
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Bruno Mégarbane | -- | 2329 | 2023-05-02 17:20:38 | | | |
| 2 | Lindsay Dong | Meta information modification | 2329 | 2023-05-03 04:49:47 | | |
Video Upload Options
In 2020, fentanyl and its analogs contributed to ~65% of drug-attributed fatalities in the USA, with a threatening increasing trend during the last ten years. These synthetic opioids used as potent analgesics in human and veterinary medicine have been diverted to recreational aims, illegally produced and sold. Like all opioids, central nervous system depression resulting from overdose or misuse of fentanyl analogs is characterized clinically by the onset of consciousness impairment, pinpoint miosis and bradypnea. However, contrasting with what observed with most opioids, thoracic rigidity may occur rapidly with fentanyl analogs, contributing to increasing the risk of death in the absence of immediate life support. Various mechanisms have been proposed to explain this particularity associated with fentanyl analogs, including the activation of noradrenergic and glutamatergic coerulospinal neurons and dopaminergic basal ganglia neurons. Due to the high affinities to the mu-opioid receptor, the need for more elevated naloxone doses than usually required in morphine overdose to reverse the neurorespiratory depression induced by fentanyl analogs has been questioned.
1. Introduction
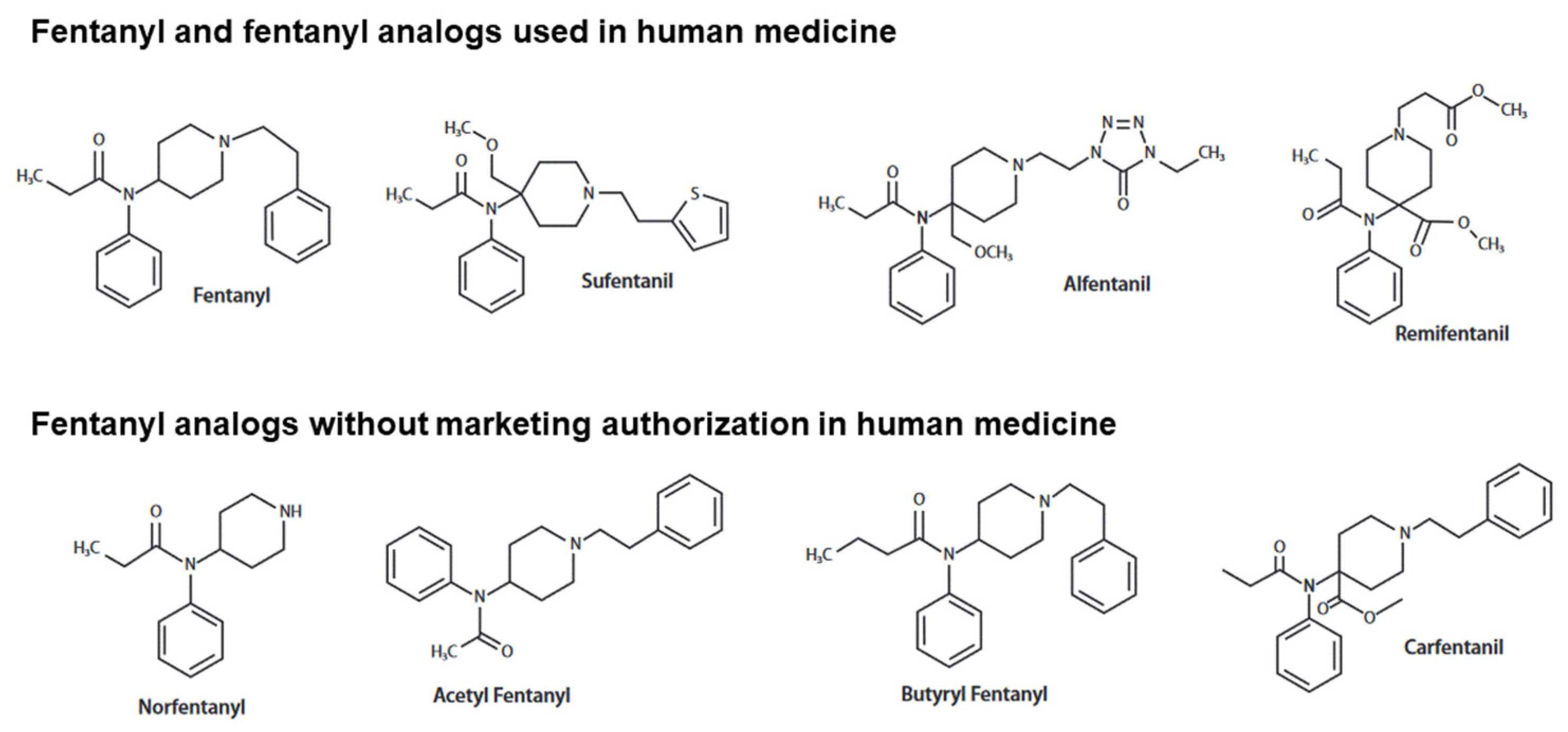
2. Neurorespiratory Effects of Fentanyl and Analogs
2.1. Depression of the Ventilation Command
2.2. Chest Wall Rigidity
3. Specificities of the Main Fentanyl Analogs
3.1. Carfentanil
3.2. Alfentanil
3.3. Sufentanil
4. Reversal of the Neurorespiratory Toxicity Induced by Fentanyl and Analogs
4.1. Effects of Naloxone
4.2. Alternative Targeted Strategies
References
- National Institute on Drug Abuse (NIDA). Overdose Death Rates. National Institute on Drug Abuse. 2023. Available online: https://www.drugabuse.gov/drug-topics/trends-statistics/overdose-death-rates (accessed on 3 February 2023).
- Jannetto, P.J.; Helander, A.; Garg, U.; Janis, G.C.; Goldberger, B.; Ketha, H. The Fentanyl Epidemic and Evolution of Fentanyl Analogs in the United States and the European Union. Clin. Chem. 2019, 65, 242–253.
- Vardanyan, R.S.; Hruby, V.J. Fentanyl-Related Compounds and Derivatives: Current Status and Future Prospects for Pharmaceutical Applications. Future Med. Chem. 2014, 6, 385–412.
- Pichini, S.; Solimini, R.; Berretta, P.; Pacifici, R.; Busardò, F.P. Acute Intoxications and Fatalities From Illicit Fentanyl and Analogues: An Update. Ther. Drug Monit. 2018, 40, 38–51.
- Shoff, E.N.; Zaney, M.E.; Kahl, J.H.; Hime, G.W.; Boland, D.M. Qualitative Identification of Fentanyl Analogs and Other Opioids in Postmortem Cases by UHPLC-Ion Trap-MSn. J. Anal. Toxicol. 2017, 41, 484–492.
- Van der Schrier, R.; Dahan, J.D.C.; Boon, M.; Sarton, E.; van Velzen, M.; Niesters, M.; Dahan, A. Advances in Reversal Strategies of Opioid-Induced Respiratory Toxicity. Anesthesiology 2022, 136, 618–632.
- Moss, R.B.; Pryor, M.M.; Baillie, R.; Kudrycki, K.; Friedrich, C.; Reed, M.; Carlo, D.J. Higher naloxone dosing in a quantitative systems pharmacology model that predicts naloxone-fentanyl competition at the opioid mu receptor level. PLoS ONE 2020, 15, e0234683.
- Moe, J.; Godwin, J.; Purssell, R.; O’Sullivan, F.; Hau, J.P.; Purssell, E.; Curran, J.; Doyle-Waters, M.M.; Brasher, P.M.A.; Buxton, J.A.; et al. Naloxone dosing in the era of ultra-potent opioid overdoses: A systematic review. Can. J. Emerg. Med. 2020, 22, 178–186.
- Manral, L.; Muniappan, N.; Gupta, P.K.; Ganesan, K.; Malhotra, R.C.; Vijayaraghavan, R. Effect of Exposure to Fentanyl Aerosol in Mice on Breathing Pattern and Respiratory Variables. Drug Chem. Toxicol. 2009, 32, 108–113.
- Chevillard, L.; Mégarbane, B.; Risède, P.; Baud, F.J. Characteristics and Comparative Severity of Respiratory Response to Toxic Doses of Fentanyl, Methadone, Morphine, and Buprenorphine in Rats. Toxicol. Lett. 2009, 191, 327–340.
- Hill, R.; Santhakumar, R.; Dewey, W.; Kelly, E.; Henderson, G. Fentanyl Depression of Respiration: Comparison with Heroin and Morphine. Br. J. Pharmacol. 2020, 177, 254–266.
- Henderson, F.; May, W.J.; Gruber, R.B.; Discala, J.F.; Puscovic, V.; Young, A.P.; Baby, S.M.; Lewis, S.J. Role of Central and Peripheral Opiate Receptors in the Effects of Fentanyl on Analgesia, Ventilation and Arterial Blood-Gas Chemistry in Conscious Rats. Respir. Physiol. Neurobiol. 2014, 191, 95–105.
- Zhang, Z.; Zhang, C.; Zhuang, J.; Xu, F. Contribution of Central μ-Receptors to Switching Pulmonary C-Fibers-Mediated Rapid Shallow Breathing into an Apnea by Fentanyl in Anesthetized Rats. Brain Res. 2012, 1469, 73–81.
- Saunders, S.E.; Baekey, D.M.; Levitt, E.S. Fentanyl effects on respiratory neuron activity in the dorsolateral pons. J. Neurophysiol. 2022, 128, 1117–1132.
- Christian, C.M.; Waller, J.L.; Moldenhauer, C.C. Postoperative Rigidity Following Fentanyl Anesthesia. Anesthesiology 1983, 58, 275–277.
- Bennett, J.A.; Abrams, J.T.; Van Riper, D.F.; Horrow, J.C. Difficult or Impossible Ventilation after Sufentanil-Induced Anesthesia Is Caused Primarily by Vocal Cord Closure. Anesthesiology 1997, 87, 1070–1074.
- Larach, D.B.; Hah, J.M.; Brummett, C.M. Perioperative Opioids, the Opioid Crisis, and the Anesthesiologist. Anesthesiology 2022, 136, 594–608.
- Dimitriou, V.; Zogogiannis, I.; Liotiri, D.; Wambi, F.; Tawfeeq, N.; Koumi, A.; Geldhof, G. Impossible Mask Ventilation after an Unusually Low Dose Fentanyl-Induced Muscle Rigidity in a Patient with Essential Tremor: A Case Report and Review of the Literature. Middle East J. Anaesthesiol. 2014, 22, 619–622.
- Lui, P.W.; Lee, T.Y.; Chan, S.H. Involvement of Coerulospinal Noradrenergic Pathway in Fentanyl-Induced Muscular Rigidity in Rats. Neurosci. Lett. 1990, 108, 183–188.
- Fu, M.J.; Tsen, L.Y.; Lee, T.Y.; Lui, P.W.; Chan, S.H. Involvement of cerulospinal glutamatergic neurotransmission in fentanyl-induced muscular rigidity in the rat. Anesthesiology 1997, 87, 1450–1459.
- Freye, E.; Kuschinsky, K. Effects of Fentanyl and Droperidol on the Dopamine Metabolism of the Rat Striatum. Pharmacology 1976, 14, 1–7.
- Tuet, W.Y.; Pierce, S.A.; Racine, M.C.; Tressler, J.; McCranor, B.J.; Sciuto, A.M.; Wong, B. Changes in Murine Respiratory Dynamics Induced by Aerosolized Carfentanil Inhalation: Efficacy of Naloxone and Naltrexone. Toxicol. Lett. 2019, 316, 127–135.
- Wong, B.; Perkins, M.W.; Tressler, J.; Rodriguez, A.; Devorak, J.; Sciuto, A.M. Effects of Inhaled Aerosolized Carfentanil on Real-Time Physiological Responses in Mice: A Preliminary Evaluation of Naloxone. Inhal. Toxicol. 2017, 29, 65–74.
- Port, J.D.; Stanley, T.H.; Steffey, E.P.; Pace, N.L.; Henrickson, R.; McJames, S.W. Intravenous Carfentanyl in the dog and Rhesus monkey. Anesthesiol. J. Am. Soc. Anesthesiol. 1984, 61, A378.
- Langston, J.L.; Moffett, M.C.; Makar, J.R.; Burgan, B.M.; Myers, T.M. Carfentanil Toxicity in the African Green Monkey: Therapeutic Efficacy of Naloxone. Toxicol. Lett. 2020, 325, 34–42.
- Moresco, A.; Larsen, R.S.; Sleeman, J.M.; Wild, M.A.; Gaynor, J.S. Use of Naloxone to Reverse Carfentanil Citrate-Induced Hypoxemia and Cardiopulmonary Depression in Rocky Mountain Wapiti (Cervus Elaphus Nelsoni). J. Zoo Wildl. Med. 2001, 32, 81–89.
- Hewson, G.; Bradley, P.B. The Effects of Anilidopiperidine Analgesics on Single Respiratory and Non-Respiratory Neurones in the Brain Stem of the Rat. Life Sci. 1982, 31, 2335–2338.
- Campbell, C.; Weinger, M.B.; Quinn, M. Alterations in Diaphragm EMG Activity during Opiate-Induced Respiratory Depression. Respir. Physiol. 1995, 100, 107–117.
- Butelman, E.R.; France, C.P.; Woods, J.H. Apparent PA2 Analysis on the Respiratory Depressant Effects of Alfentanil, Etonitazene, Ethylketocyclazocine (EKC) and Mr2033 in Rhesus Monkeys. J. Pharmacol. Exp. Ther. 1993, 264, 145–151.
- Freye, E.; Segeth, M.; Hartung, E. Somatosensory evoked potentials under alfentanyl. Anaesthesist 1984, 33, 103–107.
- Latasch, L.; Freye, E. Sufentanil-Related Respiratory Depression and Antinociception in the Dog. Mediation by Different Receptor Types. Arzneimittelforschung 2002, 52, 870–876.
- Verborgh, C.M.; Camu, F.; Meert, T.F. Interaction between Sufentanil and U-50488H with Respect to Antinociception and Respiratory Depression in Rats. Acta Anaesthesiol. Scand. 1997, 41, 895–902.
- Van den Hoogen, R.H.W.M.; Bervoets, K.J.W.; Colpaert, F.C. Respiratory effects of epidural morphine and sufentanil in the absence and presence of chlordiazepoxide. Pain 1989, 37, 103–110.
- Van den Hoogen, R.H.; Bervoets, K.J.; Colpaert, F.C. Respiratory effects of epidural and subcutaneous morphine, meperidine, fentanyl and sufentanil in the rat. Anesth. Analg. 1988, 67, 1071–1078.
- Meert, T.F.; Lu, H.R.; van Craenndonck, H.; Janssen, P.A. Comparison between Epidural Fentanyl, Sufentanil, Carfentanil, Lofentanil and Alfentanil in the Rat: Analgesia and Other in Vivo Effects. Eur. J. Anaesthesiol. 1988, 5, 313–321.
- Kuczyńska, K.; Grzonkowski, P.; Kacprzak, Ł.; Zawilska, J.B. Abuse of Fentanyl: An Emerging Problem to Face. Forensic Sci. Int. 2018, 289, 207–214.
- Marquardt, K.A.; Tharratt, R.S. Inhalation Abuse of Fentanyl Patch. J. Toxicol. Clin. Toxicol. 1994, 32, 75–78.
- Poklis, A. Fentanyl: A Review for Clinical and Analytical Toxicologists. J. Toxicol. Clin. Toxicol. 1995, 33, 439–447.
- Haouzi, P.; Mellen, N.; McCann, M.; Sternick, M.; Guck, D.; Tubbs, N. Evidence for the Emergence of an Opioid-Resistant Respiratory Rhythm Following Fentanyl Overdose. Respir. Physiol. Neurobiol. 2020, 277, 103428.
- Torralva, R.; Janowsky, A. Noradrenergic Mechanisms in Fentanyl-Mediated Rapid Death Explain Failure of Naloxone in the Opioid Crisis. J. Pharmacol. Exp. Ther. 2019, 371, 453–475.
- Brown, J.H.; Pleuvry, B.J. Antagonism of the Respiratory Effects of Alfentanil and Fentanyl by Naloxone in the Conscious Rabbit. Br. J. Anaesth. 1981, 53, 1033–1037.
- Ren, J.; Ding, X.; Funk, G.D.; Greer, J.J. Ampakine CX717 Protects against Fentanyl-Induced Respiratory Depression and Lethal Apnea in Rats. Anesthesiol. J. Am. Soc. Anesthesiol. 2009, 110, 1364–1370.
- Freye, E.; Latasch, L.; Portoghese, P.S. The Delta Receptor Is Involved in Sufentanil-Induced Respiratory Depression--Opioid Subreceptors Mediate Different Effects. Eur. J. Anaesthesiol. 1992, 9, 457–462.
- Jimenez, V.M.; Castaneda, G.; France, C.P. Methocinnamox Reverses and Prevents Fentanyl-Induced Ventilatory Depression in Rats. J. Pharmacol. Exp. Ther. 2021, 377, 29–38.
- Ren, J.; Ding, X.; Greer, J.J. Activating A4β2 Nicotinic Acetylcholine Receptors Alleviates Fentanyl-Induced Respiratory Depression in Rats. Anesthesiology 2019, 130, 1017–1031.
- Dutschmann, M.; Waki, H.; Manzke, T.; Simms, A.E.; Pickering, A.E.; Richter, D.W.; Paton, J.F.R. The Potency of Different Serotonergic Agonists in Counteracting Opioid Evoked Cardiorespiratory Disturbances. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2611–2623.
- Sahibzada, N.; Ferreira, M.; Wasserman, A.M.; Taveira-DaSilva, A.M.; Gillis, R.A. Reversal of Morphine-Induced Apnea in the Anesthetized Rat by Drugs That Activate 5-Hydroxytryptamine(1A) Receptors. J. Pharmacol. Exp. Ther. 2000, 292, 704–713.
- Manzke, T.; Dutschmann, M.; Schlaf, G.; Mörschel, M.; Koch, U.R.; Ponimaskin, E.; Bidon, O.; Lalley, P.M.; Richter, D.W. Serotonin Targets Inhibitory Synapses to Induce Modulation of Network Functions. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2589.
- Raleigh, M.D.; Baruffaldi, F.; Peterson, S.J.; Le Naour, M.; Harmon, T.M.; Vigliaturo, J.R.; Pentel, P.R.; Pravetoni, M. A Fentanyl Vaccine Alters Fentanyl Distribution and Protects against Fentanyl-Induced Effects in Mice and Rats. J. Pharmacol. Exp. Ther. 2019, 368, 282–291.
- Haile, C.N.; Baker, M.D.; Sanchez, S.A.; Lopez Arteaga, C.A.; Duddupudi, A.L.; Cuny, G.D.; Norton, E.B.; Kosten, T.R.; Kosten, T.A. An Immunconjugate Vaccine Alters Distribution and Reduces the Antinociceptive, Behavioral and Physiological Effects of Fentanyl in Male and Female Rats. Pharmaceutics 2022, 14, 2290.




