Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dario Iafusco | -- | 4126 | 2023-05-04 09:28:15 | | | |
| 2 | Lindsay Dong | Meta information modification | 4126 | 2023-05-06 05:48:28 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Iafusco, D.; Franceschi, R.; Maguolo, A.; Guercio Nuzio, S.; Crinò, A.; Delvecchio, M.; Iughetti, L.; Maffeis, C.; Calcaterra, V.; Manco, M. Type 2 Diabetes with Metabolic Syndrome in Youth. Encyclopedia. Available online: https://encyclopedia.pub/entry/43739 (accessed on 07 February 2026).
Iafusco D, Franceschi R, Maguolo A, Guercio Nuzio S, Crinò A, Delvecchio M, et al. Type 2 Diabetes with Metabolic Syndrome in Youth. Encyclopedia. Available at: https://encyclopedia.pub/entry/43739. Accessed February 07, 2026.
Iafusco, Dario, Roberto Franceschi, Alice Maguolo, Salvatore Guercio Nuzio, Antonino Crinò, Maurizio Delvecchio, Lorenzo Iughetti, Claudio Maffeis, Valeria Calcaterra, Melania Manco. "Type 2 Diabetes with Metabolic Syndrome in Youth" Encyclopedia, https://encyclopedia.pub/entry/43739 (accessed February 07, 2026).
Iafusco, D., Franceschi, R., Maguolo, A., Guercio Nuzio, S., Crinò, A., Delvecchio, M., Iughetti, L., Maffeis, C., Calcaterra, V., & Manco, M. (2023, May 04). Type 2 Diabetes with Metabolic Syndrome in Youth. In Encyclopedia. https://encyclopedia.pub/entry/43739
Iafusco, Dario, et al. "Type 2 Diabetes with Metabolic Syndrome in Youth." Encyclopedia. Web. 04 May, 2023.
Copy Citation
In the frame of metabolic syndrome, type 2 diabetes emerges along a continuum of the risk from the clustering of all its components, namely visceral obesity, high blood pressure and lipids, and impaired glucose homeostasis. Insulin resistance is the hallmark common to all the components and, in theory, is a reversible condition. Nevertheless, the load that this condition can exert on the β-cell function at the pubertal transition is such as to determine its rapid and irreversible deterioration leading to plain diabetes.
adolescents
insulin resistance
insulin secretion
youth
metabolic syndrome
obesity
type 2 diabetes
1. Introduction
The definition of metabolic syndrome (MetS) in youth remains unclear due to the absence of validated gold standard diagnostic criteria for the pediatric population [1]. Diagnostic criteria endorsed so far include the ones established by the National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III) modified for age [2], the Weiss et al. criteria [3], the de Ferranti et al. criteria [4], and the Cruz and Goran criteria [5]. The latest criteria proposed by the International Diabetes Federation for youths aged 10–16 years include abdominal obesity (defined by increased waist circumference as ≥90th percentile or adult cut-off if lower) and two or more metabolic abnormalities (elevated systolic or diastolic blood pressure (SBP ≥ 130 mmHg or DBP ≥ 85 mmHg or treatment with anti-hypertensive medication); high fasting glucose (≥100 mg/dL) or overt type 2 diabetes (T2D); high triglyceride concentrations (≥150 mg/dL); reduced HDL cholesterol (<40 mg/dL)) [6].
The prevalence of MetS in children and adolescents varies from 0.2% to 38.9% [7], with a percentage of 3.3% in the general population, 11.9% in youth with overweight, and 29.2% in those with obesity [8]. The prevalence of MetS parallels often that of obesity, and, indeed, it is remarkably higher in high-income countries where obesity rates are higher. There is a wide variation in MetS prevalence based on age, gender, race/ethnicity, and criteria used for diagnosis [9].
Disturbances of the glucose homeostasis are central in the development and progression of the MetS, with insulin resistance (IR) being the common denominator for all the components of the syndrome. On the other hand, metabolic disorders clustering into the syndrome contribute to the worsening of the glucose homeostasis up to overt T2D, whose incidence in young ages is expected to rise in the next decades.
T2D in youth is diagnosed based on plasma glucose criteria, either a fasting plasma glucose (FPG) value ≥ 126 mg/dL (7.0 mmol/L) or a 2-h plasma glucose (2hPG) value ≥ 200 mg/dL (11.1 mmol/L) following a 75 g oral glucose tolerance test (OGTT). More recently, a value of glycated hemoglobin ≥ 6.5% (hbA1c, 48 mmol/mol) has been recognized as diagnostic criterion as well. T2D is also diagnosed in individuals with classic symptoms of hyperglycemia or hyperglycemic crisis, a random plasma glucose ≥ 200 mg/dL (11.1 mmol/L) [10].
On the other hand, in a large population of Italian children and adolescents with obesity of varying severity, the prevalence of T2D diagnosed as fasting, 2-h hyperglycemia, or high hbA1c was 0.4% [11].
2. Risk Factors for T2D in Young Individuals with MetS
In adults with MetS, the transition from normal glucose homeostasis to diabetes takes decades, while in youths the evolution appears to be much more rapid [12]. Many factors are associated with the early development of T2D in young individuals with MetS, such as obesity, puberty, belonging to specific ethnic minorities, genetics, female gender, perinatal factors, nonalcoholic fatty liver disease (FLD), and polycystic ovary syndrome (PCOS).
2.1. Role of Visceral Adiposity, Inflammation, and Oxidative Stress
Obesity, especially visceral obesity, is one of the most important risk factors for the development of T2D, whose incidence increases linearly as a function of the obesity duration [13]. Up to 85% of adolescents with T2D live with overweight or obesity, and particularly with abdominal adiposity [13].
Young people with obesity have hyperinsulinemia and suffer peripheral resistance to the action of insulin (insulin resistance, IR) with an approximately 40% reduction in peripheral insulin sensitivity, meaning reduced glucose uptake in the muscle tissue and increased lipolysis in the adipose tissue [14][15].
The increased rate of inappropriate lipolysis in fasting and post-meal conditions results in an overflow of free fatty acids (FFAs) that accumulate ectopically in key metabolic organs (i.e., liver, muscle, heart, and pancreas). Adipose tissue is not just a fat storage organ but is the most expansive endocrine organ [13][16]. Adipose tissue can manifest low-grade inflammation and becomes fibrotic over time (“adiposopathy”). It releases multiple adipokines in the blood flow that can impair insulin sensitivity in peripheral organs [13].
These molecules are cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor -α (TNF-α), and hormones such as leptin and resistin. High levels of inflammatory cytokines, in particular IL-6 and TNF-α, do not just cause reduced insulin sensitivity through the modulation of insulin signaling [15][17] but also have pro-apoptotic effects on the β-cell contributing to the progressive failure in insulin secretion that ends in plain diabetes [16]. In particular, levels of leptin are generally increased in subjects with obesity, because of the obesity-induced leptin resistance, which is thought to worsen IR. Conversely, the adiposopathy causes reduced secretion of adiponectin. This molecule mediates insulin action by improving insulin signaling and its levels are inversely related to the severity of IR. In young individuals living with obesity and T2D, levels of adiponectin are significantly reduced as compared with those with obesity but no diabetes and with normal weight controls [18].
2.2. FFAs and Tissue Insulin Resistance
The release of FFAs from the adipose tissue is proportional to the subject’s fat mass. In individuals living with obesity, the adipose tissue is responsive to the lipolytic effect of catecholamines and relatively insensitive to the anti-lipolytic effect of insulin (“adipose tissue IR”). The insulin-dependent enzyme lipoprotein lipase, which limits the clearance of triglycerides and the leakage of FFAs from the adipocyte, has reduced activity in people with obesity, causing the hampered insulin-dependent esterification of FFAs in the adipose tissue [14].
In the muscle and the myocardium, FFA uptake is increased, leading to intra- and inter-fiber storage of lipids that, in turn, affect insulin signaling and result in reduced glucose uptake via down regulation of the glucose transporter 4 (GLUT4) receptor expression and in dysregulation of mitochondrial oxidation in muscle [19].
High plasma levels of FFAs also have a deleterious effect on the β-cell function (“lipotoxicity”) and cause, together with exposure to high levels of circulating glucose (“gluco-lipo-toxicity”), the progressive emergence of β-cell dysfunction in subjects genetically prone to diabetes [14][16].
The overflow of FFAs to the liver leads to an increased production and storage of triglyceride within the parenchyma up to an overt condition of FLD. The concomitant reduced synthesis of high-density lipoprotein (HDL) and very low-density lipoprotein (VLDL), and the reduced secretion of VLDL in the blood stream, favor the intrahepatic accumulation of fat. The overflow of FFAs also contributes to the increased rate of hepatic gluconeogenesis in the fasting condition (hepatic IR) [15].
2.3. Muscle–Adipose Tissue Cross-Talk
The relationship between muscle and adipose tissue was described by Raschke and Eckel as a two-edged sword [20]. They highlighted that some cytokines are released by the solo adipose tissue (i.e., adipokines) and some others by the solo muscle tissue (i.e., myokines) [20]. A number of cytokines are released by both. Known as adipo-myokines, the latter molecules serve importantly as mediators of exercise and inflammation and play a pivotal role to shape the risk of T2D onset from the very early ages of life [21]. The list of adipo-myokines includes IL-6, TNF-α, visfatin, myostatin, FSTL1, angiopoietin-like protein 4 (ANGPTL4), monocyte chemoattractant protein-1 (MCP-1), meteorin-like hormone (Metrnl), glypican-4 (GPC-4), and irisin.
2.4. Role of Puberty
The Restoring Insulin SEcretion (RISE) study demonstrated with no doubt that the natural history of T2D in children is characterized by the more rapid decline of the β-cell function as compared with adults with obesity [22][23]. More severe IR in the former individuals might make the difference. Indeed, puberty is, in itself, a physiological state of IR that leads to the compensatory increase both in basal and stimulated insulin secretion [22]. The insulin-stimulated glucose uptake (i.e., the direct estimate of insulin sensitivity) as assessed by the hyperinsulinemic-euglycemic clamp is reduced by approximately 30% during Tanner stages 2–4 as compared to stage 1 and returns to the pre-pubertal value at the completion of the pubertal transition [22]. Reduced insulin sensitivity is physiologically compensated by increased insulin secretion in a hyperbolic relationship. The product of insulin sensitivity per secretion, also termed as disposition index, is the estimate of this relationship and is the most robust predictor of T2D incidence in youth [22].
2.5. Role of Ethnicity
Ethnicity is an important risk factor for the development of T2D in young ages. Most of the patients with T2D are from ethnic minorities. In the SEARCH for diabetes in youth study, the incidence of T2D in youths aged 10–19 years ranged from 7.7% per year in Asians/Pacific Islanders, to 6.5% per years in Hispanics, 6.0% per year in non-Hispanic blacks, and 3.7% per year in Native Americans [24]. In Caucasians, the prevalence of T2D is significantly lower, accounting for approximately 5.5% of adolescents with diabetes in the United States [25] and 1.5% in Europe [26].
African Americans and Native Americans have an insulin sensitivity reduced by 35–40% as compared with Caucasian adolescents of the same age, sex, and weight [25]. The Bogalusa Heart Study showed that, among ethnic groups, African Americans are the most insulin resistant ones [27].
2.6. Role of Genetic Susceptibility
Obesity is the most important risk factor for T2D. However, some adolescents with obesity do not develop T2D, and some others do develop the disease at a relatively lower body mass index (BMI) percentile than others, consistent with differences in the genetic susceptibility to this condition in the setting of obesity and IR [28]. T2D is a polygenic disease with heritability ranging from 30 to 70%. Single nucleotide polymorphisms (SNPs) that are significantly associated with T2D susceptibility are involved in the regulation of pivotal metabolic paths such as insulin sensitivity, insulin secretion, and adipogenesis [29]. They include, for instance, the Pro12Ala peroxisome proliferator-activated receptor gamma (PPARG) polymorphism [30], the Glu23Lys KCNJ11 variant [31], and variants in ABCC8, SLC2A2, HNF4A, and INS that have been robustly associated with reduced insulin secretion [32]. The intronic variant rs7903146 (T allele) of TCF7L2 is the SNP that is the strongest genetic determinant of T2D known so far, leading to an increased risk of T2D by 41% [33].
2.7. Role of Perinatal Factors
The in utero life is paramount for shaping the offspring’s risk of T2D. Several factors can “program” such risk, mainly through epigenetic mechanisms. Children born to mothers with type 1 diabetes (T1D) or gestational diabetes have an increased risk of T2D onset [34]. Either low birth weight, which in most cases results from a delay of the intrauterine growth, or high birth weight are hallmarks of poor health during in utero life and both are associated with an increased risk of developing obesity, T2D, and all the features of the MetS [34]. Likely, either a redoubt or an excessive availability of nutrients during the in utero life cause metabolic and hormonal alterations (i.e., in the ratio between leptin and adiponectin levels at the placental site) that favor later-in-life IR, MetS, and also β-cell dysfunction [25][35].
2.8. Role of Gender
IR is more severe and T2D rates are higher in girls from childhood to mid-puberty, whilst they are greater in boys during late puberty and adulthood. Accordingly, girls develop T2D at a higher BMI than boys [36].
Boys typically have greater central fat mass, particularly visceral adipose tissue, than girls, which is associated with a higher risk for T2D, through the mechanism of decreased insulin sensitivity and β-cell function [37]. Furthermore, reduced levels of testosterone in boys with obesity at the end of puberty might have a role since testosterone levels are robustly associated with the total antioxidant capacity [38].
2.9. Role of PCOS
PCOS is a common endocrine-gynecological disorder, affecting women of reproductive age, characterized by chronic anovulation, the ultrasound finding of polycystic ovaries, and clinical and biochemical hyperandrogenism. PCOS adolescents with obesity have a 50% reduction in insulin sensitivity in peripheral tissues, hepatic IR, and increased levels of circulating insulin owing to its reduced clearance [25].
2.10. Role of FLD
With the increase in the prevalence of obesity, FLD has become among the leading causes of chronic liver disease in the pediatric age. The disease affects the liver, but it also carries multiple extrahepatic manifestations, including T2D [39]. People with FLD have higher FPG, the hallmark of the hepatic IR, while the high amount of visceral fat that accumulates within the liver and the pancreas exacerbates the risk of progressing from high fasting glucose to overt diabetes. Children with biopsy-proven fatty liver have increased IR and hampered fasting insulin secretion, both correlating with the degree of fat deposition and the severity of hepatic inflammation within the context of the MetS [40].
3. Screening for Prediabetes and T2D
While the screening for prediabetes and T2D in adults is cost-effective [41], the US Preventive Services Task Force recently concluded that, in asymptomatic children and adolescents, the evidence is insufficient to assess the balance of benefits and harms of screening for T2D, as the prevalence of T2D in children is low compared with adults [42]. Nevertheless, a risk-based screening for prediabetes or T2D is recommended in children and adolescents with overweight or obesity because of their close association [43]. In addition, T2D in youth is characterized by accelerated deterioration of insulin secretion and rapid development of complications [44]. Thus, it is crucial to screen young individuals with overweight and obesity for prediabetes and diabetes to ensure early diagnosis and targeted preventive and therapeutic interventions that could prevent or delay progression to T2D and development of its complications [45].
The guidelines of the American Diabetes Association (ADA) recommend considering testing youth over 10 years of age or after the onset of puberty, if it occurs earlier, with overweight (BMI > 85th percentile) or obesity (BMI > 95th percentile) and one or more risk factors for diabetes. Risk factors include (i) maternal history of diabetes or gestational diabetes mellitus during the child’s gestation; (ii) family history of T2D in first- or second-degree relative; (iii) high-risk race/ethnicity (i.e., Native American, African American, Latino, Asian American, Pacific Islanders); and (iv) signs of insulin resistance or associated conditions (acanthosis nigricans, hypertension, dyslipidemia, PCOS, or small-for-gestational-age birth weight). If tests are normal, it is recommended to repeat tests at a minimum of 3-year intervals or more frequently if BMI is increasing or the risk factor profile is deteriorating.
The assessment of FPG as a diagnostic tool for prediabetes and diabetes is feasible, suitable for all ages as it requires only a blood draw, and highly reproducible [46]. The disadvantage of the FPG measure is that it is influenced by stress and illness, both of which are of great relevance in pediatric age.
In contrast, HbA1c is an indirect measure of blood glucose and reflects average blood glucose levels over approximately 120 days [47]. Thus, HbA1c allows assessment of glycemic control over time, does not require fasting, and has greater pre-analytical stability and fewer daily perturbations due to stress, dietary changes, illness, or medication [48]. However, several physiological and pathological non-glycemic factors and conditions may alter HbA1c values (e.g., aging, sex, BMI, ethnicity, puberty, hemolysis, and renal function).
The OGTT consists of the collection of blood samples after the ingestion of a standard glucose load of 1.75 g/kg of body weight, up to a maximum dose of 75 g, after an overnight fast [49]. The 2hPG is used to assess glucose tolerance [49]. IGT is the hallmark of an impaired β-cell function in relation to reduced insulin sensitivity and is a high-risk condition for the development of T2D and cardiovascular disease (CVD) [48]. Thus, identifying patients with IGT is crucial, especially if FPG levels are within normal ranges [50]. The 1-h post-load glucose concentration (1hPG) during OGTT seems cost effective in identifying dysglycemia earlier than currently recommended biomarkers [51][52]. Specifically, a 1hPG level ≥ 155 mg/dl (8.6 mmol/L) may detect people with reduced β-cell function before the progression to IGT and diabetes and it is a more accurate predictor of the progression to diabetes than HbA1c or 2hPG levels in adults [51]. As regards pediatric age, a longitudinal study showed that, in youths with obesity, a value of 1hPG ≥ 155 mg/dl was associated with a greater reduction in β-cell function and, among the participants with normal value of 2hPG, with an increased risk of developing prediabetes over time [53].
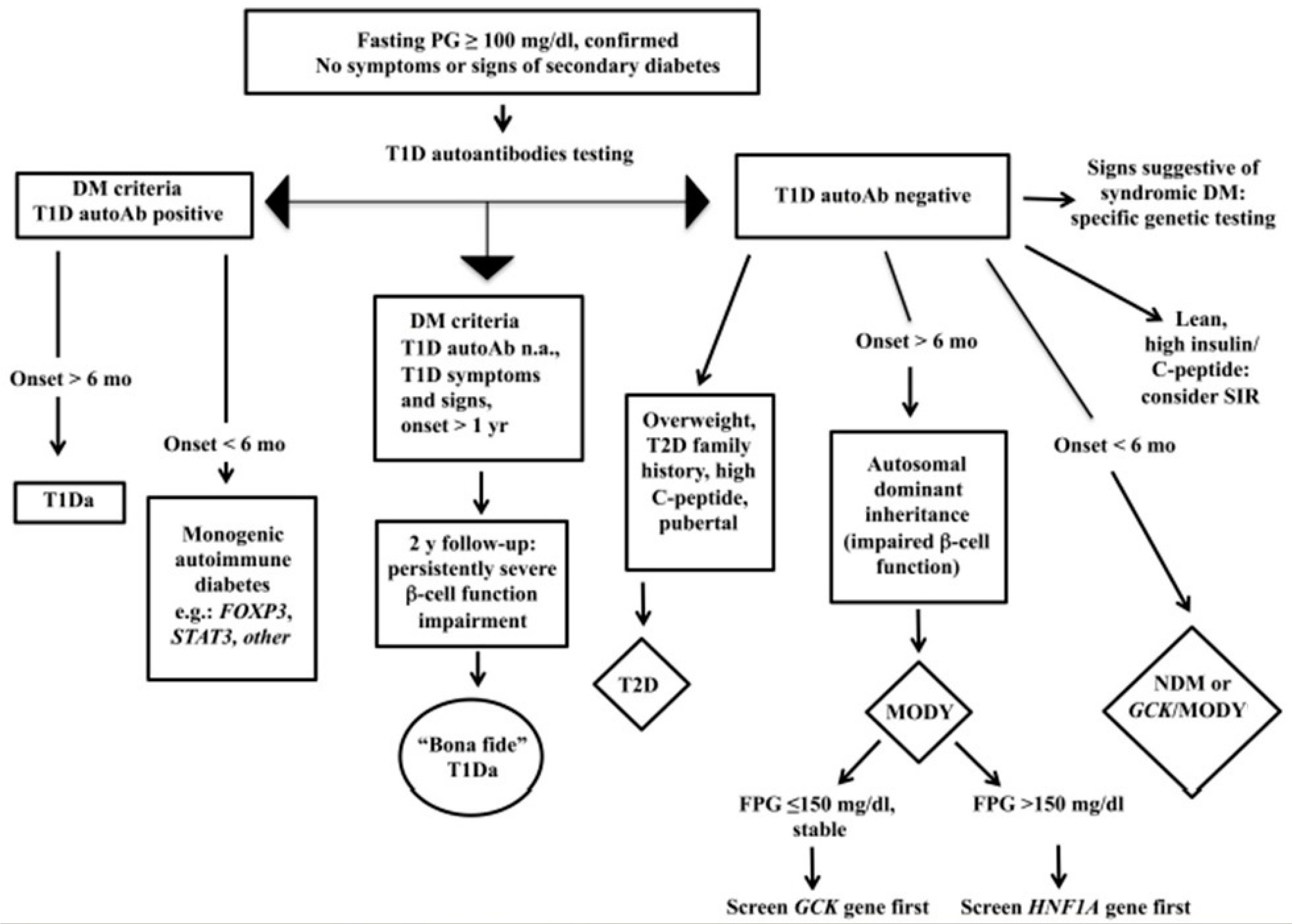
4. Diagnosis and Clinical Features at the T2D Onset
Figure 1 reports the flow chart to diagnose T2D in youth.

Figure 1. Flow chart for the diagnosis of pediatric diabetes.
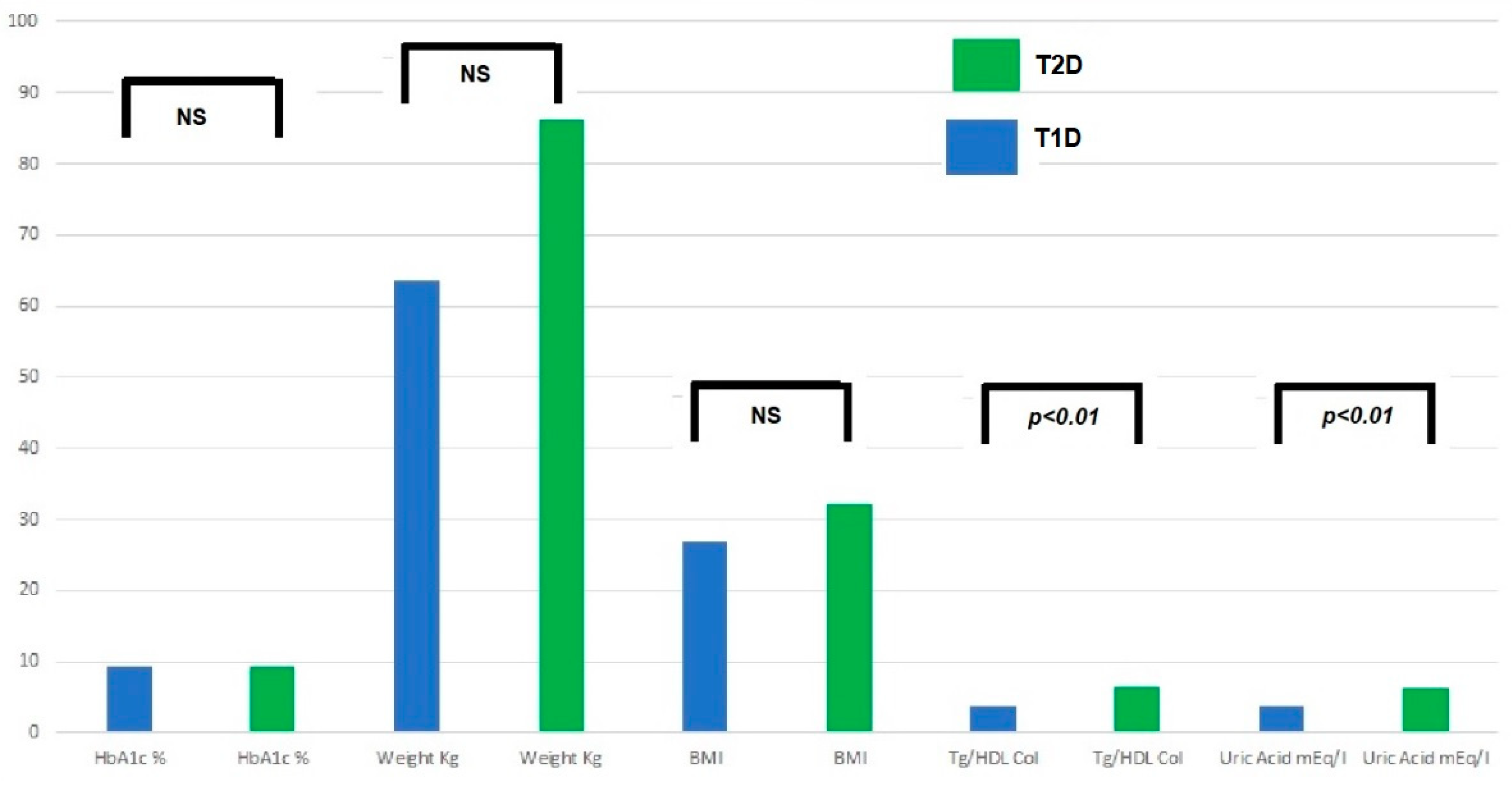
The main symptoms at the onset of T2D are the following: (a) up to 1/3 of cases onset with ketoacidosis: higher prevalence in at-risk ethnic groups and an average of 5–10%; (b) possible severe dehydration (hyperosmolarity); (c) M/F ranging from 1/4-6 North Americans and 1/1 Arabs. The increase in overweight and obesity in the general pediatric population creates troubles for the differential diagnosis with T1D. T1D is generally characterized by a lean phenotype, but in countries with a high prevalence of obesity, T1D also affects people with overweight and obesity, sometimes making the differential diagnosis cumbersome. In the case series of the Regional Center for Pediatric Diabetology “G.Stoppoloni,” which is located in Campania, the region with the highest prevalence of obesity in Italy and is the largest in the whole country for the number of referred patients in follow-up (about 1.100 up to 18 years of age) and of newly diagnosed per year (about 150), laboratory tests such as uric acid and triglyceride to HDL ratio (TG/HDL) were useful and practical means to discriminate T1D from T2D in patients with obesity at the disease onset. Figure 2 shows prevalence of T1D and T2D patients with high uric acid and TG/HDL ratio at the disease onset. In addition, a TG/HDL ratio > 2.2 was significantly correlated with hypertension, MetS, and increased waist circumference. These laboratory tests are easily available (in an admission profile) even before the results of autoimmunity markers, the positivity/negativity of which is, of course, diriment from a diagnostic point of view. Nevertheless, diagnostic accuracy of uric acid and the TG/HDL ratio must be investigated in an external population.

Figure 2. Prevalence of patients with high TG/HDL ratio and high uric acid.
5. Treatment
5.1. Nutritional Recommendations
Healthy eating patterns and habits must be encouraged [54][55][56], i.e., consumption of nutrient-dense high-quality foods and decreased consumption of calorie-dense, nutrient-poor foods as patterns and reduction in portion size, decreasing frequency of eating out, and replacing or eliminating high-calorie beverages [57]. Daily intake must be reduced by about 200 kcal per day while there is no recommendation in favor of any specific type of dietary regimen, since there is a lack of data regarding the effects on glycemic control in adolescents with T2D. Data on the short- or long-term efficacy of very low-calorie diets are lacking as well. Specific dietary intervention programs must be carried out under the supervision of an experienced nutritionist/dietitian to avoid macro- or micronutrient deficiencies. Gradual dietary changes in family habits must be recommended, and healthy parenting conducts related to diet and physical activity should be supported and encouraged. Dietary recommendations must be accustomed to the family’s cultural environment and financial constraints. As said, they must include [58][59] the elimination of sugar-sweetened soft drinks, including fruit juices; reduced intake of processed and prepackaged foods, and of refined, simple sugars and corn syrup; saturated and total fat intake; and conversely, increased intake of fruit and vegetables; of fiber-rich foods, such as whole grain products and legumes; of foods with low glycemic index; better portion control; and elimination of meals eaten away from home or while screen watching. Alike, family dietary behaviors that must be encouraged include the following: restricting the availability of high-fat, calorie-dense food and drink; understanding nutrition fact labels; emphasizing healthy parenting practices related to diet and activity but avoiding excessively restricted food intake; encouraging positive reinforcement of all goals achieved and avoiding blame for failure; promoting meals eaten on schedule, in one place, preferably as a family unit, avoiding screen activities or reading/studying while eating, and frequent snacking; maintaining food and activity logs as beneficial for raising awareness of food and activity issues and for monitoring progress. Patients are more likely to follow a diet that fits their preferences and habits and, therefore, dietary interventions must be personalized and finalized to goals that are both measurable and achievable. Frequent in-person visits with the dietitian (e.g., every 4 weeks) are recommended to assess progress and to keep both the patient and the family motivated [60].
5.2. Physical Activity
Physical activity plays a pivotal role in the management of young people living with T2D. It favors weight loss and more importantly enhances insulin sensitivity, hence improving blood glucose control [61][62]. Youth with T2D should be encouraged to engage in moderate (i.e., hiking, brisk walking, and skateboarding) to vigorous (i.e., soccer, basketball, ice or field hockey, jumping rope, and running) physical activity for at least 30–60 min at least 5 days per week [10] and at least 3 days of strength training per week. Resistance training (also called strength training) is recommended at least three times weekly and includes muscle-strengthening activities (i.e., weight lifting, push-ups, pull-ups, climbing ropes) and bone strengthening activities (i.e., skipping, running, and jumping rope). Both aerobic training and resistance training improve insulin sensitivity by enhancing muscle glucose uptake [62][63], and their combination has been found more effective for glucose control than either type of exercise alone [64]. Data are lacking on the effect of resistance training on glycemic control in youths with T2D, but evidence in those with overweight and obesity supports improvement in insulin sensitivity with resistance training in a way that is independent of changes in body composition [65][66].
Furthermore, regular physical activity leads to improvement in cardiovascular risk factors, well-being, and promotes weight loss [67].
5.3. Drugs
Metformin and insulin were, in the recent past, the only drugs approved by the US Food and Drug Administration (FDA) for the treatment of T2D in children and adolescents, until FDA approval of liraglutide in June 2019 and of semaglutide in December 2022. Therefore, to date, these drugs are the pharmacological agents approved for treatment of T2D in children and adolescents in association with diet and exercise. Phentermine/Topiramate have been recently approved by the FDA but results are still controversial. Furthermore, the use of the drug has not been approved by the European Medicines Agency.
Insulin therapy should be used soon at the onset as a first choice in patients who have ketoacidosis or severe hyper glycemia or in patients who have mixed characteristics of type 1 and type 2 diabetes (mixed forms or so-called “diabetes 1.5”). Insulin therapy is useful for these patients because they have usually inadequate insulin production (due to reduced β-cell function, which is also a consequence of the glucotoxic effect), as well as resistance to insulin itself. Guidelines recommend using insulin when blood glucose measured at random is ≥250 mg/dl or HbA1c is >9%, >75 mmol/mol [58].
Liraglutide, a GLP-1 (Glucagon-like peptide-1) analog, was approved by the FDA in 2019 [68] for use in pediatric patients with T2D, in therapeutic management in addition to diet and exercise to achieve good glycemic control, on the basis of a single clinical trial [58]. GLP-1 analogs (e.g., exenatide, liraglutide, semaglutide) are incretin mimetics that act to increase the glucose-dependent insulin secretion and help ensure adequate postprandial insulin response. These agents are administered by subcutaneous injection once daily. Liraglutide at the dose of 1.8 mg/day has the additional benefit of promoting modest weight loss, probably due to delayed gastric emptying and through central effects on appetite.
Similar to liraglutide, semaglutide is a GLP1 receptor agonist prescribed subcutaneously once-weekly at the maximal dose of 2.4 mg. It acts by decreasing appetite, thereby improving control of eating and reducing energy intake [69].
Phentermine/Topiramate (PHN/TPM) (Qsymia®) is FDA-approved for adolescents ≥ 12 years with a BMI > 95th percentile for age and sex as an adjuvant therapy to lifestyle modifications [68][70].
6. Conclusions
Type 2 diabetes in youth appears to be firmly embedded within the metabolic abnormalities belonging to the syndrome owing to the pivotal role of IR. As compared with the disease in adults, this condition is much more quickly progressive, with the early failure of oral glucose-lowering agents to maintain the metabolic and glycemic homeostasis and early onset of severe CVD. Genetic susceptibility to the disease influences β-cell function in youths as well as in adults, but exposure during prenatal life and early life to different environmental factors can influence the individual’s epigenome, explaining the faster deterioration of insulin secretion. On the other hand, the pubertal transition with exaggerated insulin resistance pulls the trigger and unravels diabetes. In this frame, reducing and even preventing excessive weight gain is a promising strategy to reduce the burden of type 2 diabetes in young people.
References
- Al-Hamad, D.; Raman, V. Metabolic syndrome in children and adolescents. Transl. Pediatr. 2017, 6, 397–407.
- Boney, C.M.; Verma, A.; Tucker, R.; Vohr, B.R. Metabolic syndrome in childhood: Association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 2005, 115, e290–e296.
- Weiss, R.; Dziura, J.; Burgert, T.S.; Tamborlane, W.V.; Taksali, S.E.; Yeckel, C.W.; Allen, K.; Lopes, M.; Savoye, M.; Morrison, J.; et al. Obesity and the metabolic syndrome in children and adolescents. N. Engl. J. Med. 2004, 350, 2362–2374.
- de Ferranti, S.D.; Gauvreau, K.; Ludwig, D.S.; Neufeld, E.J.; Newburger, J.W.; Rifai, N. Prevalence of the metabolic syndrome in American adolescents: Findings from the third national health and nutrition examination survey. Circulation 2004, 110, 2494–2497.
- Cruz, M.L.; Goran, M.I. The metabolic syndrome in children and adolescents. Curr. Diabetes Rep. 2004, 4, 53–62.
- Alberti, K.G.M.; Zimmet, P.; Shaw, J. The metabolic syndrome—A new worldwide definition. Lancet 2005, 366, 1059–1062.
- Agudelo, G.M.; Bedoya, G.; Estrada, A.; Patiño, F.A.; Muñoz, A.M.; Velásquez, C.M. Variations in the prevalence of metabolic syndrome in adolescents according to different criteria used for diagnosis: Which definition should be chosen for this age group? Metab. Syndr. Relat. Disord. 2014, 12, 202–209.
- Friend, A.; Craig, L.; Turner, S. The prevalence of metabolic syndrome in children: A systematic review of the literature. Metab. Syndr. Relat. Disord. 2013, 11, 71–80.
- Di Bonito, P.; Forziato, C.; Sanguigno, E.; Di Fraia, T.; Saitta, F.; Lardino, M.R.; Capaldo, B. Prevalence of the metabolic syndrome using ATP-derived definitions and its relation to insulin-resistance in a cohort of italian outpatient children. J. Endocrinol. Investig. 2010, 33, 806–809.
- American Diabetes Association. 2. classification and diagnosis of diabetes: Standards of medical care in diabetes—2020. Diabetes Care 2020, 43 (Suppl. S1), S14–S31.
- Pedicelli, S.; Fintini, D.; Ravà, L.; Inzaghi, E.; Deodati, A.; Spreghini, M.R.; Bizzarri, C.; Mariani, M.; Cianfarani, S.; Cappa, M.; et al. Prevalence of prediabetes in children and adolescents by class of obesity. Pediatr. Obes. 2022, 17, e12900.
- Iafusco, D. Dieci Domande Su Sindrome Metabolica e Diabete Mellito Tipo 2 Dell’adolescente. G. Ital. Diabetol. Metab. 2014, 34, 117–123.
- Shah, A.S.; Zeitler, P.S.; Wong, J.; Pena, A.S.; Wicklow, B.; Arslanian, S.; Chang, N.; Fu, J.; Dabadghao, P.; Pinhas-Hamiel, O.; et al. ISPAD clinical practice consensus guidelines 2022: Type 2 diabetes in children and adolescents. Pediatr. Diabetes 2022, 23, 872–902.
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642.
- Lee, S.-H.; Park, S.-Y.; Choi, C.S. Insulin resistance: From mechanisms to therapeutic strategies. Diabetes Metab. J. 2022, 46, 15–37.
- Manco, M.; Calvani, M.; Mingrone, G. Effects of dietary fatty acids on insulin sensitivity and secretion. Diabetes Obes. Metab. 2004, 6, 402–413.
- Deckelbaum, R.J.; Williams, C.L. Childhood obesity: The health issue. Obes. Res. 2001, 9, 239S–243S.
- Goran, M.I.; Ball, G.D.C.; Cruz, M.L. Obesity and risk of type 2 diabetes and cardiovascular disease in children and adolescents. J. Clin. Endocrinol. Metab. 2003, 88, 1417–1427.
- Greco, A.V.; Mingrone, G.; Giancaterini, A.; Manco, M.; Morroni, M.; Cinti, S.; Granzotto, M.; Vettor, R.; Camastra, S.; Ferrannini, E. Insulin resistance in morbid obesity. Diabetes 2002, 51, 144–151.
- Raschke, S.; Eckel, J. Adipo-myokines: Two sides of the same coin—Mediators of inflammation and mediators of exercise. Mediat. Inflamm. 2013, 2013, 1–16.
- Graf, C.; Ferrari, N. Metabolic health—The role of adipo-myokines. Int. J. Mol. Sci. 2019, 20, 6159.
- Magge, S.N.; Goodman, E.; Armstrong, S.C.; Daniels, S.; Corkins, M.; de Ferranti, S.; Golden, N.H.; Kim, J.H.; Schwarzenberg, S.J.; Sills, I.N.; et al. The metabolic syndrome in children and adolescents: Shifting the focus to cardiometabolic risk factor clustering. Pediatrics 2017, 140, e20171603.
- Wittcopp, C.; Conroy, R. Metabolic syndrome in children and adolescents. Pediatr. Rev. 2016, 37, 193–202.
- Divers, J.; Mayer-Davis, E.J.; Lawrence, J.M.; Isom, S.; Dabelea, D.; Dolan, L.; Imperatore, G.; Marcovina, S.; Pettitt, D.J.; Pihoker, C.; et al. Trends in incidence of type 1 and type 2 diabetes among youths—Selected counties and Indian reservations, United States, 2002–2015. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 161–165.
- Frankenberg, A.D.; von Reis, A.F.; Gerchman, F. Relationships between adiponectin levels, the metabolic syndrome, and type 2 diabetes: A literature review. Arch. Endocrinol. Metab. 2017, 61, 614–622.
- Jensen, E.T.; Dabelea, D. Type 2 diabetes in youth: New lessons from the search study. Curr. Diabetes Rep. 2018, 18, 36.
- Silverman, B.L.; Metzger, B.E.; Cho, N.H.; Loeb, C.A. Impaired glucose tolerance in adolescent offspring of diabetic mothers: Relationship to fetal hyperinsulinism. Diabetes Care 1995, 18, 611–617.
- Todd, J.N.; Srinivasan, S.; Pollin, T.I. Advances in the genetics of youth-onset type 2 diabetes. Curr. Diabetes Rep. 2018, 18, 57.
- Botnia Study Group; Almgren, P.; Lehtovirta, M.; Isomaa, B.; Sarelin, L.; Taskinen, M.R.; Lyssenko, V.; Tuomi, T.; Groop, L. Heritability and familiality of type 2 diabetes and related quantitative traits in the botnia study. Diabetologia 2011, 54, 2811–2819.
- Sarhangi, N.; Sharifi, F.; Hashemian, L.; Hassani Doabsari, M.; Heshmatzad, K.; Rahbaran, M.; Jamaldini, S.H.; Aghaei Meybodi, H.R.; Hasanzad, M. PPARG (Pro12Ala) genetic variant and risk of T2DM: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 12764.
- Hani, E.H.; Boutin, P.; Durand, E.; Inoue, H.; Permutt, M.A.; Velho, G.; Froguel, P. Missense mutations in the pancreatic islet beta cell inwardly rectifying K + channel gene (KIR6.2/BIR): A meta-analysis suggests a role in the polygenic basis of type II diabetes mellitus in caucasians. Diabetologia 1998, 41, 1511–1515.
- Barroso, I.; Luan, J.; Middelberg, R.P.S.; Harding, A.-H.; Franks, P.W.; Jakes, R.W.; Clayton, D.; Schafer, A.J.; O’Rahilly, S.; Wareham, N.J. Candidate gene association study in type 2 diabetes indicates a role for genes involved in β-cell function as well as insulin action. PLoS Biol. 2003, 1, e20.
- Ding, W.; Xu, L.; Zhang, L.; Han, Z.; Jiang, Q.; Wang, Z.; Jin, S. Meta-analysis of association between TCF7L2 polymorphism Rs7903146 and type 2 diabetes mellitus. BMC Med. Genet. 2018, 19, 38.
- Elliott, H.R.; Sharp, G.C.; Relton, C.L.; Lawlor, D.A. Epigenetics and gestational diabetes: A review of epigenetic epidemiology studies and their use to explore epigenetic mediation and improve prediction. Diabetologia 2019, 62, 2171–2178.
- Candler, T.P.; Mahmoud, O.; Lynn, R.M.; Majbar, A.A.; Barrett, T.G.; Shield, J.P.H. Continuing rise of type 2 diabetes incidence in children and young people in the UK. Diabet. Med. 2018, 35, 737–744.
- Huebschmann, A.G.; Huxley, R.R.; Kohrt, W.M.; Zeitler, P.; Regensteiner, J.G.; Reusch, J.E.B. Sex differences in the burden of type 2 diabetes and cardiovascular risk across the life course. Diabetologia 2019, 62, 1761–1772.
- Kufe, C.N.; Micklesfield, L.K.; Masemola, M.; Chikowore, T.; Kengne, A.P.; Karpe, F.; Norris, S.A.; Crowther, N.J.; Olsson, T.; Goedecke, J.H. Increased risk for type 2 diabetes in relation to adiposity in middle-aged black south African men compared to women. Eur. J. Endocrinol. 2022, 186, 523–533.
- Demirbag, R.; Yilmaz, R.; Erel, O. The association of total antioxidant capacity with sex hormones. Scand. Cardiovasc. J. 2005, 39, 172–176.
- Bush, H.; Golabi, P.; Younossi, Z.M. Pediatric non-alcoholic fatty liver disease. Children 2017, 4, 48.
- Manco, M.; Marcellini, M.; DeVito, R.; Comparcola, D.; Sartorelli, M.R.; Nobili, V. Metabolic syndrome and liver histology in paediatric non-alcoholic steatohepatitis. Int. J. Obes. 2008, 32, 381–387.
- US Preventive Services Task Force; Davidson, K.W.; Barry, M.J.; Mangione, C.M.; Cabana, M.; Caughey, A.B.; Davis, E.M.; Donahue, K.E.; Doubeni, C.A.; Krist, A.H.; et al. Screening for prediabetes and type 2 diabetes: US preventive services task force recommendation statement. JAMA 2021, 326, 736.
- US Preventive Services Task Force; Mangione, C.M.; Barry, M.J.; Nicholson, W.K.; Cabana, M.; Chelmow, D.; Coker, T.R.; Davidson, K.W.; Davis, E.M.; Donahue, K.E.; et al. Screening for prediabetes and type 2 diabetes in children and adolescents: US preventive services task force recommendation statement. JAMA 2022, 328, 963.
- Zimmermann, E.; Bjerregaard, L.G.; Gamborg, M.; Vaag, A.A.; Sørensen, T.I.A.; Baker, J.L. Childhood body mass index and development of type 2 diabetes throughout adult life-a large-scale danish cohort study: Childhood body size and adult type 2 diabetes. Obesity 2017, 25, 965–971.
- American Diabetes Association Professional Practice Committee. 14. children and adolescents: Standards of medical care in diabetes—2022. Diabetes Care 2022, 45 (Suppl. S1), S208–S231.
- Savoye, M.; Caprio, S.; Dziura, J.; Camp, A.; Germain, G.; Summers, C.; Li, F.; Shaw, M.; Nowicka, P.; Kursawe, R.; et al. Reversal of early abnormalities in glucose metabolism in obese youth: Results of an intensive lifestyle randomized controlled trial. Diabetes Care 2014, 37, 317–324.
- Brar, P.C. Update on the current modalities used to screen high risk youth for prediabetes and/or type 2 diabetes mellitus. Ann. Pediatr. Endocrinol. Metab. 2019, 24, 71–77.
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010, 33 (Suppl. S1), S62–S69.
- American Diabetes Association Professional Practice Committee. 2. classification and diagnosis of diabetes: Standards of medical care in diabetes—2022. Diabetes Care 2022, 45 (Suppl. S1), S17–S38.
- Chen, M.E.; Aguirre, R.S.; Hannon, T.S. Methods for measuring risk for type 2 diabetes in youth: The oral glucose tolerance test (OGTT). Curr. Diabetes Rep. 2018, 18, 51.
- Shaw, J.E.; Zimmet, P.Z.; McCarty, D.; de Courten, M. Type 2 diabetes worldwide according to the new classification and criteria. Diabetes Care 2000, 23 (Suppl. S2), B5.
- Bergman, M.; Jagannathan, R.; Buysschaert, M.; Pareek, M.; Olsen, M.H.; Nilsson, P.M.; Medina, J.L.; Roth, J.; Chetrit, A.; Groop, L.; et al. Lessons learned from the 1-hour post-load glucose level during OGTT: Current screening recommendations for dysglycaemia should be revised. Diabetes Metab. Res. Rev. 2018, 34, e2992.
- Andellini, M.; Manco, M.; Esposito, M.T.; Tozzi, A.E.; Bergman, M.; Ritrovato, M. A simulation model estimates lifetime health and economic outcomes of screening prediabetes using the 1-h plasma glucose. Acta Diabetol. 2022, 60, 9–17.
- Kim, J.Y.; Goran, M.I.; Toledo-Corral, C.M.; Weigensberg, M.J.; Choi, M.; Shaibi, G.Q. One-hour glucose during an oral glucose challenge prospectively predicts β-cell deterioration and prediabetes in obese Hispanic youth. Diabetes Care 2013, 36, 1681–1686.
- McGavock, J.; Dart, A.; Wicklow, B. Lifestyle therapy for the treatment of youth with type 2 diabetes. Curr. Diabetes Rep. 2015, 15, 568.
- Bacha, F.; Cheng, P.; Gal, R.L.; Kollman, C.; Tamborlane, W.V.; Klingensmith, G.J.; Manseau, K.; Wood, J.; Beck, R.W.; Pediatric Diabetes Consortium. Initial presentation of type 2 diabetes in adolescents predicts durability of successful treatment with metformin monotherapy: Insights from the pediatric diabetes consortium T2D registry. Horm. Res. Paediatr. 2018, 89, 47–55.
- Barlow, S.E.; The Expert Committee. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: Summary report. Pediatrics 2007, 120 (Suppl. S4), S164–S192.
- Styne, D.M.; Arslanian, S.A.; Connor, E.L.; Farooqi, I.S.; Murad, M.H.; Silverstein, J.H.; Yanovski, J.A. Pediatric obesity—Assessment, treatment, and prevention: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2017, 102, 709–757.
- Smart, C.E.; Annan, F.; Higgins, L.A.; Jelleryd, E.; Lopez, M.; Acerini, C.L. ISPAD clinical practice consensus guidelines 2018: Nutritional management in children and adolescents with diabetes. Pediatr. Diabetes 2018, 19, 136–154.
- Kim, J.A.; Kim, D.H.; Kim, S.M.; Park, Y.G.; Kim, N.H.; Baik, S.H.; Choi, K.M.; Han, K.; Yoo, H.J. Impact of the dynamic change of metabolic health status on the incident type 2 diabetes: A nationwide population-based cohort study. Endocrinol. Metab. 2019, 34, 406.
- The TODAY Study Group. Design of a family-based lifestyle intervention for youth with type 2 diabetes: The TODAY study. Int. J. Obes. 2010, 34, 217–226.
- Stoner, L.; Pontzer, H.; Barone Gibbs, B.; Moore, J.B.; Castro, N.; Skidmore, P.; Lark, S.; Williams, M.A.; Hamlin, M.J.; Faulkner, J. Fitness and fatness are both associated with cardiometabolic risk in preadolescents. J. Pediatr. 2020, 217, 39–45.e1.
- Fedewa, M.V.; Gist, N.H.; Evans, E.M.; Dishman, R.K. Exercise and insulin resistance in youth: A meta-analysis. Pediatrics 2014, 133, e163–e174.
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical activity/exercise and diabetes: A position statement of the American diabetes association. Diabetes Care 2016, 39, 2065–2079.
- Colberg, S.R.; Sigal, R.J.; Fernhall, B.; Regensteiner, J.G.; Blissmer, B.J.; Rubin, R.R.; Chasan-Taber, L.; Albright, A.L.; Braun, B. Exercise and type 2 diabetes. Diabetes Care 2010, 33, e147–e167.
- Shaibi, G.Q.; Ball, G.D.C.; Cruz, M.L.; Weigensberg, M.J.; Salem, G.J.; Goran, M.I. Cardiovascular fitness and physical activity in children with and without impaired glucose tolerance. Int. J. Obes. 2006, 30, 45–49.
- Dias, I.; Farinatti, P.; De Souza, M.D.G.C.; Manhanini, D.P.; Balthazar, E.; Dantas, D.L.S.; De Andrade Pinto, E.H.; Bouskela, E.; Kraemer-Aguiar, L.G. Effects of resistance training on obese adolescents. Med. Sci. Sport. Exerc. 2015, 47, 2636–2644.
- Arslanian, S.; Bacha, F.; Grey, M.; Marcus, M.D.; White, N.H.; Zeitler, P. Evaluation and management of youth-onset type 2 diabetes: A position statement by the American diabetes association. Diabetes Care 2018, 41, 2648–2668.
- United States Food and Drug Administration. FDA Approves Treatment for Chronic Weight Management in Pediatric Patients Aged 12 Years and Older. 2022. Available online: https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-treatment-chronic-weight-management-pediatric-patients-aged-12-years-and-older (accessed on 26 December 2022).
- Friedrichsen, M.; Breitschaft, A.; Tadayon, S.; Wizert, A.; Skovgaard, D. The Effect of Semaglutide 2.4 mg once weekly on energy intake, appetite, control of eating, and gastric emptying in adults with obesity. Diabetes Obes. Metab. 2021, 23, 754–762.
- Bensignor, M.O.; Kelly, A.S.; Arslanian, S. Anti-obesity pharmacotherapy for treatment of pediatric type 2 diabetes: Review of the literature and lessons learned from adults. Front. Endocrinol. 2022, 13, 1043650.
More
Information
Subjects:
Pediatrics
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
584
Revisions:
2 times
(View History)
Update Date:
06 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




