Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Eugenia Gallardo | -- | 3446 | 2023-05-05 23:27:54 | | | |
| 2 | Fanny Huang | Meta information modification | 3446 | 2023-05-06 08:19:35 | | | | |
| 3 | Fanny Huang | Meta information modification | 3446 | 2023-05-08 10:58:37 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Antunes, M.; Barroso, M.; Gallardo, E. Cannabinoids in Biological Specimens. Encyclopedia. Available online: https://encyclopedia.pub/entry/43902 (accessed on 07 February 2026).
Antunes M, Barroso M, Gallardo E. Cannabinoids in Biological Specimens. Encyclopedia. Available at: https://encyclopedia.pub/entry/43902. Accessed February 07, 2026.
Antunes, Mónica, Mário Barroso, Eugenia Gallardo. "Cannabinoids in Biological Specimens" Encyclopedia, https://encyclopedia.pub/entry/43902 (accessed February 07, 2026).
Antunes, M., Barroso, M., & Gallardo, E. (2023, May 05). Cannabinoids in Biological Specimens. In Encyclopedia. https://encyclopedia.pub/entry/43902
Antunes, Mónica, et al. "Cannabinoids in Biological Specimens." Encyclopedia. Web. 05 May, 2023.
Copy Citation
Cannabinoids are still the most consumed drugs of abuse worldwide. Despite being considered less harmful to human health, particularly if compared with opiates or cocaine, cannabis consumption has important medico-legal and public health consequences.
Cannabinoids
biological specimens
analysis
toxicology
1. Introduction
Cannabis, widely known as marijuana or hemp, is a genus in the family Cannabinaceae that grows in temperate and tropical areas such as Eastern and Central Asia. Cannabis sativa L., one of the subspecies of Cannabis, is the most controversial plant in the world. According to the United Nations Office on Drugs and Crime (UNODC), cannabis is the most popular illicit drug of the century, being consumed in similar quantities as legal drugs such as tobacco, alcohol, and caffeine [1]. It is the most widespread illicit drug in the world [2].
The medicinal properties of this plant have been known for centuries [2], but recently there has been an increased interest in the therapeutic properties of its main active secondary metabolites [1]. Medicinal cannabis has been used in the treatment of chronic pain, cancer pain, depression, sleep disturbances and anxiety, and neurological disorders. It has been widely used in cases of neurodegenerative conditions such as Parkinson’s disease, Alzheimer’s disease, and multiple sclerosis, as well as in cases of post-traumatic stress disorder, Tourette’s syndrome, epilepsy, arthritis, and nausea and vomiting due to chemotherapy, and as an appetite stimulator in HIV/AIDS patients. Cannabinoids have also gained interest in the dermatologic field [1][2]. This recent interest, however, has affected aspects such as public health and production, use, and sale of cannabis plants, which has led to legislation changes in several countries. The legalization and ethical implications of this plant are polarizing topics, so the use of this plant for medicinal purposes is a complex matter, particularly considering the psychotropic effects it also comprises [1].
There are more than five hundred forty-five different compounds in a Cannabis plant. Considering cannabinoids, over one hundred different compounds have been identified so far, belonging to different families [1]. Δ9-tetrahydrocannabinol (THC) is the primary psychoactive analyte of cannabis [3]. There are four different stereoisomers, but only the (-)-trans form exists naturally [1]. Its metabolism takes place mostly in the liver [3] but also in other tissues such as the brain, small intestine, heart, and lungs [1][4][5]. The main pathway involves hydroxylation of THC into the phase I active metabolite 1-hydroxy-Δ9-tetrahydrocannabinol (THC-OH), which is then oxidized into the phase I inactive metabolite 11-nor-9-carboxy-Δ9-tetrahydrocannabinol (THC-COOH) [3][6][7]. Then, THC and metabolites undergo phase II biotransformation to glucuronide conjugates [7]. In the plant, THC is formed by the decarboxylation of Δ9-tetrahydrocannabinolic acid A (THCA-A) [3], and, when cannabis is smoked, combustion converts THCA to THC. Cannabinol (CBN) and cannabidiol (CBD) are also important compounds that can be found in cannabis and have no psychotropic properties, showcasing different pharmacological effects than THC [1]. Δ8-tetrahydrocannabinol (Δ8-THC) is usually present in the plant in low concentrations, but fresh marijuana’s THC content can have up to 10% of this cannabinoid [8]. Figure 1 shows the chemical structures of the main cannabinoids and cannabinoid glucuronides.
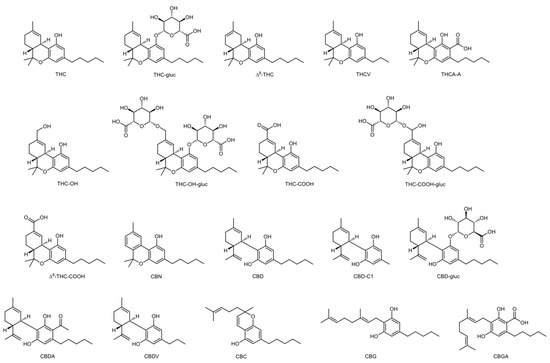
Figure 1. Naturally occurring cannabinoids and metabolites.
Cannabis is widely known for the feeling of relaxation it provides to users [1]. The most common effects of cannabis are euphoria and physical inertia—characterized by signs of ataxia, dysarthria, and incoordination. This drug also affects memory, cognition, motor function, and psychomotor performance, and it can affect the speed of thought and reaction time. Additionally, after cannabis intake, users experience an increase in blood pressure. The symptoms appear after a few minutes and can last for several hours [9][10].
Considering these effects, driving under the influence of cannabis is a major concern. Other than alcohol, cannabis is the most common drug to be detected in driving under the influence of drugs (DUID) cases [11], and its use poses an even greater risk when used concomitantly with other drugs, which is often the case [12]. The risk is higher when cannabis is consumed with alcohol, since their effects on the psychomotor impairment are additive [9][10]. Cannabis is also recurrently associated with cases of occupational accidents, child custody, or drug-facilitated crimes [2].
Cannabis sativa can be consumed in several different preparations, namely marijuana, hashish, hash oil, charas, dagga, and bhang [1][6]. There are different chemotypes of this drug that should be considered, that differ according to the amount of THC present. The fiber type (hemp) has less than 0.3% of THC and is cultivated for the textile and food industries, thus being legal in several countries. The intermediate type has 0.3–1.0% of THC. The drug type (marijuana) has 1.0–20% of THC. This amount of the psychotropic compound brands marijuana as an illicit drug in several countries, and, as such, cultivation of the plant is prohibited [13].
Cannabis consumption can be carried out in multiple ways, but most recreational users use it via the airways—by smoking or vaporization [4]. These methods allow a fast and efficient passage from the lungs to the brain [14], and THC and CBD appear in plasma just a few seconds after inhalation [1][5]. Oral administration is also quite common for both therapeutic and recreational purposes since there is no formation of harmful compounds during consumption. The intake can be carried out in the form of capsules, food, or cannabis-infused drinks [14]. Oral mucosal delivery and sublingual, dermal, rectal, and ophthalmic administrations have also been reported, mainly for therapeutic applications [4][5].
Cannabis, cannabis resin and extracts, and tinctures of cannabis were included in the list of drugs in Schedule I of the United Nations Single Convention on Narcotic Drugs in 1961, which classifies cannabis as a drug, and, thus, subjects it to all measures of control applicable to other drugs [15]. This document was the reason why cannabis’s use for medical purposes dropped in the twentieth century [1].
Over 83 million European adults are estimated to have consumed illicit drugs at some point in their lives, and a value of 78.6 million is reported for the use of cannabis alone. These statistical data vary greatly depending on the country, from 4.3% in Malta to 44.8% in France. Cannabis is still the most consumed drug on the continent, since over 22 million European adults reported using in 2021 [12], probably because it is so easily acquired, and there is low prevalence of dependence situations [1].
In the 1990s, the medicinal use of cannabis products was legalized in several states of the United States of America. Canada followed in 1999, and, since then, many other countries have implemented this condition as well [1]. Most EU countries already allow the medicinal use of cannabinoids, even though the products that are permitted and the regulatory frameworks may be different depending on the country [12].
Considering the growing market for cannabis and the several different types of products that are now easily available, analytical methodologies are constantly updated and studied to monitor this growth. A wide number of cannabinoids and their metabolites can be analysed in several different samples to access cannabis consumption and use. Numerous different cannabinoids are now analysed in the routines of toxicology laboratories around the world. These analyses are of extreme importance to differentiate between licit or illicit consumption and to determine the concentrations of active components [1]. Different aspects should be considered when studying cannabinoids’ analysis: samples, clean-up methods, and analytical instrumentation that should be applied.
Cannabinoids can be analysed in both biological and non-biological samples, and it is extremely important to choose the right matrix to perform an analysis, based on its purpose [1]. Different biological matrices provide different information about time and extent of use [2].
Sample preparation is necessary prior to analysis considering the complexity of most matrices. This procedure includes homogenization of the samples, extraction of the analytes, and a clean-up step to remove interferences. Liquid-liquid extraction (LLE) and solid-phase extraction (SPE) are the most widely used extraction methodologies for routine cannabinoid determination, although modern microextraction techniques have been emerging in the last few years since they are cheaper, faster, require fewer amounts of sample and organic solvents, and have good extraction efficiencies [1]. It is relevant to point out that the pH should be adjusted prior to LLE, considering the variation of the chemical properties of various cannabinoids (THC is neutral and THC-COOH is acidic, for example) [6]. The main advantage of SPE is the great cannabinoid recoveries, but these methodologies are also complicated, time consuming, and difficult to automate and require large amounts of sample and organic solvents [16]. In general, polar solvents are best suited to extract cannabinoids, but it is also acceptable to use a mixture of polar and non-polar solvents (such as n-hexane and ethanol). Acetone (a less polar solvent) is also a good option to extract THC, since it extracts fewer sugars and polysaccharides than methanol [17][18][19].
Usually, cannabinoids are first detected through immunoassay testing, with methods such as enzyme multiplied immunoassay technique (EMIT), enzyme-linked immunosorbent assay (ELISA) [20], fluorescence polarization, and radioimmunoassay. After these preliminary methods have been employed, confirmatory analysis should be carried out, since immunoassays have been associated with false negative and false positive results that occur due to structurally similar compounds that can be recognised by the antibodies, adulterants that affect the pH, detergents, or other surfactants [21].
Another technique used for cannabinoids’ screening is thin-layer chromatography (TLC). This technique can detect both the neutral and the acidic forms of several different cannabinoids in one assay, and several samples can be analysed simultaneously. Thus, it is an extremely cheap and high-throughput method for the screening of these compounds in crude specimens [22][23][24], but not in human biological matrices, however.
Most toxicology laboratories use gas chromatography coupled to mass spectrometry (GC-MS) methodologies for confirmatory analysis of cannabinoids in matrices such as hair and oral fluid (OF). However, GC methodologies are associated with difficulties regarding identification and quantification of acidic cannabinoids such as THC-COOH, THCA, cannabigerolic acid (CBGA), and cannabidiolic acid (CBDA), since they are decarboxylated into their neutral forms during analysis [25][26]. To surpass this problem, derivatization techniques can be employed [27]. Lately, there has been a rising interest in the use of liquid chromatography coupled to mass spectrometry (LC-MS) for confirmatory analysis, considering its high sensitivity, selectivity, and wider applicability. Unlike what happens with GC-MS methodologies, LC-MS does not require time-consuming and expensive derivatization steps to reach similar sensitivity [3][28]. LC methodologies are appropriate to analyze the native composition of the cannabis plant [25], especially considering the low concentrations in which some cannabinoids are present in the samples. Chromatographic techniques with tandem mass spectrometry (MS/MS) and time-of-flight (TOF) capabilities can simultaneously identify a wide range of analytes in a single analysis, which is a significant advantage when dealing with small sample volumes [29].
2. Cannabinoid Determination in Conventional Biological Samples
The next lines describe the determination of cannabinoids in the most commonly used biological samples, namely blood (and derivatives) and urine.
2.1. Whole Blood, Plasma, and Serum
Blood offers precious information regarding recent drug use, and its analysis allows assessing the degree of influence [30]. In general, this specimen allows correlating drug levels to the observed symptoms or to the degree of impairment. However, concentrations of the drugs are usually low, and this is particularly true for cannabinoids, being expected concentrations in the low ng/mL range. In addition, sample collection must be performed by specialized personnel, as it is extremely invasive and onerous to the donor.
In this sample, recent exposure to cannabis is monitored through the analysis of THC, THC-OH, and THC-COOH [2]. THC can be found in blood samples collected right after first inhalation, since TCH levels peak after just 8 min of exposure. Interestingly, concentration ratios of THC and metabolites can indicate the consumption timeline. A THC:THC-OH ratio of 2:1 is present 2 to 3 h after consumption [31], for example. The ratio between THC-COOH-glucuronide (THC-COOH-gluc) and THC-COOH varies from around 0.5 to 5. This variation depends on frequency and time since consumption. The Swiss Society of Legal Medicine (SGRM) has proposed a cut-off value of 40 ng/mL for THC-COOH [32].
THC, THC-OH, and THC-COOH are frequently found in plasma [33], and THC concentrations of over 3 ng/mL are recognised as a sign of recent ingestion. In addition, plasma analysis can determine the degree of CBD exposure [31].
For anti-doping analysis, THC-COOH screening in serum is usually required [31].
Even though there are several reported procedures for the analysis of cannabinoids in these matrices using GC-MS methodologies, in general, blood and plasma are difficult to analyze by GC due to matrix interferences [34]. There are low volatility components in these matrices that can obstruct the GC active sites and its injection liner or column, leading to the degradation of the analytes or an irreversible adsorption in those active sites. The result is an increased signal of the analyte in this case when compared to cases in which there are no interferents, which can overestimate cannabinoids’ concentrations. There are some reported solutions to deal with this, but they demand a laborious sample treatment process [35] and modifications to the equipment [36], can easily lead to column deterioration, and present limited effectiveness [37].
The matrix effect in GC-MS regarding the analysis of cannabinoids is mitigated in the recent work of Dawidowicz et al. [38]. The authors added oleamide to the sample extracted via a Quick, Easy, Cheap, Effective, Rugged and Safe (QuEChERS) methodology and reached values of limits of detection (LOD) exhibiting higher sensitivity and less matrix effects than most authors. The analyte response signal increased significantly, but there was also a risk of decreased analyte quantity due to polymerization of THC and its metabolites by metal ions present in blood. To minimize the risks and increase efficacy, the authors advocate that the addition of oleamide should be performed just before injection and that the magnitude of the signal depends on the ratio of the analyte to oleamide. The frequently used dilute and shot analysis for biological specimens may present some drawbacks in what concerns cannabinoid determination, since ion suppression caused by matrix interferences may hinder chromatographic peaks. Sørensen and Hasselstrøm [39] have proposed an interesting approach to overcome this problem, namely a quick filtration step to efficiently remove co-eluting phospholipids before LC-MS/MS analysis of whole blood extracts.
Products containing Δ8-THC have become more prevalent over the years. This isomer has been identified by GC-MS, but it is common to obtain interfering peaks for Δ8-THC and 11-nor-9-carboxy-Δ8-tetrahydrocannabinol (Δ8-THC-COOH) isomers [8]. Considering their structural resemblance (as seen in Figure 1), it is extremely difficult to accurately quantify Δ8 and Δ9 isomers in toxicological analysis [40].
The team of Chan-Hosokawa [8] noticed several cases of overlapping peaks in cannabinoid confirmation tests using LC-MS/MS. These cases cannot be considered since they do not meet the acceptance criteria and can lead to false positive or inconclusive results [40]. Thus, the group developed an analytical methodology to separate Δ8 and Δ9 isomers and their metabolites in blood samples. Extending the run time of analysis seemed to solve this problem, but since this is impractical for routine tests, the team decided to maintain its original run time and reanalyze cases with overlapping peaks with the longer run-time method. However, to avoid evaporation of the samples, vial tops should be replaced after the first analysis, or reinjection should take place within 24 h [8].
Likewise, Reber et al. [40] developed an analytical methodology to quantify Δ8-THC, Δ9-THC, Δ8-THC-COOH, and Δ9-THC-COOH in blood and urine samples with a SPE-LC-MS/MS methodology. While Δ8-THC and Δ9-THC results were satisfactory, a deuterated form of ∆8-THC-COOH would help increase accuracy values for the study of the carboxylated isomers. The team applied this analysis to real samples that would have been analysed with no monitorization of the Δ8 isomers and found that in some cases ∆9 cannabinoid concentrations were below established LODs, and as such would have been reported as negative.
In 2020, Hubbard et al. [41] developed an LC-MS/MS analytical methodology to quantify THC, tetrahydrocannabivarin (THCV), THC-OH, THC-COOH, THC-COOH-gluc, CBN, CBD, and cannabigerol (CBG) in whole blood samples. Extraction was performed with SPE, and the lower limits of quantification (LLOQ) ranged from 0.5 to 2 ng/mL. This method was applied by the same team one year later to study a biomarker that suggests recent cannabis consumption [42]. They learnt if it was possible to identify and quantify cannabinoids in blood, OF, or breath in the period of greatest impairment—the first 3 h after consumption. Samples were collected before and 6 h after smoking. THC, THC-COOH, and THC-COOH-gluc were identified in most samples collected after consumption. THCV and CBD were rarely detected. The team was able to conclude that THC’s concentration in cannabis is not directly related to the resulting THC concentration in blood since factors such as puff volume and puff duration may influence this value. It was concluded that CBN is the best suited cannabinoid to be used as a biomarker for recent use. This analyte—which is a primary degradation product of THC—showed the best results at the cut-off value. CBN’s concentration drops faster than THC’s, being less likely to be identified 3 h after consumption. In chronic users THC-OH, THC-COOH, and THC-COOH-gluc can be detected in blood days to several weeks after consumption, so these analytes are not suited to being biomarkers for recent use. The same happens with CBD (since its concentration depends on cannabis preparations and can be present in formulations with no THC), CBG, THCV, THC-glucuronide (THC-gluc), and THCA-A (which are not frequently detected in either blood or OF samples).
The team of da Silva et al. [33] applied for the first time a salting-out assisted liquid-liquid extraction (SALLE) and LC-MS/MS methodology to analyse THC, THC-OH, THC-COOH, CBN, and CBD in plasma. The salting-out effect of SALLE separates a water-miscible organic solvent from plasma, which induces protein precipitation and separation of the phases. This extraction technique is able to extract compounds with different polarities, which is the case of different cannabinoids.
Pichini et al. [43] validated an ultra-high-performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS) methodology to analyse THC, THC-gluc, THCA-A, THC-OH, THC-COOH, THC-COOH-gluc, CBD, and CBDA in plasma, urine, OF, and sweat samples of individuals treated with medicinal cannabis. The team developed a dilute and shoot procedure, hence avoiding time-consuming extraction methodologies. An UHPLC-MS/MS methodology operating in positive electrospray (ESI) mode allowed the team to be able to detect minimal quantities of the cannabinoids studied. The method was successfully applied to real samples.
2.2. Urine
Urine analysis has been used extensively in workplace drug testing and in abstinence control programs, as it gives important information on recent exposure [30][44][45]. However, this does not apply for cannabis [46], as several factors such as frequency of use, timing of sample collection, body fat, and urine dilution contribute to the detectability of THC metabolites in urine [31]. Samples such as OF or exhaled breath are better suited for this purpose.
This matrix is reliable and easy to collect, and its analysis is inexpensive. Thus, it is the most frequently published matrix in cannabinoids’ analysis [31]. However, one should consider the infringement of the donor’s privacy in controlled sampling, and the real possibility of sample adulteration or substitution in uncontrolled settings.
Little to no THC or THC-OH can be found in urine, so THC-COOH is the most suited cannabinoid to prove consumption. Indeed, for anti-doping analysis, THC-COOH screening in urine is usually required, as 20% of cannabis is excreted in this sample as THC-COOH and THC-COOH-gluc [31]. The THC-COOH-gluc:THC-COOH ratio varies from around 1.3 to 4.5, so monitorization of THC-COOH-gluc is often deemed necessary [32]. Another possibility of detecting the metabolites is via sample alkaline digestion [47].
Rosendo et al. [47] were the first team to extract THC, THC-OH, THC-COOH, CBN, and CBD from urine samples using a microextraction by packed sorbent (MEPS) technique to pre-concentrate the compounds, which were later analysed by GC-MS. The method was applied successfully to authentic samples and proved efficient. As mentioned before, modern microextraction techniques are cheaper, faster, require fewer amounts of sample and organic solvents, and have good extraction efficiencies.
Likewise, Morisue Sartore et al. [48] developed a new packed-in-tube solid-phase microextraction (IT-SPME) technique coupled to LC-MS/MS to automatically extract THC, THCV, THC-OH, THC-COOH, THC-COOH-gluc, CBN, and CBD from urine samples. Thus, both metabolites and neutral cannabinoids were analysed. The microcolumn for the packed IT-SPME-LC-MS/MS was extremely robust, being reused over 150 times, which dismisses the use of commercial devices. The method was applied successfully to authentic samples and proved efficient.
Urine is also a well-known specimen for the estimation of CBD exposure [31][49]. Ameline et al. [49] developed a GC-MS/MS methodology to detect CBD in urine, OF, hair, exhaled breath, and sweat after administration of a CBD capsule to a human volunteer, filling a gap when alternative matrices are concerned. Since the team expected low concentrations of the analyte, they decided not to use the correspondent deuterated compound. With the analysis of these five different matrices, the team managed to extend the detection window of CBD from 48 h (in urine) to 144 h (in sweat). Concerning urine, non-hydrolyzed samples tested negative, but enzymatic hydrolysis provided positive results, which proved that this step is vital to analyze CBD-gluc, the excreted form of CBD. Other authors share this conclusion [50][51].
References
- Gonçalves, J.; Rosado, T.; Soares, S.; Simão, A.; Caramelo, D.; Luís, Â.; Fernández, N.; Barroso, M.; Gallardo, E.; Duarte, A. Cannabis and Its Secondary Metabolites: Their Use as Therapeutic Drugs, Toxicological Aspects, and Analytical Determination. Medicines 2019, 6, 31.
- Nicolaou, A.G.; Christodoulou, M.C.; Stavrou, I.J.; Kapnissi-Christodoulou, C.P. Analysis of Cannabinoids in Conventional and Alternative Biological Matrices by Liquid Chromatography: Applications and Challenges. J. Chromatogr. A 2021, 1651, 462277.
- Cho, H.S.; Cho, B.; Sim, J.; Baeck, S.K.; In, S.; Kim, E. Detection of 11-nor-9-Carboxy-Tetrahydrocannabinol in the Hair of Drug Abusers by LC–MS/MS Analysis. Forensic Sci. Int. 2019, 295, 219–225.
- Lucas, C.J.; Galettis, P.; Schneider, J. The Pharmacokinetics and the Pharmacodynamics of Cannabinoids. Br. J. Clin. Pharmacol. 2018, 84, 2477–2482.
- Grotenhermen, F. Pharmacokinetics and Pharmacodynamics of Cannabinoids. Clin. Pharmacokinet. 2003, 42, 327–360.
- Al-Zahrani, M.A.; Al-Asmari, A.I.; Al-Zahrani, F.F.; Torrance, H.J.; Watson, D.G. Quantification of Cannabinoids in Human Hair Using a Modified Derivatization Procedure and Liquid Chromatography–Tandem Mass Spectrometry. Drug Test. Anal. 2021, 13, 1095–1107.
- Casati, S.; Angeli, I.; Ravelli, A.; Del Fabbro, M.; Minoli, M.; Orioli, M. 11-OH-THC in Hair as Marker of Active Cannabis Consumption: Estimating a Reliable Cut-off by Evaluation of 672 THC-Positive Hair Samples. Forensic Sci. Int. 2019, 304, 109951.
- Chan-Hosokawa, A.; Nguyen, L.; Lattanzio, N.; Adams, W.R. Emergence of Delta-8 Tetrahydrocannabinol in DUID Investigation Casework: Method Development, Validation and Application. J. Anal. Toxicol. 2022, 46, 1–9.
- Ashton, C.H. Adverse Effects of Cannabis and Cannabinoids. Br. J. Anaesth. 1999, 83, 637–649.
- Hall, W. The Health and Psychological Effects of Cannabis Use. Curr. Issues Crim. Justice 1994, 6, 208–220.
- Desrosiers, N.A.; Huestis, M.A. Oral Fluid Drug Testing: Analytical Approaches, Issues and Interpretation of Results. J. Anal. Toxicol. 2019, 43, 415–443.
- European Monitoring Centre for Drugs and Drug Addiction. European Drug Report 2022: Trends and Developments; European Monitoring Centre for Drugs and Drug Addiction: Luxembourg, 2022.
- Guo, T.-T.; Zhang, J.-C.; Zhang, H.; Liu, Q.-C.; Zhao, Y.; Hou, Y.-F.; Bai, L.; Zhang, L.; Liu, X.-Q.; Liu, X.-Y.; et al. Bioactive Spirans and Other Constituents from the Leaves of Cannabis Sativa f. Sativa. J. Asian Nat. Prod. Res. 2017, 19, 793–802.
- Huestis, M.A. Pharmacokinetics and Metabolism of the Plant Cannabinoids, ∆9-Tetrahydrocannibinol, Cannabidiol and Cannabinol. In Cannabinoids; Pertwee, R.G., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 657–690. ISBN 978-3-540-26573-3.
- United Nations. Single Convention on Narcotic Drugs, 1961—As Amended by the 1972 Protocol Amending the Single Convention on Narcotic Drugs, 1961; United Nations: New York, NY, USA, 1962.
- Kataoka, H.; Saito, K. Recent Advances in SPME Techniques in Biomedical Analysis. J. Pharm. Biomed. Anal. 2011, 54, 926–950.
- Namdar, D.; Mazuz, M.; Ion, A.; Koltai, H. Variation in the Compositions of Cannabinoid and Terpenoids in Cannabis Sativa Derived from Inflorescence Position along the Stem and Extraction Methods. Ind. Crops Prod. 2018, 113, 376–382.
- Richins, R.D.; Rodriguez-Uribe, L.; Lowe, K.; Ferral, R.; O’Connell, M.A. Accumulation of Bioactive Metabolites in Cultivated Medical Cannabis. PLoS ONE 2018, 13, e0201119.
- Gul, W.; Gul, S.W.; Radwan, M.M.; Wanas, A.S.; Mehmedic, Z.; Khan, I.I.; Sharaf, M.H.M.; ElSohly, M.A. Determination of 11 Cannabinoids in Biomass and Extracts of Different Varieties of Cannabis Using High-Performance Liquid Chromatography. J. AOAC Int. 2015, 98, 1523–1528.
- Spindle, T.R.; Cone, E.J.; Schlienz, N.J.; Mitchell, J.M.; Bigelow, G.E.; Flegel, R.; Hayes, E.; Vandrey, R. Acute Pharmacokinetic Profile of Smoked and Vaporized Cannabis in Human Blood and Oral Fluid. J. Anal. Toxicol. 2019, 43, 233–258.
- Sharma, P.; Murthy, P.; Bharath, M.M.S. Chemistry, Metabolism, and Toxicology of Cannabis: Clinical Implications. Iran. J. Psychiatry 2012, 7, 149–156.
- Mano-Sousa, B.J.; Maia, G.A.S.; Lima, P.L.; Campos, V.A.; Negri, G.; Chequer, F.M.D.; Duarte-Almeida, J.M. Color Determination Method and Evaluation of Methods for the Detection of Cannabinoids by Thin-Layer Chromatography (TLC). J. Forensic Sci. 2021, 66, 854–865.
- Grijó, D.R.; Olivo, J.E.; da Motta Lima, O.C. Simple Chemical Tests to Identify Cannabis Derivatives: Redefinition of Parameters and Analysis of Concepts. J. Forensic Sci. 2021, 66, 1647–1657.
- Huang, S.; Qiu, R.; Fang, Z.; Min, K.; Van Beek, T.A.; Ma, M.; Chen, B.; Zuilhof, H.; Salentijn, G.I.J. Semiquantitative Screening of THC Analogues by Silica Gel TLC with an Ag(I) Retention Zone and Chromogenic Smartphone Detection. Anal. Chem. 2022, 94, 13710–13718.
- Wang, Y.-H.; Avula, B.; Elsohly, M.A.; Radwan, M.M. Quantitative Determination of Δ9-THC, CBG, CBD, Their Acid Precursors and Five Other Neutral Cannabinoids by UHPLC-UV-MS. Planta Med. 2017, 84, 260–266.
- De Backer, B.; Debrus, B.; Lebrun, P.; Theunis, L.; Dubois, N.; Decock, L.; Verstraete, A.; Hubert, P.; Charlier, C. Innovative Development and Validation of an HPLC/DAD Method for the Qualitative and Quantitative Determination of Major Cannabinoids in Cannabis Plant Material. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 4115–4124.
- Cardenia, V.; Gallina Toschi, T.; Scappini, S.; Rubino, R.C.; Rodriguez-Estrada, M.T. Development and Validation of a Fast Gas Chromatography/Mass Spectrometry Method for the Determination of Cannabinoids in Cannabis Sativa L. J. Food Drug Anal. 2018, 26, 1283–1292.
- Mercolini, L.; Mandrioli, R.; Protti, M.; Conti, M.; Serpelloni, G.; Raggi, M.A. Monitoring of Chronic Cannabis Abuse: An LC-MS/MS Method for Hair Analysis. J. Pharm. Biomed. Anal. 2013, 76, 119–125.
- Cooper, G.A.A.; Kronstrand, R.; Kintz, P. Society of Hair Testing Guidelines for Drug Testing in Hair. Forensic Sci. Int. 2012, 218, 20–24.
- Gallardo, E.; Queiroz, J.A. The Role of Alternative Specimens in Toxicological Analysis. Biomed. Chromatogr. 2008, 22, 795–821.
- Puiu, M.; Bala, C. Affinity Assays for Cannabinoids Detection: Are They Amenable to On-Site Screening? Biosensors 2022, 12, 608.
- Frei, P.; Frauchiger, S.; Scheurer, E.; Mercer-Chalmers-Bender, K. Quantitative Determination of Five Cannabinoids in Blood and Urine by Gas Chromatography Tandem Mass Spectrometry Applying Automated On-Line Solid Phase Extraction. Drug Test. Anal. 2022, 14, 1223–1233.
- da Silva, C.P.; Dalpiaz, L.P.P.; Gerbase, F.E.; Muller, V.V.; Cezimbra da Silva, A.; Lizot, L.F.; Hahn, R.Z.; da Costa, J.L.; Antunes, M.V.; Linden, R. Determination of Cannabinoids in Plasma Using Salting-out-Assisted Liquid–Liquid Extraction Followed by LC–MS/MS Analysis. Biomed. Chromatogr. 2020, 34, e4952.
- Rahman, M.M.; Abd El-Aty, A.M.; Shim, J.H. Matrix Enhancement Effect: A Blessing or a Curse for Gas Chromatography?-A Review. Anal. Chim. Acta 2013, 801, 14–21.
- Fujiyoshi, T.; Ikami, T.; Sato, T.; Kikukawa, K.; Kobayashi, M.; Ito, H.; Yamamoto, A. Evaluation of the Matrix Effect on Gas Chromatography - Mass Spectrometry with Carrier Gas Containing Ethylene Glycol as an Analyte Protectant. J. Chromatogr. A 2016, 1434, 136–141.
- Sugitate, K.; Anazawa, H.; Nakamura, S.; Orikata, N.; Mizukoshi, K.; Nakamura, M.; Toriba, A.; Hayakawa, K. Decrease in the Matrix Effect of GC/MS by a Gold-Plated Ion Source. J. Pestic. Sci. 2012, 37, 148–155.
- Rodríguez-Ramos, R.; Lehotay, S.J.; Michlig, N.; Socas-Rodríguez, B.; Rodríguez-Delgado, M.Á. Critical Review and Re-Assessment of Analyte Protectants in Gas Chromatography. J. Chromatogr. A 2020, 1632.
- Dawidowicz, A.L.; Dybowski, M.P.; Rombel, M.; Typek, R. Oleamide as Analyte Protectant in GC Analysis of THC and Its Metabolites in Blood. J. Pharm. Biomed. Anal. 2022, 215, 114800.
- Sørensen, L.K.; Hasselstrøm, J.B. Sensitive Determination of Cannabinoids in Whole Blood by LC–MS-MS After Rapid Removal of Phospholipids by Filtration. J. Anal. Toxicol. 2017, 41, 382–391.
- Reber, J.D.; Karschner, E.L.; Seither, J.Z.; Knittel, J.L.; Dozier, K.V.; Walterscheid, J.P. An Enhanced LC-MS-MS Technique for Distinguishing Δ8- and Δ9-Tetrahydrocannabinol Isomers in Blood and Urine Specimens. J. Anal. Toxicol. 2022, 46, 343–349.
- Hubbard, J.A.; Smith, B.E.; Sobolesky, P.M.; Kim, S.; Hoffman, M.A.; Stone, J.; Huestis, M.A.; Grelotti, D.J.; Grant, I.; Marcotte, T.D.; et al. Validation of a Liquid Chromatography Tandem Mass Spectrometry (LC-MS/MS) Method to Detect Cannabinoids in Whole Blood and Breath. Clin. Chem. Lab. Med. 2020, 58, 673–681.
- Hubbard, J.A.; Hoffman, M.A.; Ellis, S.E.; Sobolesky, P.M.; Smith, B.E.; Suhandynata, R.T.; Sones, E.G.; Sanford, S.K.; Umlauf, A.; Huestis, M.A.; et al. Biomarkers of Recent Cannabis Use in Blood, Oral Fluid and Breath. J. Anal. Toxicol. 2021, 45, 820–828.
- Pichini, S.; Mannocchi, G.; Gottardi, M.; Pérez-Acevedo, A.P.; Poyatos, L.; Papaseit, E.; Pérez-Mañá, C.; Farré, M.; Pacifici, R.; Busardò, F.P. Fast and Sensitive UHPLC-MS/MS Analysis of Cannabinoids and Their Acid Precursors in Pharmaceutical Preparations of Medical Cannabis and Their Metabolites in Conventional and Non-Conventional Biological Matrices of Treated Individual. Talanta 2020, 209, 120537.
- Meier, S.I.; Koelzer, S.C.; Schubert-Zsilavecz, M.; Toennes, S.W. Analysis of Drugs of Abuse in Cerumen - Correlation of Postmortem Analysis Results with Those for Blood, Urine and Hair. Drug Test. Anal. 2017, 9, 1572–1585.
- Gallardo, E.; Barroso, M.; Queiroz, J.A. LC-MS: A Powerful Tool in Workplace Drug Testing. Drug Test. Anal. 2009, 1, 109–115.
- Samyn, N.; Van Haeren, C. On-Site Testing of Saliva and Sweat with Drugwipe and Determination of Concentrations of Drugs of Abuse in Saliva, Plasma and Urine of Suspected Users. Int. J. Legal Med. 2000, 113, 150–154.
- Rosendo, L.M.; Rosado, T.; Oliveira, P.; Simão, A.Y.; Margalho, C.; Costa, S.; Passarinha, L.A.; Barroso, M.; Gallardo, E. The Determination of Cannabinoids in Urine Samples Using Microextraction by Packed Sorbent and Gas Chromatography-Mass Spectrometry. Molecules 2022, 27, 5503.
- Morisue Sartore, D.; Costa, J.L.; Lanças, F.M.; Santos-Neto, Á.J. Packed In-Tube SPME–LC–MS/MS for Fast and Straightforward Analysis of Cannabinoids and Metabolites in Human Urine. Electrophoresis 2022, 43, 1555–1566.
- Ameline, A.; Raul, J.S.; Kintz, P. Characterization of Cannabidiol in Alternative Biological Specimens and Urine, after Consumption of an Oral Capsule. J. Anal. Toxicol. 2020, 46, 170–175.
- Bergamaschi, M.M.; Barnes, A.; Queiroz, R.H.C.; Hurd, Y.L.; Huestis, M.A. Impact of Enzymatic and Alkaline Hydrolysis on CBD Concentration in Urine. Anal. Bioanal. Chem. 2013, 405, 4679–4689.
- Kraemer, M.; Broecker, S.; Madea, B.; Hess, C. Decarbonylation: A Metabolic Pathway of Cannabidiol in Humans. Drug Test. Anal. 2019, 11, 957–967.
More
Information
Subjects:
Medical Laboratory Technology; Medicine, Legal
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
607
Revisions:
3 times
(View History)
Update Date:
09 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




