Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Amanda Lynne Kornel | -- | 3013 | 2023-05-08 16:47:00 | | | |
| 2 | Sirius Huang | Meta information modification | 3013 | 2023-05-10 02:44:49 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kornel, A.; Nadile, M.; Retsidou, M.I.; Sakellakis, M.; Gioti, K.; Beloukas, A.; Sze, N.S.K.; Klentrou, P.; Tsiani, E. Effects of Ursolic Acid Against Prostate Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/43988 (accessed on 08 February 2026).
Kornel A, Nadile M, Retsidou MI, Sakellakis M, Gioti K, Beloukas A, et al. Effects of Ursolic Acid Against Prostate Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/43988. Accessed February 08, 2026.
Kornel, Amanda, Matteo Nadile, Maria Ilektra Retsidou, Minas Sakellakis, Katerina Gioti, Apostolos Beloukas, Newman Siu Kwan Sze, Panagiota Klentrou, Evangelia Tsiani. "Effects of Ursolic Acid Against Prostate Cancer" Encyclopedia, https://encyclopedia.pub/entry/43988 (accessed February 08, 2026).
Kornel, A., Nadile, M., Retsidou, M.I., Sakellakis, M., Gioti, K., Beloukas, A., Sze, N.S.K., Klentrou, P., & Tsiani, E. (2023, May 08). Effects of Ursolic Acid Against Prostate Cancer. In Encyclopedia. https://encyclopedia.pub/entry/43988
Kornel, Amanda, et al. "Effects of Ursolic Acid Against Prostate Cancer." Encyclopedia. Web. 08 May, 2023.
Copy Citation
Prostate cancer is the second most diagnosed form of cancer in men worldwide (second to lung cancer). Finding novel approaches to prevent and treat prostate and other urogenital cancers effectively is of major importance. Chemicals derived from plants, such as docetaxel and paclitaxel, have been used in cancer treatment. Ursolic acid, found in high concentrations in cranberries, is a pentacyclic triterpenoid compound demonstrated to have anti-inflammatory, antioxidant, and anticancer properties.
ursolic acid
prostate
urogenital
cancer
survival
apoptosis
proliferation
signaling cascades
1. Effects of Ursolic Acid against Prostate Cancer: Evidence from In Vitro Studies
The treatment of human androgen-sensitive LNCaP and hormone refractory PC-3 prostate cancer cells with UA (55 µM for 24 and 48 h) reduced viability (MTT assay) and induced apoptosis (Annexin assay) and these effects were associated with a downregulation of the anti-apoptotic protein B-cell lymphoma 2 (Bcl-2) [1]. These results provide evidence that UA initiates apoptosis in prostate cancer cells through downregulating the anti-apoptotic protein Bcl-2.
Zhang et al. showed that UA dose-dependently decreased viability and induced apoptosis in both LNCaP androgen-dependent and LNCaP-A1 androgen-independent prostate cancer cells [2]. Western blot analysis revealed that UA treatment increased the phosphorylation/activation of c-Jun N-terminal kinase (JNK) and its downstream target transcription factor c-Jun without affecting the activation of other mitogen activated proteins kinases (MAPKs) such as the extracellular signal-regulated protein kinase (ERK) and p38. Fluorometric assays demonstrated an increase in caspase-3 and caspase-9 activity with no change in caspase-8 activity indicated activation of the intrinsic apoptotic pathway. UA treatment in both cell lines caused an increase in phosphorylation of Bcl-2 protein resulting in its degradation. The involvement of the JNK pathway in UA-induced apoptosis was confirmed using the JNK inhibitor, SP600125. The UA-induced activation of the examined apoptotic markers was abolished when cells were pretreated with SP600125. These data indicate apoptosis induction by UA is triggered by increased activation of JNK and downstream activation of the intrinsic apoptotic pathway [2].
LNCaP prostate cancer cells treated with UA (40 µM) had suppressed proliferation and increased apoptosis which occurred via mediation of the ROCK1/PTEN signaling pathway. UA induced cleavage of ROCK1 and phosphorylation of PTEN leading to increased protein expression of cytochrome c and cofilin-1. Increased cytochrome c due to UA treatment lead to increased activity of caspases-3 and -9 [3].
The treatment of primary malignant tumor (RC-58T/h/SA#4)-derived human prostate cancer cells with UA inhibited survival, reduced cell density, and increased apoptosis. Cell treatment with UA (30 or 40 µg/mL, 24 h) caused nuclear condensation, formation of apoptotic bodies, and DNA fragmentation, all markers of apoptosis. Further investigation of the cell cycle with flow cytometry showed an increased number of cells in the subG1 phase. An increase of active caspase-3, -8, and -9 was observed with UA treatment (investigated by fluorometric assays). Western blot analysis showed upregulation of the pro-apoptotic protein Bax, downregulation of the anti-apoptotic protein Bcl-2, and induction of Bid cleavage. These data suggest that UA treatment stimulates the activation of caspase-8 leading to Bid cleavage and downstream activation of caspase-9. UA also increased the expression of mitochondrial apoptosis factor (AIF) and caused its translocation into the nucleus. Together these data suggest that treatment of RC-58T/h/SA#4 prostate cancer cells with UA induces apoptosis through both caspase-dependent and independent pathways [4].
Exposure of PC-3 prostate cancer cells to UA resulted in reduced viability and increased apoptosis through activation of both intrinsic and extrinsic apoptotic pathways. Fluorometric assays showed increased caspase-3, -8, and -9 activity while Western blotting demonstrated increased cleavage of caspase-8 and -9. Inhibiting either caspase-8 or -9 (using Z-IETD-FMK and Z-LEHD-FMK, specific inhibitors for caspase-8 and -9, respectively) prevented the UA-induced apoptosis. In addition, UA treatment lead to activation of JNK and subsequent Bcl-2 phosphorylation. UA reduced Akt phosphorylation and increased the levels of Fas ligand (FasL). FasL knockdown by a small interfering RNA (siRNA) approach attenuated the UA-induced caspase-8 activation and apoptosis. These data suggest FasL involvement in the UA-induced apoptosis of PC-3 prostate cancer cells. Cell invasion was also inhibited as evidenced by the downregulation of the matrix metalloproteinase-9 (MMP-9). Taken together, the data of this study support UA as a potential therapeutic agent against prostate cancer due to its ability to induce apoptosis via both the intrinsic and extrinsic pathways and to inhibit invasion and metastasis [5].
Shin et al. demonstrated that PC-3 prostate cancer cells treated with UA had reduced viability associated with cell cycle arrest at the G1 phase [6]. In addition, UA treatment enhanced the expression of the autophagosome marker LC3-II, clearly indicating induction of autophagy. These effects on autophagy occurred through the Beclin-1 and Akt/mTOR signaling pathways. Inhibition of autophagy with 3-methyladenine, and siRNA silencing of Belclin-1 and Atg5 lead to enhanced UA-induced apoptosis. These data suggest that in PC-3 cells, autophagy is a survival mechanism against UA-induced apoptosis and the use of autophagy inhibitors in combination with UA resulted in greater cancer cell inhibition. These data indicate that a combination of UA and autophagy inhibitors may provide a novel cancer therapy [6].
Meng et al. showed that prostate cancer cells LNCaP and PC-3 treated with UA had reduced proliferation and increased apoptosis [7]. UA caused a decrease in protein expression of Bcl-2, Bcl-xl, and survivin and an increase in activated caspase-3. Increased apoptosis in these cells was associated with reduced expression of phosphatidylinositol-3-kinase (PI3K) and reduced phosphorylation of the signaling proteins Akt and mTOR [7].
Androgen-independent DU145 and androgen-dependent LNCaP prostate cancer cells treated with UA had decreased proliferation and increased DNA fragmentation, an indicator of apoptosis [8]. NF-κB activity was suppressed through inhibiting TNF-α-induced IkB kinase (IKK) activation as well as IkBα and p65 phosphorylation. UA treatment of these cells also resulted in the suppression of STAT3 activation associated with suppression of the upstream kinases Src and JAK2. In these cell lines, UA treatment lead to the downregulation of NF-κB and STAT3 gene products. Importantly, this study was the first to demonstrate UA’s ability to suppress NF-κB activation in DU145 cells and TNF-α-induced NF-κB activation in LNCaP cells [8].
In a study by Zhang et al., human DU145 androgen-refractory prostate cancer cells were treated with UA resulting in a concentration-dependent decrease in cell viability. In addition, UA induced apoptosis as evidenced by fluorescence microscopy showing nuclear shrinkage, condensation, and fragmentation, all morphological changes typical of apoptotic cells [9]. In these prostate cancer cells, UA increased the phosphorylation of JNK indicating increased activation, with no effects on ERK1/2 or p38 MAPK. UA also increased the phosphorylation of the JNK activated transcription factor c-Jun. Pre-treatment of the cells with the JNK inhibitor SP600125 (10 µM, 2 h) abolished the UA-induced modulation of p-c-Jun, caspase-3, and p-Bcl-2, indicating that UA induces apoptosis through JNK activation in these cells [9].
DU145 prostate cancer cells had an increase in intracellular ATP and P2Y2 transcript levels when treated with UA (25 µM) [10]. Activation of P2Y2 led to activation of Src and phosphorylation of p38 leading to downstream overexpression of COX-2; COX-2 overexpression caused the cells to become resistant to apoptosis [10]. COX-2 overexpression was attenuated with suramin, a broad-spectrum P2Y inhibitor, added to the UA treatment; suramin added to the UA increased apoptosis, suggesting that P2Y is involved in UA resistance. Together these results suggest that initiation of apoptosis in DU145 prostate cancer cells is dependent on protein kinase C (PKC) activation after UA treatment. This study showed evidence that P2Y2 activation and subsequent COX-2 overexpression led to UA-induced apoptosis resistance. An important finding was the dual role of UA as a cancer treatment and its ability to both induce apoptosis and create resistance to it. Having a better understanding of the mechanisms involved in apoptosis resistance is important in designing cancer drugs [10].
Ursolic acid-induced apoptosis of DU145 prostate cancer cells is based on the increased activity of the pro-apoptotic proteins caspase-3 and caspase-9. Protein levels of cytochrome c were increased in the cytoplasm and suppressed in the mitochondria suggesting mitochondrial apoptosis pathway activation. UA in these cells suppressed the rho-associated protein kinase/phosphatase and tensin homolog (ROCK/PTEN) signaling pathway which led to the inhibition of cofilin-1 protein expression. Together, these data suggest that, in DU145 prostate cancer cells, UA induces apoptosis through ROCK/PTEN-mediated mitochondrial translocation of cofilin-1 [11].
Park et al. also found that treatment of PC-3, LNCaP, and DU145 prostate cancer cells with UA resulted in a dose-dependent increase in apoptosis [12]. In addition, UA treatment of these prostate cancer cells suppressed Wnt5α/β and β-catenin expression and increased phosphorylation of glycogen synthase kinase 3 β (GSK3β). Inhibiting GSK3β (SB216763) or using Wnt3a-conditioned medium resulted in reversal of the activation of apoptosis markers (cleaved caspase-3 and PARP) that was observed with UA treatment [12].
Ursolic acid treatment sensitized tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-resistant prostate cancer cells (LNCaP and DU145) allowing TRAIL-induced apoptosis to occur [13]. UA + TRAIL treatment caused a significant increase in caspase-3 activity and increased protein expression of cleaved PARP and cleaved caspase-9. UA treatment led to the upregulation of DR5 via CHOP. The overall results identified the use of UA as a sensitizer for TRAIL-induced apoptosis suggesting its potential as a combination treatment against prostate cancer [13].
Ventral prostate tumor cells derived from mice, HMVP2, were treated with 20 µM UA and a series of other natural compounds, including curcumin (CUR) and resveratrol (RES), to determine the most effective combinations [14]. This screening procedure was also performed with human prostate cancer cell lines DU145, PC-3, and C42B. Combination of UA with CUR resulted in the greatest suppression of cell viability. In addition, UA combined with either CUR or RES showed comparable suppression of cell growth/survival to the clinical agents docetaxel and enzalutamide. Treatment with UA alone or in combination with CUR or RES caused changes in the intracellular glutamine flux, suggesting changes to the citric acid cycle and therefore metabolic changes. Western blot analysis of the HMVP2 cells showed that UA (20 µM) caused changes to signaling molecules relevant to glutamate transport and apoptosis; UA decreased protein expression of ASCT2, p-Src (Tyr416), and p-STAT (Ser705). UA + CUR caused increased protein expression of p-AMPK; however, neither compound alone had any effect. UA caused decreased expression of p-S6 Ribo (Ser235/236) alone and with CUR while p-S6 Ribo (Ser240/244) was only decreased when treated with both UA + CUR. Both UA alone and with CUR lead to a significantly increased number of apoptotic cells (measured by Annexin V) and increased levels of cleaved PARP protein [14].
Shanmugam et al. showed that human prostate cancer cells (DU145, LNCaP, and PC-3) treated with UA not only had reduced viability, as measured by MTT assay, but also reduced cell migration [15]. In addition, treatment with UA resulted in dose-dependent downregulation of CXCR4 expression. This downregulation was found to be due to the downregulation of mRNA levels and was associated with reduced levels of NF-κB activation. UA inhibited the binding of NF-κB to the CXCR4 promoter. In addition, treatment with UA suppressed the CXCL12-induced migration and invasion of prostate cancer cells. [15].
UA extracted from Vaccinium macrocarpon (cranberries) inhibited the growth of DU145 prostate cancer cells. UA treatment reduced the activity of matrix metalloproteinases MMP-2 and MMP-9 as examined by gelatin gel electrophoresis [16]. These data demonstrate the anti-migratory and antimetastatic properties of UA.
In experiments by Wang et al., prostate cancer cells were treated with UA (20 µM) or phenethyl isothiocyanate (PEITC, 5 µM) and analyzed using qPCR and Western blotting. UA treatment resulted in changes to the mRNA levels of Setd7, Nrf2, quinone oxidoreductase 1 (Nqo1), and glutathione S-transferase theta 2 (Gstt2). In LNCaP cells, but not PC-3, UA treatment increased the protein expression of Setd7. Setd7 and Nqo1 protein expression were increased with PEITC (5 µM, 24 h). Short hairpin-RNA (shRNA) was used to knockdown Setd7 leading to the inhibition of colony formation (LNCaP and PC-3) and increased ROS (LNCaP cells). Chromatin immunoprecipitation assays showed that knockdown of Setd7 decreased H3K4me1 at the Nrf2 and Gstt2 promoter region and this effect was attenuated with either UA or PEITC treatments [17]. These data suggest that UA is able to induce Setd7 expression, activating the Nrf2/antioxidant response element signaling pathway, which protects DNA from damage due to oxidative stress.
DU145 prostate cancer cells treated with either UA (30 µM) or exposed to 5 Gy of irradiation had decreased survival. However, when irradiation and UA were combined, a greater decrease in cell viability and induction of apoptosis was seen. The increased apoptosis was associated with the activation of caspase-3 [18].
Overall, the articles presented here provide strong evidence for the effects of ursolic acid against proliferation and survival and induction of apoptosis of prostate cancer cells. In addition, the treatment of prostate cancer cells with UA resulted in inhibition of JNK, Akt, mTOR, p70 S6K, 4EBP1, and NF-κB (Figure 1). Moreover, cleaved caspases and PARP, indicators of apoptosis, were increased as well as the tumor suppressor PTEN. In addition, UA inhibited MMP-2 and -9 indicating antimetastatic effects.
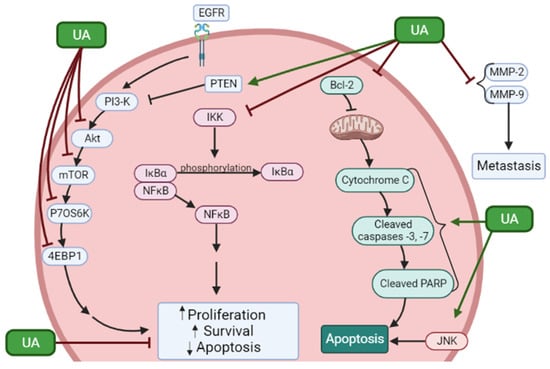
Figure 1. Effects of ursolic acid on signaling molecules in prostate cancer cells. UA reduced proliferation and survival and induced apoptosis of prostate cancer cells. These effects were associated with inhibition of phosphorylation/activation of JNK, Akt, mTOR, p70 S6K, and 4EBP1 and inhibition of the NF-κB pathway. Increased levels of PTEN and apoptosis markers cleaved caspases and PARP were seen. In addition, UA inhibited MMP-2 and -9 indicating antimetastatic effects. The figure was created using BioRender.com. ↑ = Increased; ↓ = Decreased.
2. Effects of Ursolic Acid against Prostate Cancer: Evidence from In Vivo Studies
Only a few studies have utilized mouse xenograft models of prostate cancer to examine the effects of ursolic acid in vivo (Figure 2). HMVP2 prostate cancer cells, grown as spheroids, were subcutaneously injected into the flanks of immunocompromised mice and allowed to grow for 13 days. UA and the polyphenols resveratrol and curcumin alone or in combination were then added to the diet of the animals and the treatment continued for 32 days. Tumor volume was monitored starting on day one and throughout the experimental time. Administration of UA into the diet had no effect on animal body weight or daily food consumption. Treatment with UA alone caused small reductions in tumor volume and weight. However, when UA was combined with curcumin or resveratrol, a greater reduction was seen, with the combination of UA and curcumin showing the greatest effect [14]. Unfortunately, whether such a combination treatment affects the bioavailability of either of the chemicals used is not known (was not examined) and hopefully it will be addressed in future studies.
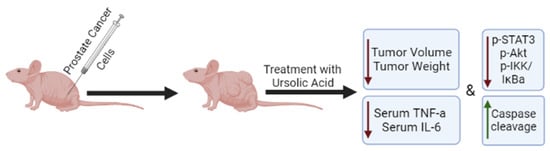
Figure 2. Effects of ursolic acid treatment on prostate cancer in vivo. Mice xenografted with prostate cancer cells and treated with ursolic acid, had reduced tumor volume and weight compared to untreated animals. The overall survival was also increased in UA-treated animals in one study. Ursolic acid treatment reduced the serum levels of TNF-α and IL-6 and the phosphorylation/activation of STAT3, AKT, and IKKα/β in tumor tissues. ↑ = Increased; ↓ = Decreased.
In another study, immunodeficient mice transplanted with human VCaP-Luc prostate cancer cells had reduced tumor growth when administered ursolic acid. Cellular metabolites and metabolism-related signaling pathways were regulated by UA including S-adenosylmethionine (SAM), suggesting potential methylation reprogramming resulting in overall anticancer effects [19].
Shanmugam et al. fed transgenic adenocarcinoma of mouse prostate (TRAMP) mice a diet containing 1% w/w UA continuously for 36 weeks, which initially resulted in delayed formation of prostate intraepithelial neoplasia (PIN). During weeks 12–18 of the experiment, there was an inhibited progression of the PIN to adenocarcinoma. UA treatment reduced tumor growth and prolonged overall survival without affecting body weight. Subsequent experiments revealed that UA treatment led to downregulated activity of pro-inflammatory mediators such as NF-κB, STAT3, AKT, and IKKα/β in prostate tissues and reduced serum levels of TNF-α and IL-6. Immunohistochemical analysis of tumor tissue samples revealed reduced expression of cyclin D1 and COX-2 and increased levels of caspase-3. The serum samples had nanogram levels of detectable UA. Taken together these data support the use of UA for both prevention and treatment of prostate cancer [20].
Nude mice were injected subcutaneously with DU145 prostate cancer cells and then fed a diet containing 200 mg/kg UA twice a week for 6 weeks [8]. Mice fed UA had a lower tumor volume than the vehicle control group (DMSO), while overall body weight remained the same. Immunohistochemical analysis of the removed tumor tissues showed a decrease in expression of VEGF and increased expression of caspase-3 [8].
Transgenic adenocarcinoma of mouse prostate (TRAMP) mice treated with UA showed suppressed CXCR4 expression in the prostate and inhibited metastasis of prostate cancer to distal organs including the liver and lungs. CXCR4 is an important signaling molecule in cancer metastasis and its downregulation with this treatment suggests UA has potential as a treatment against prostate cancer cell metastasis [15].
A transgenic line of mice was created in which the PTEN tumor suppressor gene was specifically knocked out in prostate tissues (PTEN KO). PTEN KO mice had an age dependent increase in the size of the prostate lobes, and this increase was attenuated in mice fed a diet containing UA. Epigenomic CpG methyl-seq analysis showed that UA was able to attenuate the differentially methylated regions induced in PTEN KO mice [21].
Furthermore, UA abrogated the PTEN KO-induced prostate cancer-related oncogenes Has3, Cfh, and Msx1. Association analysis of these studies identified a correlation between methylation status and mRNA expression of the tumor suppressor gene BDH2 and oncogenes Ephas, Isg15, and Nos2. These data suggest that UA may regulate oncogenes and/or tumor suppressor genes through modulation of their promoter methylation at an early stage of tumorigenesis. A metabolomic study was also performed and found that UA attenuated the PTEN KO-induced cancer-associated metabolic changes. UA attenuated purine metabolism/metabolites as well as glycolysis/gluconeogenesis metabolism and pyruvate and lactate levels. These changes in metabolism suggest UA has an important role in PTEN KO-mediated metabolic and epigenetic reprogramming and therefore, UA protects against PTEN knockout-induced tumorigenesis [21]. This is an important finding as PTEN mutations drive many cancers including prostate cancer.
Female athymic nude mice treated with 20–40 mg/kg of UA intraperitoneally for 3 weeks resulted in a decrease in tumor volume and weight. Inhibition of proliferation and induction of apoptosis was evident. Immunohistochemical analysis revealed a decrease in p-Akt, p-mTOR, and Ki67 [7].
References
- Kassi, E.; Papoutsi, Z.; Pratsinis, H.; Aligiannis, N.; Manoussakis, M.; Moutsatsou, P. Ursolic Acid, a Naturally Occurring Triterpenoid, Demonstrates Anticancer Activity on Human Prostate Cancer Cells. J. Cancer Res. Clin. Oncol. 2007, 133, 493–500.
- Zhang, Y.-X.; Kong, C.-Z.; Wang, L.-H.; Li, J.-Y.; Liu, X.-K.; Xu, B.; Xu, C.-L.; Sun, Y.-H. Ursolic Acid Overcomes Bcl-2-Mediated Resistance to Apoptosis in Prostate Cancer Cells Involving Activation of JNK-Induced Bcl-2 Phosphorylation and Degradation. J. Cell. Biochem. 2009, 109, 764–773.
- Mu, D.; Zhou, G.; Li, J.; Su, B.; Guo, H. Ursolic Acid Activates the Apoptosis of Prostate Cancer via ROCK/PTEN Mediated Mitochondrial Translocation of Cofilin-1. Oncol. Lett. 2017, 15, 3202–3206.
- Kwon, S.-H.; Park, H.-Y.; Kim, J.-Y.; Jeong, I.-Y.; Lee, M.-K.; Seo, K.-I. Apoptotic Action of Ursolic Acid Isolated from Corni Fructus in RC-58T/h/SA#4 Primary Human Prostate Cancer Cells. Bioorg. Med. Chem. Lett. 2010, 20, 6435–6438.
- Zhang, Y.; Kong, C.; Zeng, Y.; Wang, L.; Li, Z.; Wang, H.; Xu, C.; Sun, Y. Ursolic Acid Induces PC-3 Cell Apoptosis via Activation of JNK and Inhibition of Akt Pathways in Vitro. Mol. Carcinog. 2010, 49, 374–385.
- Shin, S.W.; Kim, S.Y.; Park, J.-W. Autophagy Inhibition Enhances Ursolic Acid-Induced Apoptosis in PC3 Cells. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2012, 1823, 451–457.
- Meng, Y.; Lin, Z.-M.; Ge, N.; Zhang, D.-L.; Huang, J.; Kong, F. Ursolic Acid Induces Apoptosis of Prostate Cancer Cells via the PI3K/Akt/mTOR Pathway. Am. J. Chin. Med. 2015, 43, 1471–1486.
- Shanmugam, M.K.; Rajendran, P.; Li, F.; Nema, T.; Vali, S.; Abbasi, T.; Kapoor, S.; Sharma, A.; Kumar, A.P.; Ho, P.C.; et al. Ursolic Acid Inhibits Multiple Cell Survival Pathways Leading to Suppression of Growth of Prostate Cancer Xen-ograft in Nude Mice. J. Mol. Med. 2011, 89, 713–727.
- Zhang, Y.-X.; Kong, C.-Z.; Wang, H.-Q.; Wang, L.-H.; Xu, C.-L.; Sun, Y.-H. Phosphorylation of Bcl-2 and Activation of Caspase-3 via the c-Jun N-Terminal Kinase Pathway in Ursolic Ac-id-Induced DU145 Cells Apoptosis. Biochimie 2009, 91, 1173–1179.
- Limami, Y.; Pinon, A.; Leger, D.Y.; Pinault, E.; Delage, C.; Beneytout, J.-L.; Simon, A.; Liagre, B. The P2Y2/Src/P38/COX-2 Pathway Is Involved in the Resistance to Ursolic Acid-Induced Apoptosis in Colorectal and Prostate Cancer Cells. Biochimie 2012, 94, 1754–1763.
- Gai, W.-T.; Yu, D.-P.; Wang, X.-S.; Wang, P.-T. Anti-Cancer Effect of Ursolic Acid Activates Apoptosis through ROCK/PTEN Mediated Mitochondrial Transloca-tion of Cofilin-1 in Prostate Cancer. Oncol. Lett. 2016, 12, 2880–2885.
- Park, J.-H.; Kwon, H.-Y.; Sohn, E.J.; Kim, K.A.; Kim, B.; Jeong, S.-J.; Song, J.H.; Koo, J.S.; Kim, S.-H. Inhibition of Wnt/β-Catenin Signaling Mediates Ursolic Acid-Induced Apoptosis in PC-3 Prostate Cancer Cells. Pharmacol. Rep. 2013, 65, 1366–1374.
- Shin, S.W.; Park, J.-W. Ursolic Acid Sensitizes Prostate Cancer Cells to TRAIL-Mediated Apoptosis. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2013, 1833, 723–730.
- Lodi, A.; Saha, A.; Lu, X.; Wang, B.; Sentandreu, E.; Collins, M.; Kolonin, M.G.; DiGiovanni, J.; Tiziani, S. Combinatorial Treatment with Natural Compounds in Prostate Cancer Inhibits Prostate Tumor Growth and Leads to Key Modulations of Cancer Cell Metabolism. NPJ Precis. Oncol. 2017, 1, 18.
- Shanmugam, M.K.; Manu, K.A.; Ong, T.H.; Ramachandran, L.; Surana, R.; Bist, P.; Lim, L.H.K.; Prem Kumar, A.; Hui, K.M.; Sethi, G. Inhibition of CXCR4/CXCL12 Signaling Axis by Ursolic Acid Leads to Suppression of Metastasis in Transgenic Adenocarcinoma of Mouse Prostate Model. Int. J. Cancer 2011, 129, 1552–1563.
- Kondo, M.; MacKinnon, S.L.; Craft, C.C.; Matchett, M.D.; Hurta, R.A.R.; Neto, C.C. Ursolic Acid and Its Esters: Occurrence in Cranberries and Other Vaccinium Fruit and Effects on Matrix Metallo-proteinase Activity in DU145 Prostate Tumor Cells: Anti-Tumor Activity and Content of Ursolic Acid from Vac-cinium Fruit. J. Sci. Food Agric. 2011, 91, 789–796.
- Wang, C.; Shu, L.; Zhang, C.; Li, W.; Wu, R.; Guo, Y.; Yang, Y.; Kong, A.-N. Histone Methyltransferase Setd7 Regulates Nrf2 Signaling Pathway by Phenethyl Isothiocyanate and Ursolic Acid in Human Prostate Cancer Cells. Mol. Nutr. Food Res. 2018, 62, e1700840.
- Koh, S.J.; Tak, J.K.; Kim, S.T.; Nam, W.S.; Kim, S.Y.; Park, K.M.; Park, J.-W. Sensitization of Ionizing Radiation-Induced Apoptosis by Ursolic Acid. Free. Radic. Res. 2012, 46, 339–345.
- Li, S.; Wu, R.; Wang, L.; Kuo, H.D.; Sargsyan, D.; Zheng, X.; Wang, Y.; Su, X.; Kong, A. Triterpenoid Ursolic Acid Drives Metabolic Rewiring and Epigenetic Reprogramming in Treatment/Prevention of Human Prostate Cancer. Mol. Carcinog. 2022, 61, 111–121.
- Shanmugam, M.K.; Ong, T.H.; Kumar, A.P.; Lun, C.K.; Ho, P.C.; Wong, P.T.H.; Hui, K.M.; Sethi, G. Ursolic Acid Inhibits the Initiation, Progression of Prostate Cancer and Prolongs the Survival of TRAMP Mice by Modulating Pro-Inflammatory Pathways. PLoS ONE 2012, 7, e32476.
- Wang, L.; Wang, C.; Sarwar, M.S.; Chou, P.; Wang, Y.; Su, X.; Kong, A.-N.T. PTEN-Knockout Regulates Metabolic Rewiring and Epigenetic Reprogramming in Prostate Cancer and Chemoprevention by Triterpenoid Ursolic Acid. FASEB J. 2022, 36, e22626.
More
Information
Subjects:
Cell Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
918
Revisions:
2 times
(View History)
Update Date:
10 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




