Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dan Yaniv | -- | 1446 | 2023-05-10 21:13:07 | | | |
| 2 | Peter Tang | + 1 word(s) | 1447 | 2023-05-11 03:51:08 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Chiang, E.; Stafford, H.; Buell, J.; Ramesh, U.; Amit, M.; Nagarajan, P.; Migden, M.; Yaniv, D. Tumor Microenvironment in Basal and Squamous Cell Carcinoma. Encyclopedia. Available online: https://encyclopedia.pub/entry/44124 (accessed on 07 February 2026).
Chiang E, Stafford H, Buell J, Ramesh U, Amit M, Nagarajan P, et al. Tumor Microenvironment in Basal and Squamous Cell Carcinoma. Encyclopedia. Available at: https://encyclopedia.pub/entry/44124. Accessed February 07, 2026.
Chiang, Elizabeth, Haleigh Stafford, Jane Buell, Uma Ramesh, Moran Amit, Priyadharsini Nagarajan, Michael Migden, Dan Yaniv. "Tumor Microenvironment in Basal and Squamous Cell Carcinoma" Encyclopedia, https://encyclopedia.pub/entry/44124 (accessed February 07, 2026).
Chiang, E., Stafford, H., Buell, J., Ramesh, U., Amit, M., Nagarajan, P., Migden, M., & Yaniv, D. (2023, May 10). Tumor Microenvironment in Basal and Squamous Cell Carcinoma. In Encyclopedia. https://encyclopedia.pub/entry/44124
Chiang, Elizabeth, et al. "Tumor Microenvironment in Basal and Squamous Cell Carcinoma." Encyclopedia. Web. 10 May, 2023.
Copy Citation
It is widely known that tumor cells of basal and squamous cell carcinoma interact with the cellular and acellular components of the tumor microenvironment to promote tumor growth and progression. While this environment differs for basal and squamous cell carcinoma, the cellular players within both create an immunosuppressed environment by downregulating effector CD4+ and CD8+ T cells and promoting the release of pro-oncogenic Th2 cytokines. Understanding the crosstalk that occurs within the tumor microenvironment has led to the development of immunotherapeutic agents, including vismodegib and cemiplimab to treat BCC and SCC, respectively.
tumor microenvironment
basal cell carcinoma
squamous cell carcinoma
immunotherapy
1. Introduction
Skin cancer is the most common cancer diagnosed in the United States and represents a worldwide threat as its incidence steadily rises [1]. Risk factors for skin cancer development include both genetic predisposition and environmental factors, specifically, exposure to UV rays [2]. Basal cell carcinoma and squamous cell carcinoma are the most common skin cancer types [3].
The tumor microenvironment (TME) refers to the cellular environment of tumors or cancer stem cells [4][5]. In addition to tumor cells themselves, the TME includes nerve cells, epithelial cells, fibroblasts, plasmacytoid cells, dendritic cells, Langerhans cells, macrophages, and lymphocytes (Figure 1). This composition varies based on the type of cancer and a patient’s immune status [6]. Each of these cell types may act in either an anti- or pro-oncogenic manner, depending on their interactions with other components of the TME [7]. In turn, tumor cells stimulate cellular and molecular crosstalk in the TME to promote an immunosuppressive state, allow tumor progression, and even change the phenotype of cells in the TME [8][9].
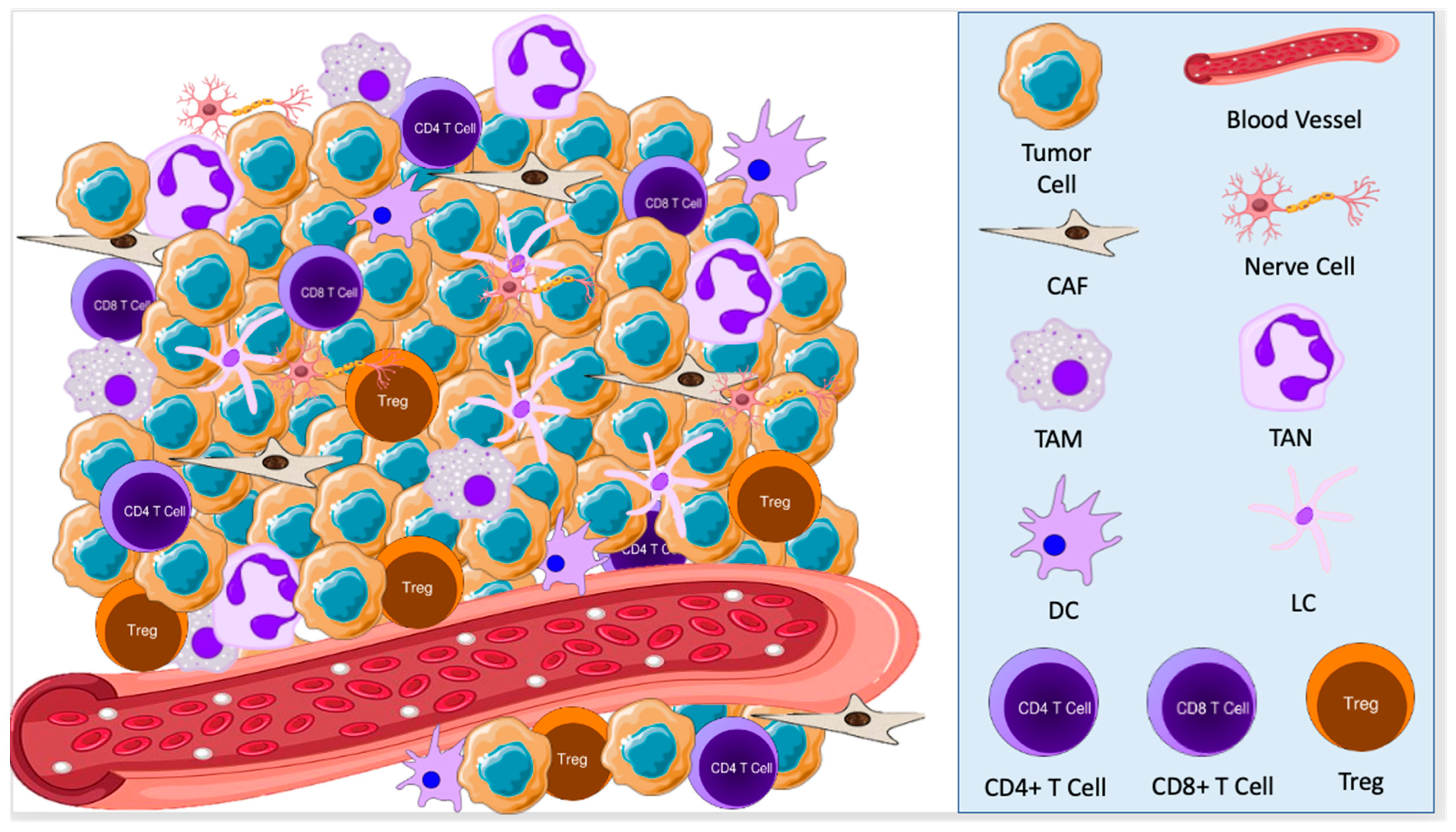
Figure 1. The tumor microenvironment consists of tumor cells, nerve cells, cancer-associated fibroblasts (CAFs), tumor-associated macrophages (TAMs), tumor-associated neutrophils (TANs), dendritic cells (DCs), Langerhans cells (LCs), and lymphocytes. These cells interact with each other to promote a pro-oncogenic state, allowing for tumor growth and development.
2. Basal Cell Carcinoma
Basal cell carcinoma (BCC) is the most prevalent skin cancer, making up approximately 80% of all non-melanoma skin cancer cases [10]. Patients are typically over 60 years old at first presentation with BCC, and these lesions tend to present on body areas exposed to the sun. BCC pathogenesis is complex and has been traced to several risk-factors. Sun exposure is the usual culprit; however, recent research has connected increased physiologic exposure to hormone replacement therapy (HRT) and oral contraceptives (OCs) to aggressive BCC subtypes [11][12]. The molecular mechanism behind this connection is not well understood, but, conventionally, BCC pathogenesis has been traced back to aberrant sonic hedgehog (Shh) signaling [10]. Irreversible activation of Shh signaling creates high levels of oncogenic glioma-associated oncogene homolog (GLI) transcription factors, which initiate and promote BCC tumor growth [13]. Activation of Shh signaling in the TME has been associated with tumor growth and metastatic activity via its contribution to an immunosuppressed environment [10]. The BCC tumor develops the TME in a dense surrounding fibromyxoid stroma, protecting the tumor from the host’s immune system and promoting tumor angiogenesis and progression [14][15].
3. Squamous Cell Carcinoma
Cutaneous squamous cell carcinoma (SCC) is the second most common type of non-melanoma skin cancer, accounting for approximately 20% of skin cancers [16]. Risk factors for developing SCC include advanced age, extensive sun exposure, and immunosuppression [16]. Typically, most SCC lesions can be successfully treated with excision; however, some metastasize, potentially becoming life-threatening. Thus, about 75% of all deaths due to skin cancer are caused by SCC [16]. The development of SCC is gradual, stemming from an uncontrolled proliferation of epidermal keratinocytes and progression through dysplasia and actinic keratosis as the tumor cells accumulate genetic mutations in genes such as TP53, CDKN21, NOTCH, and RAS [16][17]. During tumorigenesis, the cancer cells enable growth, angiogenesis, and metastasis through autocrine and paracrine signaling [18]. Recent research has shown that the loss of p53, which commonly occurs in SCC tumors, allows for increased expression of type 2 Deiodinase (D2), an enzyme that activates the thyroid hormone (TH) [19][20]. In turn, TH induces VEGF-A transcription in SCC tumor cells, fostering tumor angiogenesis, nutrient delivery, and cancer progression [21].
4. Therapeutic Considerations for Skin Cancer
For both BCC and SCC, surgical excision is the preferred treatment [22][23]. However, for advanced or metastatic non-resectable cancers, management has transitioned to an immunotherapeutic approach [24]. The treatment of advanced BCC focuses on Shh inhibition, while therapy for SCC interferes with the PD-1/PD-L1 checkpoint [25][26].
Early treatment of BCC with surgical resection is curative in most cases, but, for some patients, locally advanced or metastatic tumors can be life-threatening or, at the very least, can negatively impact a patient’s quality of life [22]. Therefore, clinicians have turned to Shh pathway inhibitors such as vismodegib or sonidegib as an alternative treatment for more severe cases [22][25]. Administration of these Shh inhibitors triggers increases in tumor-infiltrating CD8+ T cells and tumor-cell major histocompatibility complex I (MHC class I), stimulating an anti-tumoral response within the TME [25]. The ERIVANCE trial, studying the efficacy of vismodegib in advanced BCC, showed overall response rates of 48.5% in metastatic cohorts and 60.3% in locally advanced patients [27]. However, that still leaves many cases of BCC that are unresponsive to this treatment, despite its seemingly beneficial immunologic effects [25]. Recent studies have shown that vismodegib-resistant tumor cells transform their cell identity toward a mesenchymal stem cell-like profile, becoming resistant to Shh pathway inhibitors but providing a potential target for therapy [14].
For patients with SCC, surgical excision is the treatment modality of choice; however, for patients with increased risks of local recurrence, perineural spread, and metastasis, immunotherapy with cemiplimab, pembrolizumab, or nivolumab have been considered [23][26]. These drugs are PD-1 inhibitors, which inhibit the PD-1/PD-L1 immune checkpoint that typically decreases T cell functionality in the TME, suppresses the immune system, and accelerates cancer cell proliferation [26][28]. Therefore, treatment with these immunotherapy agents increases the volume of cytotoxic CD8+ T cells in the TME, allowing for tumor cell destruction [26]. In advanced-stage patients who responded to neoadjuvant cemiplimab, the drug had an overwhelming response, with many patients achieving complete pathologic responses [29]. The most suitable combination of this drug with other treatment modalities such as surgery and radiation, the clinical meaning of a pathologic complete response after immunotherapy, and the need for further treatment are still being explored. However, similar to the treatment of BCC with vismodegib, SCC therapy with cemiplimab achieved an objective response rate of approximately 50% of patients [30]. Therefore, further research must be conducted to determine more effective treatment methods for advanced SCC.
A better understanding of the TME provides many potential sites for intervention. For example, finding a way to polarize TAMs toward their M1 anti-tumorigenic phenotypes through immune modulation mechanisms may be a nonsurgical method to treat advanced BCC in the future [31]. Likewise, TANs in SCC could be polarized more toward the N1 phenotype through the inhibition of TGF-β in the TME [32]. As new research further explores the intricacies of the TME of BCC and SCC, there will be more opportunities to develop targeted therapies to treat advanced cancers.
Research into TME therapeutic targets is ongoing. Lu et al. discovered that the overexpression of the brain-derived neurotrophic factor (BDNF) and its p75 pan-neurotrophin receptor could induce BCC tumor cell death by stimulating M1 macrophages and T cell recruitment on a mouse model, providing a potential therapeutic intervention for Shh inhibitor-resistant tumors [33]. Moreover, by studying cancers with similar TME profiles to that of BCC, researchers have begun investigating the use of therapies for other cancers, such as kidney chromophobe cancers or myxofibrosarcoma, in treating advanced BCC. For example, Zhang et al. suggested targeting the RB pathway, as one would treat myxofibrosarcoma to modulate the TME contained within the BCC fibromyxoid stroma, rendering it less suitable for tumor growth [34]. The researchers conducted a search on clinicaltrials.gov using the search terms “Skin Cancer, Non-Melanomta”, “SCC”, and “BCC” to identify clinical trials targeting components of the TME. Some of these innovative trials include a few institutions who are attempting to use genetically modified viruses to enhance host immune responses against BCC and cutaneous SCC lesions. Researchers are studying the type I herpes simplex virus genetically modified to preferentially replicate in tumor cells talimogene laherparepvec (T-VEC) because it can be injected directly into tumors to enhance antigen presentation by DCs, promoting an anti-oncogenic immune response [ID: NCT03458117, NCT02978625, NCT04163952] [35]. Other institutions are researching similar biologics including Imvamune, a modified smallpox vaccine, and IFx-Hu2.0, an immunomodulatory agent that triggers innate and adaptive immune responses in non-melanoma skin cancer tumors [ID: NCT04410874, NCT04160065]. Specifically for treating cutaneous SCC, a non-coding RNA called CV8102 has been developed to mimic a viral infection of the tumor, which serves to recruit and activate APCs and, subsequently, T cells to kill tumor cells at the site of injection [ID: NCT03291002]. Thus, a deeper understanding of the cellular players and interactions within the TME has allowed for the development of novel immunotherapies.
References
- Gruber, P.; Zito, P.M. Skin Cancer. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022.
- Leiter, U.; Eigentler, T.; Garbe, C. Epidemiology of Skin Cancer. In Sunlight, Vitamin D and Skin Cancer; Springer: New York, NY, USA, 2014; pp. 120–140. ISBN 978-1-4939-0436-5.
- Bashline, B. Skin Cancer: Squamous and Basal Cell Carcinomas. FP Essent 2019, 481, 17–22.
- Arneth, B. Tumor Microenvironment. Medicina 2019, 56, 15.
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, Inflammation, and Cancer. Cell 2010, 140, 883–899.
- Capasso, A.; Viggiano, D.; Lee, M.W.; Palladino, G.; Bilancio, G.; Simeoni, M.; Capolongo, G.; Secondulfo, C.; Ronchi, A.; Caputo, A.; et al. Kidney Transplant Modifies the Architecture and Microenvironment of Basal Cell Carcinomas. Kidney Blood Press Res. 2020, 45, 368–377.
- Amôr, N.G.; Santos, P.S.d.S.; Campanelli, A.P. The Tumor Microenvironment in SCC: Mechanisms and Therapeutic Opportunities. Front. Cell Dev. Biol. 2021, 9, 636544.
- Ji, A.L.; Rubin, A.J.; Thrane, K.; Jiang, S.; Reynolds, D.L.; Meyers, R.M.; Guo, M.G.; George, B.M.; Mollbrink, A.; Bergenstråhle, J.; et al. Multimodal Analysis of Composition and Spatial Architecture in Human Squamous Cell Carcinoma. Cell 2020, 182, 497–514.e22.
- Amit, M.; Takahashi, H.; Dragomir, M.P.; Lindemann, A.; Gleber-Netto, F.O.; Pickering, C.R.; Anfossi, S.; Osman, A.A.; Cai, Y.; Wang, R.; et al. Loss of P53 Drives Neuron Reprogramming in Head and Neck Cancer. Nature 2020, 578, 449–454.
- Moisejenko-Golubovica, J.; Volkov, O.; Ivanova, A.; Groma, V. Analysis of the Occurrence and Distribution of Primary and Recurrent Basal Cell Carcinoma of Head and Neck Coupled to the Assessment of Tumor Microenvironment and Sonic Hedgehog Signaling. Rom. J. Morphol. Embryol. 2020, 61, 821–831.
- Cahoon, E.K.; Kitahara, C.M.; Ntowe, E.; Bowen, E.M.; Doody, M.M.; Alexander, B.H.; Lee, T.; Little, M.P.; Linet, M.S.; Freedman, D.M. Female Estrogen-Related Factors and Incidence of Basal Cell Carcinoma in a Nationwide US Cohort. J. Clin. Oncol. 2015, 33, 4058–4065.
- Kuklinski, L.F.; Zens, M.S.; Perry, A.E.; Gossai, A.; Nelson, H.H.; Karagas, M.R. Sex Hormones and the Risk of Keratinocyte Cancers among Women in the United States: A Population-Based Case-Control Study. Int. J. Cancer 2016, 139, 300–309.
- Grund-Gröschke, S.; Ortner, D.; Szenes-Nagy, A.B.; Zaborsky, N.; Weiss, R.; Neureiter, D.; Wipplinger, M.; Risch, A.; Hammerl, P.; Greil, R.; et al. Epidermal Activation of Hedgehog Signaling Establishes an Immunosuppressive Microenvironment in Basal Cell Carcinoma by Modulating Skin Immunity. Mol. Oncol. 2020, 14, 1930–1946.
- Lefrançois, P.; Xie, P.; Gunn, S.; Gantchev, J.; Villarreal, A.M.; Sasseville, D.; Litvinov, I.V. In Silico Analyses of the Tumor Microenvironment Highlight Tumoral Inflammation, a Th2 Cytokine Shift and a Mesenchymal Stem Cell-like Phenotype in Advanced in Basal Cell Carcinomas. J. Cell Commun. Signal. 2020, 14, 245–254.
- Ressler, J.M.; Zila, N.; Korosec, A.; Yu, J.; Silmbrod, R.; Bachmayr, V.; Tittes, J.; Strobl, J.; Lichtenberger, B.M.; Hoeller, C.; et al. Myofibroblast Stroma Differentiation in Infiltrative Basal Cell Carcinoma Is Accompanied by Regulatory T-cells. J. Cutan. Pathol. 2023, cup.14381.
- Fania, L.; Didona, D.; Di Pietro, F.R.; Verkhovskaia, S.; Morese, R.; Paolino, G.; Donati, M.; Ricci, F.; Coco, V.; Ricci, F.; et al. Cutaneous Squamous Cell Carcinoma: From Pathophysiology to Novel Therapeutic Approaches. Biomedicines 2021, 9, 171.
- Quadri, M.; Marconi, A.; Sandhu, S.K.; Kiss, A.; Efimova, T.; Palazzo, E. Investigating Cutaneous Squamous Cell Carcinoma in Vitro and in Vivo: Novel 3D Tools and Animal Models. Front. Med. 2022, 9, 875517.
- Flemming, J.P.; Hill, B.L.; Haque, M.W.; Raad, J.; Bonder, C.S.; Harshyne, L.A.; Rodeck, U.; Luginbuhl, A.; Wahl, J.K.; Tsai, K.Y.; et al. MiRNA- and Cytokine-associated Extracellular Vesicles Mediate Squamous Cell Carcinomas. J. Extracell. Vesicles 2020, 9, 1790159.
- Nappi, A.; Miro, C.; Pezone, A.; Tramontano, A.; Di Cicco, E.; Sagliocchi, S.; Cicatiello, A.G.; Murolo, M.; Torabinejad, S.; Abbotto, E.; et al. Loss of P53 Activates Thyroid Hormone via Type 2 Deiodinase and Enhances DNA Damage. Nat. Commun. 2023, 14, 1244.
- Nappi, A.; Di Cicco, E.; Miro, C.; Cicatiello, A.G.; Sagliocchi, S.; Mancino, G.; Ambrosio, R.; Luongo, C.; Di Girolamo, D.; De Stefano, M.A.; et al. The NANOG Transcription Factor Induces Type 2 Deiodinase Expression and Regulates the Intracellular Activation of Thyroid Hormone in Keratinocyte Carcinomas. Cancers 2020, 12, 715.
- Miro, C.; Nappi, A.; Cicatiello, A.G.; Di Cicco, E.; Sagliocchi, S.; Murolo, M.; Belli, V.; Troiani, T.; Albanese, S.; Amiranda, S.; et al. Thyroid Hormone Enhances Angiogenesis and the Warburg Effect in Squamous Cell Carcinomas. Cancers 2021, 13, 2743.
- Puig, S.; Berrocal, A. Management of High-Risk and Advanced Basal Cell Carcinoma. Clin. Transl. Oncol. 2015, 17, 497–503.
- Work Group; Invited Reviewers; Kim, J.Y.S.; Kozlow, J.H.; Mittal, B.; Moyer, J.; Olenecki, T.; Rodgers, P. Guidelines of Care for the Management of Cutaneous Squamous Cell Carcinoma. J. Am. Acad. Dermatol. 2018, 78, 560–578.
- Shalhout, S.Z.; Emerick, K.S.; Kaufman, H.L.; Miller, D.M. Immunotherapy for Non-Melanoma Skin Cancer. Curr. Oncol. Rep. 2021, 23, 125.
- Lipson, E.J.; Lilo, M.T.; Ogurtsova, A.; Esandrio, J.; Xu, H.; Brothers, P.; Schollenberger, M.; Sharfman, W.H.; Taube, J.M. Basal Cell Carcinoma: PD-L1/PD-1 Checkpoint Expression and Tumor Regression after PD-1 Blockade. J. Immunother. Cancer 2017, 5, 23.
- Ansary, T.M.; Hossain, M.D.R.; Komine, M.; Ohtsuki, M. Immunotherapy for the Treatment of Squamous Cell Carcinoma: Potential Benefits and Challenges. Int. J. Mol. Sci. 2022, 23, 8530.
- Sekulic, A.; Migden, M.R.; Oro, A.E.; Dirix, L.; Lewis, K.D.; Hainsworth, J.D.; Solomon, J.A.; Yoo, S.; Arron, S.T.; Friedlander, P.A.; et al. Efficacy and Safety of Vismodegib in Advanced Basal-Cell Carcinoma. N. Engl. J. Med. 2012, 366, 2171–2179.
- Jin, H.-T.; Ahmed, R.; Okazaki, T. Role of PD-1 in Regulating T-Cell Immunity. Curr. Top. Microbiol. Immunol. 2011, 350, 17–37.
- Gross, N.D.; Miller, D.M.; Khushalani, N.I.; Divi, V.; Ruiz, E.S.; Lipson, E.J.; Meier, F.; Su, Y.B.; Swiecicki, P.L.; Atlas, J.; et al. Neoadjuvant Cemiplimab for Stage II to IV Cutaneous Squamous-Cell Carcinoma. N. Engl. J. Med. 2022, 387, 1557–1568.
- Migden, M.R.; Rischin, D.; Schmults, C.D.; Guminski, A.; Hauschild, A.; Lewis, K.D.; Chung, C.H.; Hernandez-Aya, L.; Lim, A.M.; Chang, A.L.S.; et al. PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. N. Engl. J. Med. 2018, 379, 341–351.
- Tjiu, J.-W.; Chen, J.-S.; Shun, C.-T.; Lin, S.-J.; Liao, Y.-H.; Chu, C.-Y.; Tsai, T.-F.; Chiu, H.-C.; Dai, Y.-S.; Inoue, H.; et al. Tumor-Associated Macrophage-Induced Invasion and Angiogenesis of Human Basal Cell Carcinoma Cells by Cyclooxygenase-2 Induction. J. Investig. Dermatol. 2009, 129, 1016–1025.
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of Tumor-Associated Neutrophil Phenotype by TGF-Beta: “N1” versus “N2” TAN. Cancer Cell 2009, 16, 183–194.
- Lu, Q.; Qu, Y.; Ding, Y.; Kang, X. P75NTR/ProBDNF Modulates Basal Cell Carcinoma (BCC) Immune Microenvironment via Necroptosis Signaling Pathway. J. Immunol. Res. 2021, 2021, 1–10.
- Zhang, E.-R.; Ghezelbash, S.; Xie, P.; Fotovati, M.; Litvinov, I.V.; Lefrançois, P. Comparison of the Basal Cell Carcinoma (BCC) Tumour Microenvironment to Other Solid Malignancies. Cancers 2023, 15, 305.
- Conry, R.M.; Westbrook, B.; McKee, S.; Norwood, T.G. Talimogene Laherparepvec: First in Class Oncolytic Virotherapy. Hum. Vaccines Immunother. 2018, 14, 839–846.
More
Information
Subjects:
Dermatology; Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
966
Revisions:
2 times
(View History)
Update Date:
11 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




