Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Esteban Durán-Lara | -- | 3309 | 2023-05-16 16:06:19 | | | |
| 2 | Beatrix Zheng | Meta information modification | 3309 | 2023-05-17 02:49:58 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Rafael, D.; Guerrero, M.; Marican, A.; Arango, D.; Sarmento, B.; Ferrer, R.; Durán-Lara, E.F.; Clark, S.J.; Schwartz, S. Intravitreal Delivery Systems for Sustained Drug Release. Encyclopedia. Available online: https://encyclopedia.pub/entry/44383 (accessed on 08 February 2026).
Rafael D, Guerrero M, Marican A, Arango D, Sarmento B, Ferrer R, et al. Intravitreal Delivery Systems for Sustained Drug Release. Encyclopedia. Available at: https://encyclopedia.pub/entry/44383. Accessed February 08, 2026.
Rafael, Diana, Marcelo Guerrero, Adolfo Marican, Diego Arango, Bruno Sarmento, Roser Ferrer, Esteban F. Durán-Lara, Simon J. Clark, Simo Schwartz. "Intravitreal Delivery Systems for Sustained Drug Release" Encyclopedia, https://encyclopedia.pub/entry/44383 (accessed February 08, 2026).
Rafael, D., Guerrero, M., Marican, A., Arango, D., Sarmento, B., Ferrer, R., Durán-Lara, E.F., Clark, S.J., & Schwartz, S. (2023, May 16). Intravitreal Delivery Systems for Sustained Drug Release. In Encyclopedia. https://encyclopedia.pub/entry/44383
Rafael, Diana, et al. "Intravitreal Delivery Systems for Sustained Drug Release." Encyclopedia. Web. 16 May, 2023.
Copy Citation
Slow-release delivery systems are needed to ensure long-term sustained treatments for retinal diseases such as age-related macular degeneration and diabetic retinopathy, which are currently treated with anti-angiogenic agents that require frequent intraocular injections. These can cause serious co-morbidities for the patients and are far from providing the adequate drug/protein release rates and required pharmacokinetics to sustain prolonged efficacy.
hydrogels
stimuli-responsive
thermo-responsive
retinopathies
delivery systems
1. Intraocular Implants
An alternative to prolonged IVT injections is the use of long-term intraocular implants to improve the sustained release of small drugs to the retina [1][2][3][4][5][6][7]. In fact, intraocular implants are currently the only delivery systems approved by the FDA for intraocular use. Drugs are conjugated to a polymer-based platform to allow their sustained release inside the vitreal cavity. According to the nature of the polymers used, implants can be divided into: (i) non-biodegradable implants, most of which use poly(ethylene–co-vinyl acetate) (pEVA), poly(dimethyl siloxane) (PDMS), or poly(vinyl alcohol) (PVA); and (ii) biodegradable implants mostly made of poly(lactic–co-glycolic acid) (PLGA), poly(glycolic acid) (PGA), or poly(lactic acid) (PLA) [2][3][4][5]. Whereas most biodegradable implants might sustain drug release and efficacy up to 6 months due to progressive IVT scaffold degradation and an exponential decrease in IVT drug concentration over time, non-biodegradable implants might extend drug release up to 2–3 years following a linear decrease in IVT drug concentration, while the scaffolds remain intact within the vitreous cavity.
An example of a biodegradable implant is Ozurdex® (dexamethasone corticosteroid conjugated to a polymer backbone of poly(lactic–co-glycolic acid) indicated for the treatment of macular edema following branch retinal vein occlusion (BRVO) or central retinal vein occlusion (CRVO) and moreover, for the treatment of inflammation (non-infectious uveitis) of the posterior segment of the eye. The implant allows the controlled release of Dexamethasone up to 4–6 months and degrades entirely in vivo [1][6]. Other examples are Brimo DDS® (a brimonidine drug delivery system in a poly(D,L-lactide) biodegradable polymer matrix), a selective α 2-adrenoceptor agonist currently approved in the United States and Europe for the treatment of open-angle glaucoma and ocular hypertension, which is currently in phase III clinical trials for the treatment of patients with geographic atrophy secondary to neovascular AMD [7][8]. Both from Allergan Inc. (Irvine, CA, USA). Moreover, the FDA has recently approved Durysta® (Bimatoprost, a prostaglandin analogue in a biodegradable polymer matrix consisting of a poly(D,L-lactide), poly(D,L-lactide–co-glycolide), poly(D,L-lactide) acid end, and poly(ethylene glycol) 3350. Allergan; FDA approval March 2020) to reduce the intraocular pressure caused by open-angle glaucoma, thereby preventing further retinal damage.
Furthermore, Retisert® (Fluocinolone acetonide corticosteroid. Bausch & Lomb, Rochester, NY, USA), an implant consisting of a tablet within a silicone elastomer cup with a release orifice and a polyvinyl alcohol membrane (RETISERT [Package Insert]. Rochester, NY, USA: Bausch & Lomb Incorporated.; May 2019. 2. U.S. Food & Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021737s000TOC.cfm, accessed on 4 October 2017) and Illuvien® (Fluocinolone acetonide corticosteroid in a solid matrix made of silicone, polyimide and poly(vinyl alcohol). Alimera Sciences Inc., Aldershot, UK) are non-biodegradable implants approved by the FDA to treat diabetic macular edema, macular edema secondary to retinal vascular occlusion, and posterior uveitis [9][10][11]. Another non-biodegradable implant is Vitrasert® (Ganciclovir in a silicone-based matrix from Bausch & Lomb, Rochester, NY, USA) for the treatment of retinitis caused by cytomegalovirus.
Unfortunately, the use of implants involves invasive procedures and requires surgical implantation. In addition, non-biodegradable implants also require surgical removal after drug release is completed. For this reason, they carry a higher risk of infection, an associated rise of intraocular pressure, and an increased incidence of post-operative cataracts [2]. Furthermore, most intraocular implants are not designed to provide a sustained release of intraocular therapeutic proteins because of their large size (high MW), hydrophilicity, and subsequent protein stability, which hampers their suitability for the administration of the current VEGF inhibitors [12].
2. Hydrogels for Intravitreal Drug Delivery
New delivery systems are required to ensure the long-term sustained release of therapeutic payloads to prolong injection intervals and to reduce morbidity risks and side effects while targeting the retina. Among them, the use of injectable aqueous biodegradable gels formulated using hydrophilic polymers (hydrogels, HGs) and of in situ-forming HGs (stimuli-responsive HGs, SRHGs) are gaining particular attention as delivery vehicles for intraocular treatments as long-term release platforms for drugs and proteins [13][14][15][16][17][18][19] (Figure 1). Generally speaking, HGs are water-swollen tridimensional networks made of polymers that are produced by the conjugation/reaction of one or more monomers. Moreover, HGs contain high amounts of water due to the presence of numerous hydrophilic functional groups, allowing the entrapment and/or conjugation of high payloads of proteins and drugs. In addition, HGs can be adapted through the modification of certain properties such as their biodegradability, swelling degree, pore size, permeability, viscoelasticity and hydrophilicity. The selection of monomers and polymers, their concentration, and the degree of cross-linkage and the chosen linkers directly impact the physicochemical attributes of the HGs and have a strong influence on their degradation profile and the release of protein payloads. As an example, hydrophilicity and cross-linking density can alter the pore size and protein diffusion rates, which strongly influence drug and protein release by changing the swelling degree of the HG. Moreover, cross-linking density can also be tailored by modifying the molecular weight, concentration and architecture of the chosen polymers, which together with the type of selected biodegradable linkers directly affects the degradation rate of the HG [13][14][15][16].
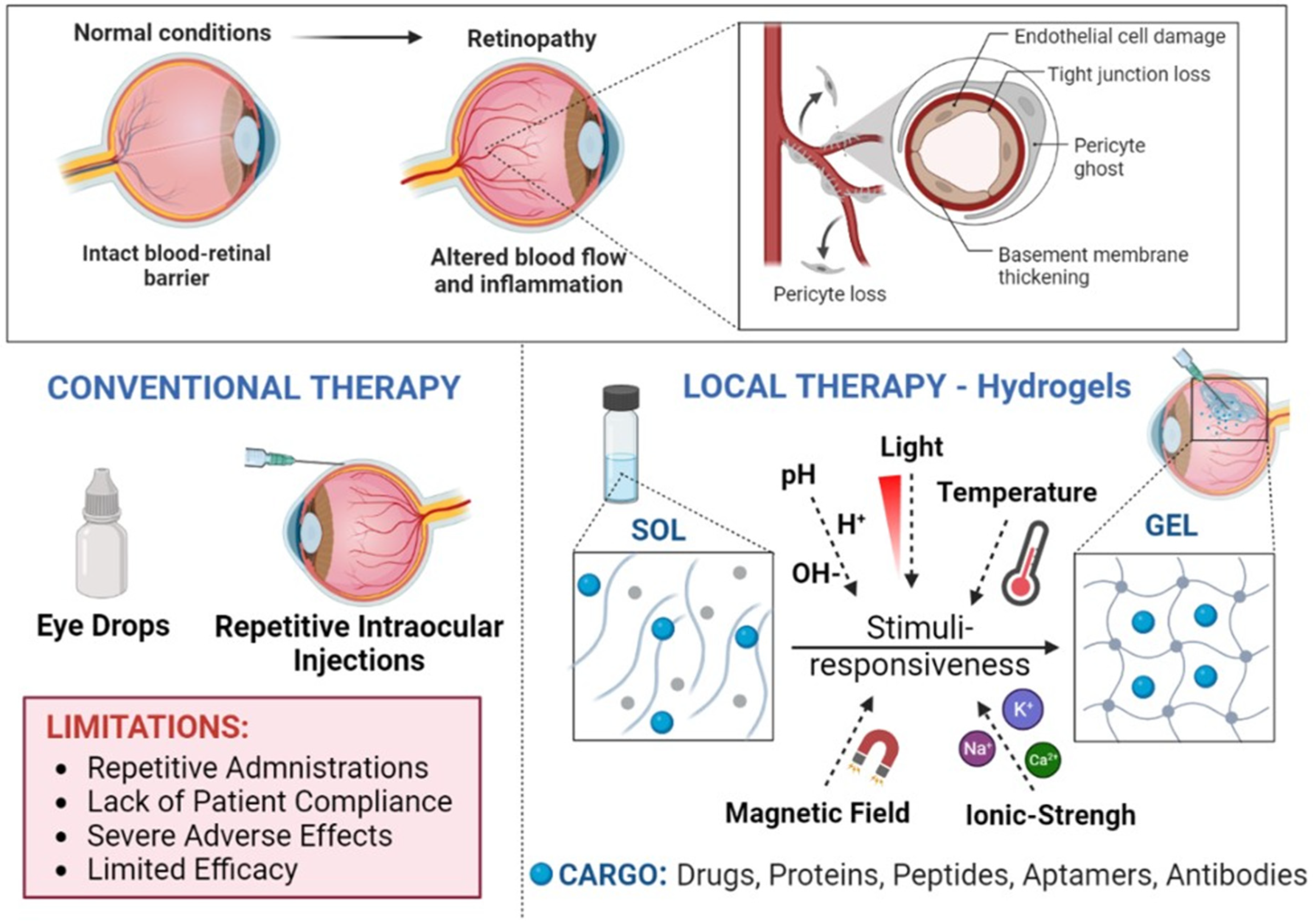
Figure 1. Graphical representation of inflammation and vascular alterations in retinopathies and the use of stimuli-responsive hydrogels as slow-release scaffolds for intraocular treatments.
According to the nature of the polymers used to produce the HGs, polymers can be classified into three main categories: synthetic polymers such as poly(N-isopropylacrylamide) (PNIPAAm), poly(lactic–co-glycolic acid) (PLGA), poly(ethylene glycol) (PEG) and its derivatives poly(ethylene glycol) diacrylate (PEGDA) and poly(ethylene glycol) methacrylate (PEGMA), and poly(caprolactone) (PCL); non-synthetic (natural) polymers such as hyaluronic acid (HA), alginate, chitosan, cellulose, dextran and silk; and hybrid polymers, which contain a mix of synthetic and non-synthetic polymers [13][17][18]. The presence of hydrolysable linkages within the polymers makes these HGs biodegradable by chemical and/or enzymatic hydrolysis. Furthermore, HGs can also be classified according to the type of cross-linking as covalent and non-covalent cross-linked HGs [19]. While non-covalent forces (hydrogen bonds, electrostatic charges and hydrophobic interactions) are weak and reversible depending on environmental conditions, covalent links are based on strong chemical binding between polymer chains (small cross-linking molecules, polymer-to-polymer chemical reactions). Of note, non-covalent cross-linked HGs are more difficult to tune up as their interaction and reactivity with the environment as well as their biodegradability and payload release are far from predictable [19][20][21][22][23][24][25]. On the other hand, covalent cross-linked HGs require chemical reactions that might cause the inactivation of bioactive molecules and protein payloads. In addition, the use of cross-linking molecules can also increase their in vivo toxicity and strongly affect protein release.
3. Release Kinetics of Hydrogels at the Vitreal Cavity
In these regards, the release of bioactive molecules such as anti-VEGF proteins into the vitreal cavity can be achieved either by the direct diffusion of the protein from the HG (diffusion-controlled) and/or by the progressive degradation of the gellified structure (degradation-controlled) [20][21][22]. Both mechanisms are often overlapped. In fact, the release kinetics of most HGs show an initial burst-type effect (burst-release phase) produced just before the onsite cross-linkage of the gelation state is completed. This phase can be responsible for releasing up to 50% of the total protein payload into the vitreous [16][23][24]. Thus, a large percentage of the payload can experience a faster clearance than initially desired. A second phase characterized by a slow, diffusion-controlled release of the remaining protein payload follows. This diffusion depends on the pore size of the HG and on the remaining payload concentration, and it is progressively affected by the chemical and/or enzymatic degradation (degradation-phase) of the polymer network. Because of the high content of water and the sponge-like architecture of the HG, this degradation takes place from both the inside and the outside of its three-dimensional structure. In time, the release of the payload decreases, as it does its concentration within the hydrophilic spaces of the polymeric network, until a final burst-release phase occurs when the collapse of the HG matrix takes place [22][25][26][27][28]. Improved fine-tuning of the attributes and rheology of the HGs is required in order to better conceal the dynamics of their progressive structural changes and degradation with the clinical demand of slow, sustained-release systems for bioactive proteins in the vitreal cavity. Nonetheless, the delivery of anti-VEGF proteins is particularly challenging because chemical treatments, heat and/or pH modifications, among other environmental factors, might strongly alter their tertiary and quaternary structure, which are determinant of their bioactivity [29]. In this regard, the porous tridimensional matrix structure of the HGs not only allows the loading of high amounts of anti-VEGF proteins but also protects them from environmental aggressions that can cause their denaturation, thus preserving their activity. Subsequently, HGs are nowadays considered ideal delivery systems for the sustained release of bioactive hydrophilic agents into the vitreal cavity, including current anti-VEGF protein treatments. Yu et al. developed a vinyl sulfone functionalized hyaluronic acid (HA–VS)-thiolated dextran (Dex-SH) in situ-forming HG for the IVT controlled release of Bevacizumab that showed good biocompatibility in rabbit eyes by binocular indirect ophthalmoscopy, full-field electroretinogram, and histology. Bioactivity of Bevacizumab was seen for up to 6 months with IVT concentrations up to 107 times higher than for the bolus injection [18]. Zhang et al. described an in situ-forming HG that undergoes gelation upon exposure to water through hydrophobic forces and physical cross-links. The HG was prepared via simple free-radical polymerization using poly(ethylene-glycol) methyl ether methylacrylate (PEGMA) and a vitamin E (Ve) methacrylate copolymer (PEGMA–co-Ve). The HGs showed a slow degradation process determined by the water content over a two-month period in vitro, with the lower-water-content gels showing the slowest degradation times [30]. Lovett et al. reported the good biocompatibility and sustained release of high doses of Bevacizumab (Avastin™) (50 μg/mL) for at least 3 months in Dutch-belted rabbits using silk-based HGs. Remarkably, the dose release concentration of Bevacizumab at day 90 was equivalent to the dose concentration of a standard single injection of 1.25 mg of Bevacizumab at day 30. Signs of HG biodegradation were detected after 3 months [17].
Another interesting example of a biodegradable HG for IVT use is OTX-IVT, an implantable biodegradable HG for the IVT sustained release of Aflibercept or Bevacizumab for up to 4–6 months, and OTX-TKI for the sustained release of axitinib, a small-molecule tyrosine kinase inhibitor with anti-angiogenic properties, for up to 12 months, for the treatment of wet AMD, designed by Ocular Therapeutix, Bedford, MA, USA in collaboration with Regeneron Pharmaceuticals, Tarrytown, NY, USA [31]. Multicentric clinical studies are underway to evaluate the safety, tolerability, and efficacy of OTX-TKI for IVT use in comparison to an on-label 8-week Aflibercept bolus injection in subjects with neovascular age-related macular degeneration (NCT03630315; NCT04989699). In September 2022, the company made public its 7-month interim report showing the good safety profile of OTX-TKI and sustained visual acuity in treated patients. Its 10-month provisional report presented in February 2023 also showed good tolerance, safety and sustained efficacy as compared with the Aflibercept bolus. Another clinical study is underway to evaluate the safety, tolerability, and efficacy of OTX-TKI in subjects with moderately severe to severe non-proliferative diabetic retinopathy (NCT05695417).
4. Stimuli-Responsive Hydrogels in Retinopathies
SRHGs are in situ gel-forming HGs considered smart materials because they modify their tridimensional structural conformation in response to different external stimuli such as pH, temperature, ionic strength, light, biomolecules, or when subjected to a magnetic field [32][33][34][35][36][37][38][39]. Because of this, it is possible to design HGs with specific rheological attributes that allow their use as injectable solutions through small gauge needles for IVT administration. This is a clear advantage over HGs that lack syringeability and have to be suspended in water prior to their administration. Accurate dosing, simple formulation processes, and easy sterilization are additional advantages to consider [40]. Furthermore, the movement of macroscopic HGs when gellified is significantly restricted or even absent in the vitreal cavity as they are larger than the average mesh size in the vitreous network of hyaluronic acid (500 nm) [41][42].
5. Temperature-Responsive Hydrogels for Intraocular Delivery
An example of SRHGs are thermo-responsive HGs (TRHGs) (Figure 2). They are designed to undergo the sol–gel phase transition in response to the existing environmental temperature of the vitreal cavity. The transition to a gel state (gelation by polymerization, self-assembly and/or cross-linking) occurs when polymers in solution are above or below the so-called lower critical solution temperature (LCST) and the upper critical solution temperature (UCST), when the solution separates into a gel phase and a solvent phase (often water) [40][43][44][45]. The use of TRHGs for intravitreal applications is under extensive study because of their good syringeability and fast in situ gelation in the vitreous. In addition, TRHGs can also be useful as tamponade agents in the vitreal cavity for the treatment/prevention of retinal detachment, as showed by Liu et al. using a urethane-based TRHG in vitrectomized non-human primates for up to 12 months [46]. Nonetheless, challenges remain regarding how to develop in situ gelling HGs that are able to sustain protein delivery for a period of months and not just days or weeks, and also regarding how to avoid/reduce the fast burst release of the protein payload to avoid toxicity and sustain efficacy.
In this context, Drapala et al. in 2011 used free-radical polymerization to develop TRHGs made of PNIPAAm cross-linked with either poly(ethylene glycol)–co-(L-lactic acid) diacrylate (PEG–PLLADA) or poly(ethylene glycol) diacrylate (PEGDA) and showed that the cross-linking density has a direct influence on protein release and degradation. Their TRHGs provided the sustained IVT release of Bevacizumab or Ranibizumab for a month [47]. These TRHGs did not show long-term adverse effects in the retina [33]. These same authors also showed in 2014 that the controlled degradation of the system was also influenced by the addition of biodegradable copolymers and other additives such as glutathione, as a chain transfer agent, for the delivery of immunoglobulin G (IgG) and the recombinant proteins Bevacizumab (Avastin®) and Ranibizumab (Lucentis®). These authors showed that increased concentrations of glutathione accelerated the degradation rate of the TRHGs and subsequent protein release, compared with TRHGs prepared without glutathione that showed complete protein release after 3 weeks. Moreover, the PEGylation of IgG also significantly reduced the protein burst release [48].
Other examples of biodegradable TRHGs for the extended release of Bevacizumab have been previously reported. Using a copolymer poly(2-ethyl-2-oxazoline)–b-poly(ε-caprolactone)–b-poly(2-ethyl-2-oxazoline) (PEOz–PCL–PEOz) HG, Wang et al. demonstrated the extended release of bioactive Bevacizumab in vitro for up to a month using a human retinal pigment epithelial cell line. The HG also had a good temperature-sensitive sol–gel phase transition and in vivo biocompatibility in the rabbit neuroretina, and showed well-preserved histomorphology and electrophysiology after 2 months of IVT injection [49]. Park et al. created a TRHG composed of poly(ethylene glycol)–poly(serinol hexamethylene urethane) (ESHU) capable of providing the sustained in vitro release of Bevacizumab for up to 4 months. Later on, Rauck et al. demonstrated the in vivo sustained release of Bevacizumab from ESHU after its IVT administration, for over 9 weeks. No toxicity was reported using a bovine corneal endothelial cell model, nor was any significant inflammatory response elicited over a 9-week period in vivo using male New Zealand white rabbit models. Remarkably, ESHU was able to maintain a 4.7 times higher intraocular drug concentration of Bevacizumab than the repeated administration of an IVT-injected bolus [50][51]. Furthermore, Xie et al. reported sustained IVT release of Avastin(R) using injectable TRHGs composed of poly(lactic acid–co-glycolic acid)–poly(ethylene glycol)–poly(lactic acid–co-glycolic acid) (PLGA–PEG–PLGA). Sustained Avastin® release was seen in vitro over a period of up to 14 days. Good biocompatibility, preserved retinal function, and the extended release of Avastin® were also detected in vivo for up to 2 months [52]. Similarly, López-Cano et al. recently reported the successful use of PLGA–PEG–PLGA TRHGs for the sustained release of the neuroprotective agents Dexamethasone and ketorolac to the retina, using in vitro models [53]. In 2019, Xue et al. reported promising results using multiblock poly(ether ester urethane) thermo-responsive HGs composed of poly(ethylene glycol), poly(propylene glycol) and poly(ε-caprolactone), with HMDI (1,6-hexamethylene diisocyanate) as a coupling agent. The TRHG showed good encapsulation of anti-VEGFs into polyurethane thermogel depots and moreover, that the anti-VEGF release rates were dependent on the hydrophilic–hydrophobic balance within the copolymer. Anti-VEGF release was seen in vitro for up to 40 days, and anti-angiogenic bioactivity was detected in rat ex vivo choroidal explants and in a VEGF-driven neovascularization rabbit model [54].
6. Thermo-Responsive Hydrogels Containing Nanoformulations
Whereas it has been shown that microparticles can be cleared from the vitreous in 50 days [55], the use of mixtures of TRHGs with nano- and microparticles can reduce the initial release burst of the protein payloads and allow their localized and extended release after IVT administration [56][57]. Nanogels are HGs composed of a hydrophilic polymer network and a therapeutic payload at the nanoscale. Therapeutic payloads can be conjugated and/or encapsulated into a variety of different nanoformulations (e.g., nanoparticles and polymer-based nanoparticulate systems, liposomes, solid lipid nanoparticles, dendrimers, etc.) within the nanogel, and they can be designed as SRHGs. Therefore, the influence of the addition of nano/microparticulate systems during protein release from TRHGs has been extensively studied [56][58][59].
Li et al. demonstrated that nano- and microspheres fabricated from poly(DL-lactide–co-glycolide) (PLGA) and poly(ethylene glycol)–b-poly(D,L-lactic acid) (PEGLA), were capable of sustaining the release of Bevacizumab (Avastin®) for 90 days in 10 mM phosphate-buffered saline (PBS). Interestingly, changes in the drug/polymer ratio also altered the protein release rates [60]. Therefore, the potential use of microspheres in TRHGs has also been explored in order to achieve the controlled and extended release of the protein payloads. In this regard, Kang-Mieler’s group showed that the addition of biodegradable PLGA microspheres loaded with the VEGF inhibitors Ranibizumab (Lucentis™) and Aflibercept (Eylea™) in a thermo-responsive PNIPAAm–PEG diacrylate (PEGDA) HG were able to achieve a controlled and extended release of the inhibitors for 6 months in vitro. The phase transition temperature of the TRHG could also be modified by adjusting the concentration of PEGDA in the cross-linking reaction [61][62][63][64]. The group also showed the in vivo efficacy and anti-angiogenic bioactivity of the released anti-VEGF payloads in a laser-induced rat model of choroidal neovascularization for up to 12 weeks [65].
Another example of an amphiphilic thermo-sensitive PLGA nanogel as an IVT delivery system was reported by Hu et al. A TRHG composed of a methoxy poly(ethylene glycol)–block poly(lactic–co-glycolic acid)–BOX (2,2′-bis(2-Oxazoline) (mPEG–PLGA–BOX) diblock copolymer was synthesized to deliver Bevacizumab for wet AMD using Rex rabbit models. In vitro sustained anti-VEGF activity was detected using human umbilical vein endothelial cells and rhesus choroid–retina endothelial cells [66][67]. Further experiments showed in vivo anti-angiogenic activity for up to 30 days in a Rex rabbit neoangiogenic model by using laser retinal photocoagulation. In addition, good cytocompatibility and preserved retinal function were observed [68].
Other similar examples have been published mixing liposomes or nanocomposites with TRHGs to extend protein release. Pachis et al. used liposomes loaded with Flurbiprofen (Flu), a non-steroidal anti-inflammatory drug (NSAID), mixed with Pluronic® F127 (a polymer sensitive to temperature that gellifies at body temperature) in IVT injections. Good biocompatibility extended the intraocular release of Flu, and tissue safety was demonstrated [69]. Furthermore, Sapino et al., also using Pluronic® F127, created TRHGs containing either solid lipid nanoparticles (SLNs) obtained by cold microemulsion dilution, or nanoemulsions (NEs) made with IPM as the lipid phase and Epikuron®200 as the surfactant (µE1-based thermosensitive nanocomposite). Both designs showed good syringeability properties and reduced initial burst release of cefuroxime, which is a cephalosporin antibiotic for the treatment of endophthalmitis, a severe IVT inflammatory disease, using a two-compartment in vitro eye flow model (PK-Eye) to estimate ocular drug clearance through the anterior aqueous outflow [70].
References
- Cao, Y.; Samy, K.E.; Bernards, D.A.; Desai, T.A. Recent advances in intraocular sustained-release drug delivery devices. Drug Discov. Today 2019, 24, 1694–1700.
- Bourges, J.L.; Bloquel, C.; Thomas, A.; Froussart, F.; Bochot, A.; Azan, F.; Gurny, R.; BenEzra, D.; Behar-Cohen, F. Intraocular implants for extended drug delivery: Therapeutic applications. Adv. Drug Deliv. Rev. 2006, 58, 1182–1202.
- Haller, J.A.; Dugel, P.; Weinberg, D.V.; Chou, C.; Whitcup, S.M. Evaluation of the safety and performance of an applicator for a novel IVT dexamethasone drug delivery system for the treatment of macular edema. Retina 2009, 29, 46–51.
- Lee, S.S.; Hughes, P.; Ross, A.D.; Robinson, M.R. Biodegradable implants for sustained drug release in the eye. Pharm. Res. 2010, 27, 2043–2053.
- Haghjou, N.; Soheilian, M.; Abdekhodaie, M.J. Sustained release intraocular drug delivery devices for treatment of uveitis. J. Ophthal. Vis. Res. 2011, 6, 317–329.
- Chan, A.; Leung, L.-S. Critical appraisal of the clinical utility of the dexamethasone intravitreal implant (Ozurdex-R) for the treatment of macular edema related to branch retinal vein occlusion or central retinal vein occlusion. Clin. Ophthalmol. 2011, 5, 1043–1049.
- Kuppermann, B.D.; Patel, S.S.; Boyer, D.S.; Augustin, A.J.; Freeman, W.R.; Kerr, K.J.; Guo, Q.; Schneider, S.; López, F.J. Phase 2 study of the safety and efficacy of brimonidine drug delivery system (brimo dds) generation 1 in patients with geographic atrophy secondary to age-related macular degeneration. Retina 2021, 41, 144–155.
- Freeman, W.R.; Bandello, F.; Souied, E.; Guymer, R.H.; Garg, S.J.; Chen, F.K.; Rich, R.; Holz, F.G.; Patel, S.S.; Kim, K.; et al. Randomized Phase IIb Study of Brimonidine Drug Delivery System Generation 2 for Geographic Atrophy in Age-Related Macular Degeneration. Ophthalmol. Retin. 2023, in press.
- Jaffe, G.J.; McCallum, R.M.; Branchaud, B.; Skalak, C.; Butuner, Z.; Ashton, P. Long-term follow-up results of a pilot trial of a fluocinolone acetonide implant to treat posterior uveitis. Ophthalmology 2005, 112, 1192–1198.
- Kuppermann, B.D.; Blumenkranz, M.S.; Haller, J.A.; Williams, G.A.; Weinberg, D.; Chou, C.; Whitcup, S.M. Randomized controlled study of an intravitreous dexamethasone drug delivery system in patients with persistent macular edema. Arch. Ophthalmol. 2007, 125, 309–317.
- Kane, F.E.; Burdan, J.; Cutino, A.; Green, K.E. Iluvien: A new sustained delivery technology for posterior eye disease. Expert Opin. Drug. Deliv. 2008, 5, 1039–1046.
- Frokjaer, S.; Otzen, D.E. Protein drug stability: A formulation challenge. Nat. Rev. Drug Discov. 2005, 4, 298.
- Ilochonwu, B.C.; Urtti, A.; Hennink, W.E.; Vermonden, T. Intravitreal hydrogels for sustained release of therapeutic proteins. J. Control. Release 2020, 326, 419–441.
- Vermonden, T.; Censi, R.; Hennink, W.E. Hydrogels for protein delivery. Chem. Rev. 2012, 112, 2853–2888.
- Kirchhof, S.; Abrami, M.; Messmann, V.; Hammer, N.; Goepferich, A.M.; Grassi, M.; Brandl, F.P. Diels-alder hydrogels for controlled antibody release: Correlation between mesh size and release rate. Mol. Pharm. 2015, 12, 3358–3368.
- Lin, C.C.; Metters, A.T. hydrogels in controlled release formulations: Network design and mathematical modeling. Adv. Drug Deliv. Rev. 2006, 58, 1379–1408.
- Lovett, M.L.; Wang, X.; Yucel, T.; York, L.; Keirstead, M.; Haggerty, L.; Kaplan, D.L. Silk hydrogels for sustained ocular delivery of anti-vascular endothelial growth factor (anti-VEGF) therapeutics. Eur. J. Pharm. Biopharm. 2015, 95, 271–278.
- Yu, Y.; Lau, L.C.; Lo, A.C.; Chau, Y. Injectable chemically crosslinked hydrogel for the controlled release of bevacizumab in vitreous: A 6-month in vivo study. Transl. Vis. Sci. Technol. 2015, 4, 5.
- Seah, I.; Zhao, X.; Lin, Q.; Liu, Z.; Su, S.Z.Z.; Yuen, Y.S.; Hunziker, W.; Lingam, G.; Loh, X.J.; Su, X. Use of biomaterials for sustained delivery of anti-VEGF to treat retinal diseases. Eye 2020, 34, 1341–1356.
- Franssen, O.; Vandervennet, L.; Roders, P.; Hennink, W.E. Degradable dextran hydrogels: Controlled release of a model protein from cylinders and microspheres. J. Control. Release 1999, 60, 211–221.
- Buwalda, S.J.; Bethry, A.; Hunger, S.; Kandoussi, S.; Coudane, J.; Nottelet, B. Ultrafast in situ forming poly(ethylene glycol)-poly(amido amine) hydrogels with tunable drug release properties via controllable degradation rates. Eur. J. Pharm. Biopharm. 2019, 139, 232–239.
- Censi, R.; Vermonden, T.; van Steenbergen, M.J.; Deschout, H.; Braeckmans, K.; De Smedt, S.C.; van Nostrum, C.F.; di Martino, P.; Hennink, W.E. Photopolymerized thermosensitive hydrogels for tailorable diffusion-controlled protein delivery. J. Control. Release 2009, 140, 230–236.
- Huang, X.; Brazel, C.S. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J. Control. Release 2001, 73, 121–136.
- Yu, J.; Xu, X.; Yao, F.; Luo, Z.; Jin, L.; Xie, B.; Shi, S.; Ma, H.; Li, X.; Chen, H. In situ covalently cross-linked PEG hydrogel for ocular drug delivery applications. Int. J. Pharm. 2014, 470, 151–157.
- Weber, L.M.; Lopez, C.G.; Anseth, K.S. Effects of PEG hydrogel crosslinking density on protein diffusion and encapsulated islet survival and function. J. Biomed. Mater. Res. Part A 2009, 90, 720–729.
- Kirchhof, S.; Gregoritza, M.; Messmann, V.; Hammer, N.; Goepferich, A.M.; Brandl, F.P. Diels-Alder hydrogels with enhanced stability: First step toward controlled release of bevacizumab. Eur. J. Pharm. Biopharm. 2015, 96, 217–225.
- Ikada, Y.; Tabata, Y. Protein release from gelatin matrices. Adv. Drug Deliv. Rev. 1998, 31, 287–301.
- Mellott, M.B.; Searcy, K.; Pishko, M.V. Release of protein from highly cross-linked hydrogels of poly(ethylene glycol) diacrylate fabricated by UV polymerization. Biomaterials 2001, 22, 929–941.
- Oo, C.; Kalbag, S.S. Leveraging the attributes of biologics and small molecules, and releasing the bottlenecks: A new wave of revolution in drug development. Expert Rev. Clin. Pharmacol. 2016, 9, 747–749.
- Zhang, J.; Muirhead, B.; Dodd, M.; Liu, L.; Xu, F.; Mangiacotte, N. An injectable hydrogel prepared using a PEG/Vitamin E copolymer facilitating aqueous-driven gelation. Biomacromolecules 2016, 17, 3648–3658.
- Elhayek, R.F.; Jarrett, T.; Lattrell, Z.; Takach, S.; Jarrett, P.K.; McGrath, M.; Talamo, J.H.; Sawhney, A. Efficacy of a 6 month sustained hydrogel delivery system for Tyrosine kinase inhibitors in a VEGF induced retinal leakage model. ARVO Abstract. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1968.
- Lee, S.C.; Kwon, I.K.; Park, K. Hydrogels for delivery of bioactive agents: A historical perspective. Adv. Drug Deliv. Rev. 2013, 65, 17–20.
- Turturro, S.B.; Guthrie, M.J.; Appel, A.A.; Drapala, P.W.; Brey, E.M.; Pérez-Luna, V.H.; Mieler, W.F.; Kang-Mieler, J.J. The effects of cross-linked thermo-responsive PNIPAAm-based hydrogel injection on retinal function. Biomaterials 2011, 32, 3620–3626.
- Qu, J.; Zhao, X.; Liang, Y.; Zhang, T.; Ma, P.X.; Guo, B. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials 2018, 183, 185–199.
- Kushwaha, S.K.; Saxena, P.; Rai, A. Stimuli sensitive hydrogels for ophthalmic drug delivery: A review. Int. J. Pharm. Investig. 2012, 2, 54.
- Thrimawithana, T.; Rupenthal, I.; Young, S.; Alany, R. Environment-sensitive polymers for ophthalmic drug delivery. J. Drug Deliv. Sci. Technol. 2012, 22, 117–124.
- Tanihara, M.; Suzuki, Y.; Nishimura, Y.; Suzuki, K.; Kakimaru, Y. Thrombin-sensitive peptide linkers for biological signal-responsive drug release systems. Peptides 1998, 19, 421–425.
- Shigemitsu, H.; Fujisaku, T.; Onogi, S.; Yoshii, T.; Ikeda, M.; Hamachi, I. Preparation of supramolecular hydrogel-enzyme hybrids exhibiting biomolecule-responsive gel degradation. Nat. Protoc. 2016, 11, 1744–1756.
- Miyata, T.; Uragami, T.; Nakamae, K. Biomolecule-sensitive hydrogels. Adv. Drug Del. Rev. 2002, 54, 79–98.
- Agrawal, A.K.; Das, M.; Jain, S. In situ gel systems as ‘smart’ carriers for sustained ocular drug delivery. Expert Opin. Drug Deliv. 2012, 9, 383–402.
- Xu, Q.; Boylan, N.J.; Suk, J.S.; Wang, Y.Y.; Nance, E.A.; Yang, J.C.; McDonnell, P.J.; Cone, R.A.; Duh, E.J.; Hanes, J. Nanoparticle diffusion in and microrheology of the bovine vitreous ex vivo. J. Control. Release 2013, 167, 76–84.
- Peeters, L.; Sanders, N.N.; Braeckmans, K.; Boussery, K.; Van de Voorde, J.; De Smedt, S.C.; Demeester, J. Vitreous: A barrier to nonviral ocular gene therapy. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3553–3561.
- Ruel-Gariépy, E.; Leroux, J.-C. In situ-forming hydrogels—Review of temperature-sensitive systems. Eur. J. Pharm. Biopharm. 2004, 58, 409–426.
- Cao, Y.; Zhang, C.; Shen, W.; Cheng, Z.; Yu, L.; Ping, Q. Poly(N-isopropylacrylamide)–chitosan as thermosensitive In Situ gel-forming system for ocular drug delivery. J. Control. Release 2007, 120, 186–194.
- Klouda, L. Thermoresponsive hydrogels in biomedical applications a seven-year update. Eur. J. Pharm. Biopharm. 2015, 97, 338–349.
- Liu, Z.; Liow, S.S.; Lai, S.L.; Alli-Shaik, A.; Holder, G.E.; Parikh, B.H.; Krishnakumar, S.; Li, Z.; Tan, M.J.; Gunaratne, J.; et al. Retinal-detachment repair and vitreous-like-body reformation via a thermogelling polymer endotamponade. Nat. Biomed. Eng. 2019, 3, 598–610.
- Drapala, P.W.; Brey, E.M.; Mieler, W.F.; Venerus, D.C.; Derwent, J.J.K.; Pérez-Luna, V.H. Role of thermo-responsiveness and poly (ethylene glycol) diacrylate cross-link density on protein release from poly(N-isopropylacrylamide) hydrogels. J. Biomater. Sci. Polym. Ed. 2011, 22, 59–75.
- Drapala, P.W.; Jiang, B.; Chiu, Y.-C.; Mieler, W.F.; Brey, E.M.; Kang-Mieler, J.J.; Pérez-Luna, V.H. The effect of glutathione as chain transfer agent in PNIPAAm-based thermo-responsive hydrogels for controlled release of proteins. Pharm. Res. 2014, 31, 742–753.
- Wang, C.H.; Hwang, Y.S.; Chiang, P.R.; Shen, C.R.; Hong, W.H.; Hsiue, G.H. Extended release of bevacizumab by thermosensitive biodegradable and biocompatible HG. Biomacromolecules 2012, 13, 40–48.
- Park, D.; Shah, V.; Rauck, B.M.; Friberg, T.R.; Wang, Y. An antiangiogenic reverse thermal gel as a drug-delivery system for agerelated wet macular degeneration. Macromol. Biosci. 2013, 13, 464–469.
- Rauck, B.M.; Friberg, T.R.; Medina Mendez, C.A.; Park, D.; Shah, V.; Bilonick, R.A.; Wang, Y. Biocompatible reverse thermal gel sustains the release of intravitreal bevacizumab in vivo. Investig. Ophthalmol. Vis. Sci. 2014, 55, 469–476.
- Xie, B.; Jin, L.; Luo, Z.; Yu, J.; Shi, S.; Zhang, Z.; Shen, M.; Chen, H.; Li, X.; Song, Z. An injectable thermosensitive polymeric hydrogel for sustained release of Avastin(R) to treat posterior segment disease. Int. J. Pharm. 2015, 490, 375–383.
- López-Cano, J.J.; Sigen, A.; Andrés-Guerrero, V.; Tai, H.; Bravo-Osuna, I.; Molina-Martínez, I.T.; Wang, W.; Herrero-Vanrell, R. Thermo-responsive PLGA-PEG-PLGA hydrogels as novel injectable platforms for neuroprotective combined therapies in the treatment of retinal degenerative diseases. Pharmaceutics 2021, 13, 234.
- Xue, K.; Zhao, X.; Zhang, Z.; Qiu, B.; Tan, Q.S.W.; Ong, K.H.; Liu, Z.; Parikh, B.H.; Barathi, V.A.; Yu, W.; et al. Sustained delivery of anti-VEGFs from thermogel depots inhibits angiogenesis without the need for multiple injections. Biomater. Sci. 2019, 7, 4603–4614.
- Moritera, T.; Ogura, Y.; Hondo, Y.; Wadaf, R.; Hyoaf, S.-H.; Ikadaf, Y. Microspheres of biodegradable polymers as a drug-delivery system in the vitreous. Investig. Ophthalmol. Vis. Sci. 1991, 32, 1785–1790.
- Buwalda, S.J.; Vermonden, T.; Hennink, W.E. Hydrogels for therapeutic delivery: Current developments and future directions. Biomacromolecules 2017, 18, 316–330.
- Kang-Mieler, J.J.; Dosmar, E.; Liu, W.; Mieler, W.F. Extended ocular drug delivery systems for the anterior and posterior segments: Biomaterial options and applications. Expert Opin. Drug Deliv. 2017, 14, 611–620.
- Kompella, U.B.; Amrite, A.C.; Ravi, R.P.; Durazo, S.A. Nanomedicines for back of the eye drug delivery, gene delivery, and imaging. Prog. Retin. Eye Res. 2013, 36, 172–198.
- Sepahvandi, A.; Eskandari, M.; Moztarzadeh, F. Drug Delivery Systems to the Posterior Segment of the Eye: Implants and Nanoparticles. BioNanoScience 2016, 6, 276–283.
- Li, F.; Hurley, B.; Liu, Y.; Leonard, B.; Griffith, M. Controlled Release of Bevacizumab through Nanospheres for Extended Treatment of Age-Related Macular Degeneration. Open Ophthalmol. J. 2012, 6, 54–58.
- Osswald, C.R.; Kang-Mieler, J.J. Controlled and extended release of a model protein from a microsphere-hydrogel drug delivery system. Ann. Biomed. Eng. 2015, 43, 2609–2617.
- Osswald, C.R.; Kang-Mieler, J.J. Controlled and extended in vitro release of bioactive anti-vascular endothelial growth factors from a microsphere-hydrogel drug delivery system. Curr. Eye Res. 2016, 41, 1216–1222.
- Liu, W.; Lee, B.S.; Mieler, W.F.; Kang-Mieler, J.J. Biodegradable microsphere-hydrogel ocular drug delivery system for controlled and extended release of bioactive aflibercept in vitro. Curr. Eye Res. 2019, 44, 264–274.
- Liu, W.; Borrell, M.A.; Venerus, D.C.; Mieler, W.F.; Kang-Mieler, J.J. Characterization of biodegradable microsphere-hydrogel ocular drug delivery system for controlled and extended release of ranibizumab. Transl. Vis. Sci. Technol. 2019, 8, 12.
- Osswald, C.R.; Guthrie, M.J.; Avila, A.; Valio, J.A., Jr.; Mieler, W.F.; Kang-Mieler, J.J. In vivo efficacy of an injectable microsphere hydrogel ocular drug delivery system. Curr. Eye Res. 2017, 42, 1293–1301.
- Hu, C.C.; Chaw, J.R.; Chen, C.F.; Liu, H.W. Controlled release bevacizumab in thermoresponsive hydrogel found to in-hibit angiogenesis. Bio-Med. Mater. Eng. 2014, 24, 1941–1950.
- Hu, C.C.; Chaw, J.R.; Chen, Y.C.; Chen, C.F.; Liu, H.W. A novel thermo-responsive nanogel for intraocular drug delivery. J. Comput. Theory Nanosci. 2015, 12, 762–768.
- Hu, C.C.; Chiu, Y.C.; Chaw, J.R.; Chen, C.F.; Liu, H.W. Thermo-responsive hydrogel as an anti-VEGF drug delivery system to inhibit retinal angiogenesis in Rex rabbits. Technol. Health Care 2019, 27, 153–163.
- Pachis, K.; Blazaki, S.; Tzatzarakis, M.; Klepetsanis, P.; Naoumidi, E.; Tsilimbaris, M.; Antimisiaris, S.G. Sus-tained release of intravitreal flurbiprofen from a novel drug-in-liposome-in-hydrogel formulation. Eur. J. Pharm. Sci. 2017, 109, 324–333.
- Sapino, S.; Peira, E.; Chirio, D.; Chindamo, G.; Guglielmo, S.; Oliaro-Bosso, S.; Barbero, R.; Vercelli, C.; Re, G.; Brunella, V.; et al. Thermosensitive Nanocomposite hydrogels for intravitreal Delivery of Cefuroxime. Nanomaterials 2019, 9, 1461.
More
Information
Subjects:
Pharmacology & Pharmacy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
17 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




