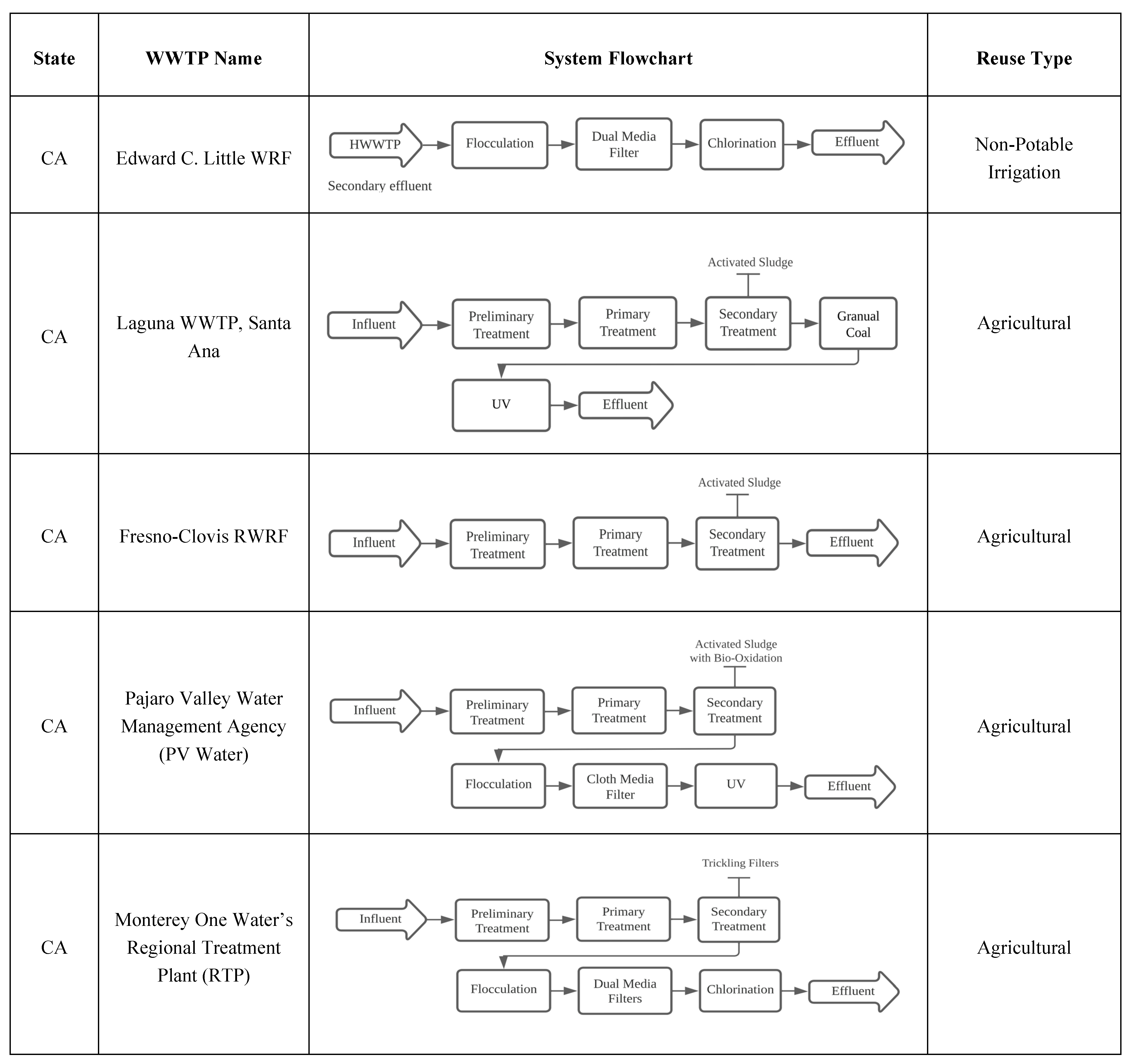
Figure 1. Wastewater treatment technologies for agricultural purposes based on various case studies in the US.
3. Potable Reuse Treatment Technologies
Potable reuse can be divided into two categories, which are direct potable reuse (DPR) and indirect potable reuse (IPR). Typically, complex treatment processes are used to remove organics, pathogens, and other impurities to fulfill potable water requirements. IPR refers to a system in which recycled effluent or advanced treated effluent is delivered to an environmental buffer prior to withdrawal for potable uses [28]. Direct potable reuse (DPR) refers to a system in which there is no environmental barrier between recycled effluent and potable water; nevertheless, mixing processes can be employed and still be classified as DPR [2]. Different treatment systems for IPR and DPR are depicted in Figure 2. In 2017, the EPA published the Potable Reuse Compendium which serves as a supplement to the 2012 guidelines and highlights current practices and treatment technologies in potable reuse.
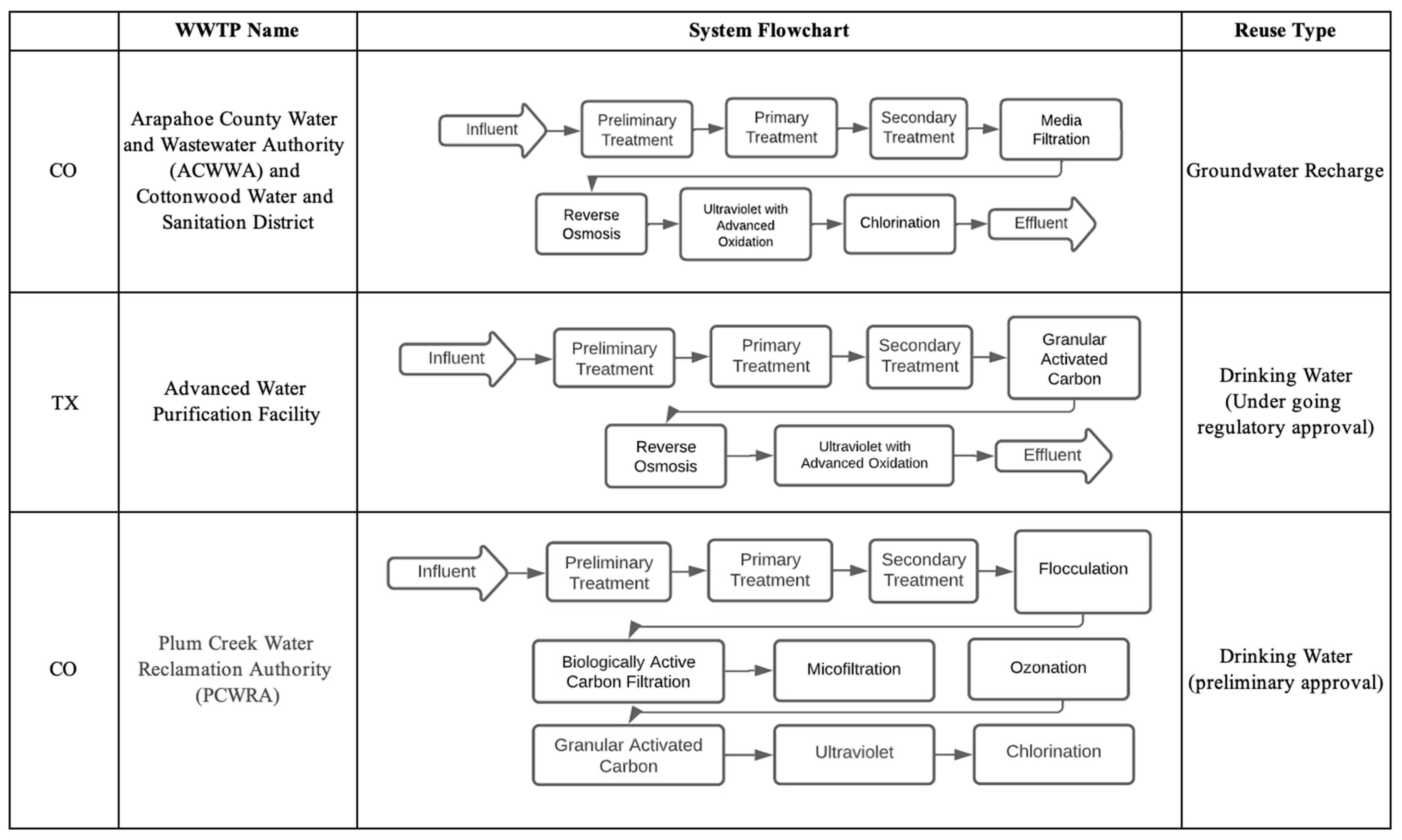
Figure 2. Wastewater treatment technologies for IPR and DPR purposes based on case studies in the US.
4. Costs of Treatment Technologies
As recycled water is a relatively new source of supply, the water sector has not yet adopted a pricing strategy for recycled water. Moreover, the assessment and distribution of costs associated with the production of recycled water are inherently complicated, reflecting both water and wastewater functions and necessitating judgments regarding the optimal management of shared costs [29]. Table 4a provides approximate costs using information from previous water reuse projects in 2009 USD [30], along with a comparison of reclaimed (recycled) water rates for various communities in the US (Table 4b) [3].
Table 4. (a) Water reuse projects financial costs. (b) Comparison of reclaimed (recycled) water rates [3].
| (a) | |||||||
| Capacity (million gallons per day, MGD) | Treatment Technologies | Total Capital Cost (USD /kgal per year) | Annualized Capital Cost (USD /kgal) | Capital Cost (USD /kgal) | Annual Capital Cost + O&M Cost (USD /kgal) | End Uses | Facility |
| 5 | Secondary treated water–Filtration–UV | 5.73 | 0.5 | 0.35 | 0.85 | Landscape irrigation | Desert Breeze, NV, USA |
| 10 | Secondary treated water–Filtration–UV | 4.23 | 0.37 | 0.68 | 1.05 | Landscape irrigation | Durango Hills, NV, USA |
| 16.4 | Advanced Activated Sludge Treatment | 1.14 | 0.1 | 0.05 | 0.15 | Landscape irrigation, amenity reservoir | Trinity River Authority, TX, USA |
| 30 | Biologically aerated filters–Flocculation–Sedimentation–Filtration–Disinfection | 13.57 | 1.18 | 1.06 | 2.24 | Landscape irrigation, Industrial cooling, zoo | Denver Water, CO, USA |
| 40 | Biological Nutrient Removal (BNR) secondary treated water–Filtration–Chlorine Disinfection | 18.75 | 1.63 | 1.02 | 2.65 | Irrigation, industrial cooling, laundry, paper processing | West Basin, CA, USA |
| 12.5 | Microfiltration-Reverse Osmosis (RO)–Advanced Oxidation | 30.72 | 2.68 | 2.38 | 5.6 | Indirect Potable Reuse | West Basin, CA, USA |
| 10 | Activated Sludge Secondary Treatment with Denitrification–Anaerobic Digestion–Lime Treatment–Sand Filtration-Ozonation-Biologically Active Granular Activated Carbon Filtration–Final Disinfection |
23.46 | 2.05 | 0.33 | 2.38 | Indirect Potable Reuse | El Paso Water, TX, USA |
| 20 | Biological Nutrient Removal (BNR) secondary treated water–Filtration–Chlorine Disinfection–Soil Aquifer Treatment | 11.26 | 0.98 | 1.18 | 2.16 | Indirect Potable Reuse | Inland Empire, CA, USA |
| 24 | Biological Nutrient Removal (BNR) secondary treated water–Sodium Hypochlorite Disinfection–Treatment Wetlands | 3.92 | 0.34 | 0.35 | 0.69 | Indirect Potable Reuse | Casey WRF/Huie Wetlands Clayton Co., GA, USA |
| 70 | Enhanced Primary Treatment–Activated Sludge and Trickling Filter Secondary Treatment–Microfiltration (MF)–Reverse Osmosis (RO)–Advanced Oxidation (ultraviolet light and hydrogen peroxide) | 20.0 | 1.74 | 1.16 | 2.90 | Indirect Potable Reuse | Orange Co. GWRS, CA, USA |
| (b) | |||||||
| Community | Potable Water Rates (First Tiers Only) | Reclaimed Water Rates | |||||
| Rate per 1000 gal | Use | Rater per 1000 gal | Use | ||||
| Tucson, AZ, USA | 2.19 | 1–15 ccf | 2.45 | Variable on all use | |||
| 7.82 | 16–30 ccf | ||||||
| Dublin San Ramon Services District, CA, USA | 3.28 | Tier 1 Volume charge, first 22,440 gallons | 3.19 | Flat rate volume charge | |||
| 3.48 | Tier 2 Volume Charge over 22,440 gallons | ||||||
| Eastern Municipal Water District, CA, USA | 2.07 | Tier 1 Indoor use | 0.8 | R-452 Non-Ag, Secondary, Disinfected-2009 | |||
| 3.79 | Tier 2 Outdoor use | 0.88 | R-452 Non-Ag, Tertiary, Disinfected, Filtered-2009 | ||||
| Glendale Water and Power, CA, USA | 3.18 | Commercial Rate | 2.39 | Non-potable purposes | |||
| Irvine Ranch Water District, CA 1, USA | 1.62 | Residential Detached Base Rate 5–9 ccf | 1.44 | Landscape Irrigation Base Index 41–100% ET | |||
| 3.34 | Residential Detached Inefficient Rate 10–14 ccf | 3.01 | Landscape Irrigation Inefficient Index 101–110% ET | ||||
| 5.78 | Residential Detached Excessive Rate 15–19 ccf | 5.2 | Landscape Irrigation Excessive Index 111–120% ET | ||||
| Orange Country, FL, USA | 1.04 | 0–3000 gal | 0.74 | Variable on >4000 gal/month | |||
| 1.39 | 4000–10,000 gal | ||||||
| St. Petersburg, FL, USA | 3.45 | 0–5600 gal | 17.63 | Unmetered–First acre | |||
| 10.1 | Unmetered > 1 acre | ||||||
| 0.5 | Metered | ||||||
| El Paso, TX, USA | 1.94 | Over 4 ccf | 1.24 | Variable on all use | |||
Notes: ccf = 100 cubic feet; 1 Irvine Ranch Water District employs a steep inclined rate based on watering in excess of the evapotranspiration (ET) rate.
5. Water Reuse Distribution Infrastructure
According to Asano and Mills (1990), the network of the reclaimed water distribution system comprises all pipeline routes, storage reservoir locations, sizes, types, and pumping station locations and their capabilities. When elevation changes exist, it may be essential to divide the distribution system into two or more pressure zones; each pressure zone should be able to meet peak water demands. Therefore, redundant infrastructures are needed [31]. Figure 3 depicts a conceptual diagram of several distribution system configurations. Asano et al. (2007) discussed the distribution system types of loop, grid, and tree systems (Table 5). With a grid or loop system, each major reuse area is supplied from multiple directions, ensuring that all demands will be met even if a portion of the distribution system is disrupted. While in a tree system, a failure in the main supply line will interrupt service to all or a portion of the users. A tree system is generally not advised to be used for the distribution of water reuse due to the possibility of odors developing in the dead-end outlets [5].
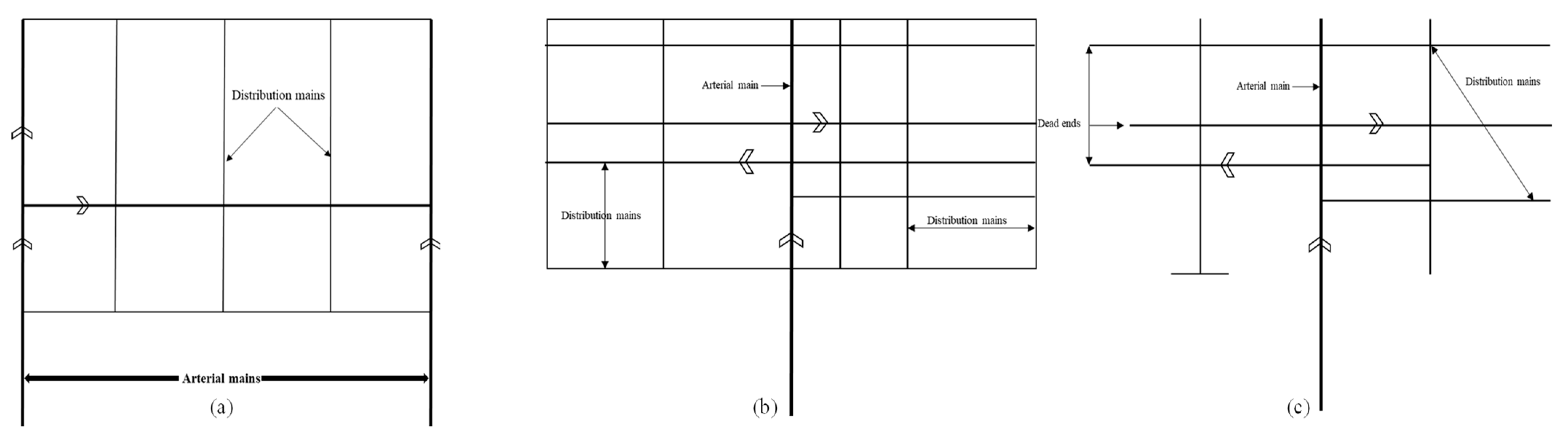
Figure 3. Pipelines distribution configurations: (a) loop, (b) grid, (c) tree.
Table 5. Types of distribution systems.
| System Type | Description | Notes |
|---|---|---|
| Loop | The areas that are going to be served are surrounded by large feeder mains, and smaller cross feed lines are connected to the main loop. | Reclaimed water is distributed from two directions to the main reuse area. Looped systems have less head loss than tree system. |
| Grid | The piping is set out in a checkerboard arrangement, and the size of the pipe typically decreases as the distance from the source increases. | Pipe size reduction will reduce material costs and has similar advantages as the loop system. |
| Tree | It utilizes a single main that decreases in size the further away it is from the source. | Usually used for systems that do not need the higher level of reliability that loop and grid systems offer. The accumulation of build-up in dead ends can be avoided with regular line flushing. |
The majority of states mandate that recycled water distribution pipelines to be purple; Pantone 512 or 522 is typically preferred for this purpose. Reclaimed water piping should be identified in accordance with state design guidelines, which may include labeling, tagging, and signs along the piping’s alignment. PVC is a popular material for constructing reclaimed water pipes, as it is easy to infuse color during the manufacturing process. Reclaimed water distribution systems will contain all components characteristic of potable water distribution systems. Most standard system components are now available in purple, to facilitate the expanded installation of reclaimed water systems with purple color coding [3].
6. Water Reuse Planning Model
Planning a rational project requires well-defined objectives. The conventional framework for analysis begins with determining if a project has a single-purpose or multi-purpose, i.e., designed to serve two or more fundamental functions. The typical wastewater reclamation projects are intended for control or water supply. Water reuse planning generally consists of three stages [31]:
1. Conceptual level planning;
2. Preliminary feasibility investigation;
3. Facilities planning.
According to Asano and Mills (1990), a proposed project is drawn out during conceptual planning, then approximate costs are assessed and a potential market for recovered water is identified. If the conceptual planning seems viable, a preliminary feasibility analysis is conducted. Preliminary feasibility includes the following steps:
-
Performing a market evaluation, i.e., identifying a market for recycled water and specifying the criteria that must be met (e.g., user needs for water quality and pricing);
-
Evaluating the current water supply and wastewater facilities and creating some preliminary options that might service the entire market, in parts or in full, while meeting its technical and water quality needs;
-
Comparing a wastewater reclamation and reuse option with other non-reclamation facilities, such as wastewater treatment for stream discharge or the construction of a reservoir for water supply;
-
Considering technical needs, economics, financial advantages, marketability of recovered water, and other restrictions such as health protection of recycled water.
If wastewater reclamation and reuse look feasible, and desired based on the previous preliminary feasibility research, deeper planning may be explored, revised facilities options can be produced, and a final facilities’ designs can be suggested [30]. The Water Environment Federation (WEF) also highlights the importance of holistic planning and decision-making frameworks, including but not limited to triple-bottom-line, “one water”, and life cycle analysis. The WEF defines three components of water reuse planning, such as establishing a long-term vision for integrated water resource; setting strategic planning goals to create an integrated, reliable, resilient and sustainable water supply; and lastly, mapping the water resource supply/demand and infrastructure capacity [2].
References
- Crini, G.; Lichtfouse, E. Advantages and Disadvantages of Techniques Used for Wastewater Treatment. Environ. Chem. Lett. 2019, 17, 145–155.
- Water Environment Federation (WEF). The Water Reuse Roadmap. Available online: https://ebookcentral-proquest-com.proxy.cc.uic.edu/lib/uic/detail.action?docID=6186983 (accessed on 1 March 2022).
- U.S. Environmental Protection Agency (EPA). 2012 Guidelines for Water Reuse; EPA/600/R-12/618; U.S. Environmental Protection Agency (EPA): Washington, DC, USA. Available online: https://www.epa.gov/sites/default/files/2019-08/documents/2012-guidelines-water-reuse.pdf (accessed on 28 January 2022).
- Tchobanoglous, G.; Stensel, H.D.; Tsuchihashi, R.; Burton, F. Wastewater Engineering Treatment and Resource Recovery; McGraw-Hill: New York, NY, USA, 2014.
- Asano, T. Water Reuse: Issues, Technology, and Applications; McGraw-Hill: New York, NY, USA, 2007.
- Tricas, M.; Albert, R.; Bastian, R.; Nappier, S.; Regli, S.; Kasparek, L.; Gorke, R. 2017 Potable Reuse Compendium; United States Environmental Protection Agency: Washington, DC, USA, 2018.
- Berefield, L.D.; Judkins, J.F.; Weand, B.L. Process Chemistry for Water and Wastewater Treatment; Prentice-Hall: Hoboken, NJ, USA, 1982.
- Henze, M.; Harremoes, P.; Arvin, E.; la Cour Jansen, J. Wastewater Treatment. Biological and Chemical Processes; Springer: Berlin/Heidelberg, Germany, 1997.
- Sonune, A.; Ghate, R. Developments in Wastewater Treatment Methods. Desalination 2004, 167, 55–63.
- Chen, G. Electrochemical Technologies in Wastewater Treatment. Sep. Purif. Technol. 2004, 38, 11–41.
- Pokhrel, D.; Viraraghavan, T. Treatment of Pulp and Paper Mill Wastewater—A Review. Sci. Total Environ. 2004, 333, 37–58.
- Parsons, S. (Ed.) Advanced Oxidation Processes for Water and Wastewater Treatment; IWA Publishing: London, UK, 2004.
- Anjaneyulu, Y.; Sreedhara Chary, N.; Samuel Suman Raj, D. Decolourization of Industrial Effluents—Available Methods and Emerging Technologies—A Review. Environ. Sci. Bio/Technol. 2005, 4, 245–273.
- Chuah, T.G.; Jumasiah, A.; Azni, I.; Katayon, S.; Choong, S.T. Rice Husk as A Potentially Low-Cost Biosorbent for Heavy Metal and Dye Removal: An Overview. Desalination 2005, 175, 305–316.
- Crini, G. Recent Developments in Polysaccharide-Based Materials Used as Adsorbents In Wastewater Treatment. Prog. Polym. Sci. 2005, 30, 38–70.
- Crini, G. Non-Conventional Low-Cost Adsorbents for Dye Removal: A Review. Bioresour. Technol. 2006, 97, 1061–1085.
- Bratby, J. Coagulation and Flocculation In Water And Wastewater Treatment. IWA Publishing: London, UK, 2006.
- Crini, G.; Montiel, A.J.; Badot, P.M. Traitement Et Épuration Des Eaux Industrielles Polluées: Procédés Membranaires, Bioadsorption Et Oxydation Chimique; Presses Universitaires de Franche-Comté: Besançon, France, 2007; Volume 352.
- Crini, G.; Badot, P.M. (Eds.) Sorption Processes and Pollution: Conventional and Non-Conventional Sorbents For Pollutant Removal From Wastewaters; Presses Universitaires de Franche-Comté: Besançon, France, 2010.
- Cox, M.; Négré, P.; Yurramendi, L. Industrial Liquid Effluents; INASMET Tecnalia: San Sebastian, Spain, 2007; Volume 283.
- Mohan, D.; Pittman, C.U., Jr. Arsenic Removal from Water/Wastewater Using Adsorbents—A Critical Review. J. Hazard. Mater. 2007, 142, 1–53.
- Hai, F.I.; Yamamoto, K.; Fukushi, K. Hybrid Treatment Systems for Dye Wastewater. Crit. Rev. Environ. Sci. Technol. 2007, 37, 315–377.
- Wojnárovits, L.; Takács, E. Irradiation Treatment of Azo Dye Containing Wastewater: An Overview. Radiat. Phys. Chem. 2008, 77, 225–244.
- Barakat, M.A. New Trends in Removing Heavy Metals from Industrial Wastewater. Arab. J. Chem. 2011, 4, 361–377.
- Sharma, S.K.; Sanghi, R. (Eds.) Advances In Water Treatment and Pollution Prevention; Springer Science+Business Media: Berlin, Germany, 2012.
- Rathoure, A.K. (Ed.) Toxicity and Waste Management Using Bioremediation; IGI Global: Hershey, PA, USA, 2015.
- Morin-Crini, N.; Crini, G.; Roy, L. Eaux industrielles contaminées. PUFC Besanço 2017, 513, 37–47.
- WateReuse Research Foundation; American Water Works Association; Water Environment Federation; National Water Research Institute. Framework for Direct Potable Reuse; WateReuse Research Foundation: Alexandria, VA, USA, 2015.
- American Water Works Association (AWWA). Water Reuse Cost Allocations and Pricing Survey; AWWA: Denver, CO, USA, 2019.
- National Research Council (NRC). Water Reuse: Potential for Expanding the Nation’s Water Supply Through Reuse of Municipal Wastewater; The National Academies Press: Washington, DC, USA, 2012.
- Asano, T.; Mills, R.A. Planning and Analysis for Water Reuse Projects. J.-Am. Water Work. Assoc. 1990, 82, 38–47.
More
Information
Subjects:
Engineering, Environmental
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.7K
Revisions:
3 times
(View History)
Update Date:
18 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




