Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rejeena Jha | -- | 1172 | 2023-05-01 18:21:35 | | | |
| 2 | Conner Chen | Meta information modification | 1172 | 2023-05-04 02:43:48 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Jha, R.; Mayanovic, R.A. Structural and Physiochemical Characteristics of Chitosan. Encyclopedia. Available online: https://encyclopedia.pub/entry/43659 (accessed on 05 March 2026).
Jha R, Mayanovic RA. Structural and Physiochemical Characteristics of Chitosan. Encyclopedia. Available at: https://encyclopedia.pub/entry/43659. Accessed March 05, 2026.
Jha, Rejeena, Robert A. Mayanovic. "Structural and Physiochemical Characteristics of Chitosan" Encyclopedia, https://encyclopedia.pub/entry/43659 (accessed March 05, 2026).
Jha, R., & Mayanovic, R.A. (2023, May 01). Structural and Physiochemical Characteristics of Chitosan. In Encyclopedia. https://encyclopedia.pub/entry/43659
Jha, Rejeena and Robert A. Mayanovic. "Structural and Physiochemical Characteristics of Chitosan." Encyclopedia. Web. 01 May, 2023.
Copy Citation
Chitosan is a fibrous compound derived from chitin, which is the second most abundant natural polysaccharide and is produced by crustaceans, including crabs, shrimps, and lobsters. Chitosan has all of the important medicinal properties, including biocompatibility, biodegradability, and hydrophilicity, and it is relatively nontoxic and cationic in nature.
chitosan
chitin
chitosan nanoparticle
1. Introduction
Significant developments have been made in the recent past in medical imaging, drug delivery systems, advanced therapy, and the treatment of fatal diseases. In particular, the discovery of nanomaterials and nanomedicine has dramatically improved the precision and efficacy of a significant number of medical procedures and treatments. Nanoparticles (NPs) are nano-sized (on a scale of ~10−9 m) particles of matter that may have very unusual mechanical, physical, optical, and chemical properties compared to their larger-sized or bulk counterparts. Due to our increased capability of tuning desired characteristics and properties at various sizes and dimensions, the uses of nanomaterials have widened across a wide extent of industrial applications, including for medicine, cosmetics, air purification, agriculture, and environmental remediation. In recent years, the application of nanoparticles has increased significantly to the point of creating a nascent field of medicine, generally termed nanomedicine. Nanoparticles are widely used as contrast agents for medical imaging applications and as transport agents for drug and gene delivery in vivo [1]. Due to their small size, nanoparticles can enter the body and reach the specific tissue more efficiently and in a more direct fashion. Nanoparticles also have the capability to deliver molecules, such as from drugs, in less time and with lower pain to detect and cure diseases [2]. For nanomaterials to be used in the treatment of disease, many factors need to be considered, such as biodegradability, biocompatibility, size, hydrophilicity, and conjugation power with various drugs. Among the water-soluble materials that are currently available, chitosan has all of the properties mentioned above. Furthermore, in part due to being inexpensive, chitosan nanoparticles are widely investigated for their implementation as drug delivery systems to cure various fatal diseases.
Chitosan is a polysaccharide or fibrous compound prepared by the N-deacetylation of chitin. Chitin is a biopolymer naturally produced within crustaceans’ shells, such as those of shrimps, lobsters, and crabs. Chitosan also occurs in microorganisms, such as fungi and yeast [1]. The molecular structure of chitosan consists of glucosamine and N-acetyl-glucosamine units: The repeatability of the units is determined by the degree of deacetylation (DD) (see Figure 1). Having an equilibrium acid association constant pKa value of ~6.5 on the amine groups makes chitosan insoluble at neutral pH values; however, chitosan is soluble at acidic pH values < 6.5, whereas the chitosan molecule is positively charged [3]. Notably, compounds in their un-ionized form tend to be less soluble, but can more easily penetrate lipophilic barriers between them, and are a biological target of interest [4]. The degree of deacetylation directly affects the occurrence of amine groups in the chitosan molecular structure, which can be protonated [3]. This has a direct bearing on the solubility, the degree of hydrophilicity vs. hydrophobicity, and the nature of interactions of chitosan with various polyanions. Chitosan is soluble in acetic, formic, citric, tartaric, and other organic acids [5]. Chitosan, however, is insoluble in some inorganic acids, including phosphoric and sulfuric acids [6]. Chitosan can be obtained in a broad range of molecular weights and degrees of deacetylation [1]. For considerations of the synthesis of chitosan nanoparticles, molecular weight and the degree of deacetylation have a direct bearing on their particle size, the nature of particle formation, and their degree aggregation in solution [1].
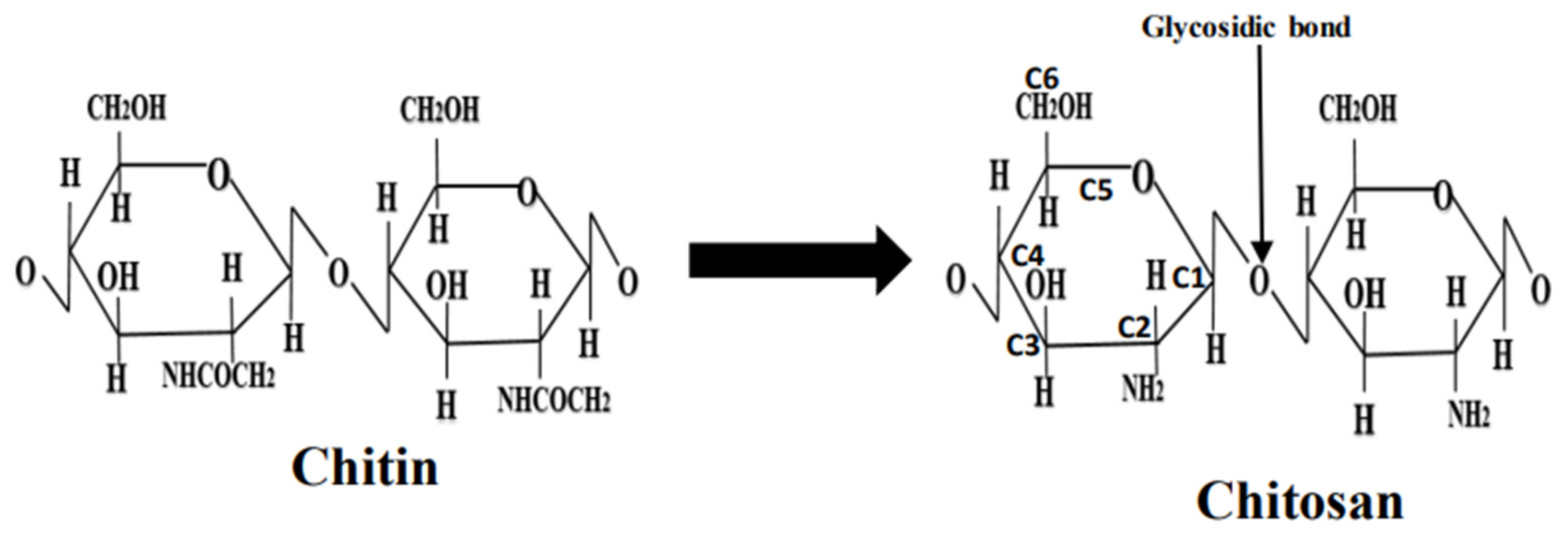
Figure 1. Molecular conversion of chitin to chitosan.
Chitosan nanoparticles (CNPs) can be produced with the desirable nano-scale characteristics, such as a small size, certain surface and interface effects, and quantum size effects [7].
2. Structural and Physiochemical Characteristics of Chitosan
The structural and physicochemical properties of chitosan have been investigated in detail by various researchers. The discovery of chitin was first made by Henri Braconnot in 1811 during his investigations on mushrooms [8]. In 1859, Charles Rouget discovered that the alkali treatment of chitin could form a very different organic polysaccharide that can be dissolved in acids [7]. This organic polysaccharide was termed chitosan by Hoppe Seiler [9].
Chitin is among the (i.e., second) most abundant polysaccharides in nature. It is present in the crustacean shells of shrimps, lobsters, and crabs [10]. It is also the primary constituent of insects’ cuticles, fungal cell walls, yeasts, and green algae [11]. Unlike chitin, chitosan has a much lower occurrence in nature; however, it has been discovered to occur within the cell wall of certain types of fungi [12]. Chitin is a polymer that comprises [β-(1-4)-2-acetamido-2deoxy-D-glucopyranose] units. The idealized structure of chitin is similar to that of cellulose with the exception that an acetamido group substitutes for the C(2) hydroxyl group [13]. There are three types of chitin: α, β, and γ [7]. Specifically, α-chitin has an antiparallel chain, β-chitin has intrasheet hydrogen bonding within parallel chains, and γ-chitin is the combination of both α- and β- chitin [14]. Chitosan is predominantly a derivative from chitin [13].
Chitosan contains 60% or more glucosamine (D units) [15]. The D unit content (and the free amine groups) in chitosan enables its solubility in aqueous acidic solution. The degree of deacetylation (DD) value is a reflection of the fractional molar content of D units in chitosan [16]. Thus, the DD value has a direct bearing on the performance of chitosan in a wide variety of applications [17]. The DD value of chitosan can be determined by using infrared radiation (IR) spectroscopy [18], UV-visible spectrophotometry [19], potentiometric titration [20], H-liquid-state nuclear magnetic resonance (NMR), and solid-state NMR spectroscopy [21]. Chitin is a comparatively intractable polymer that has sufficient structural dissimilarity to cellulose so that it is insoluble in solvents used to dissolve cellulose, including cuprammonium hydroxide (Scheweizer’s reagent), cadoxe, and cupriethylene diamine [22].
The molecular structures of chitin and chitosan are shown in Figure 1. The solubility of chitosan depends on various factors such as DD, pH, temperature, polymer crystallinity, and the type of solvent. Chitosan solubility in aqueous media is determined by the extent of protonated NH2 groups: for example, chitosan is soluble in aqueous solutions when ~50% of the protonation of amino groups occurs [23]. If DD is ~28%, chitosan is soluble in an acetic acid solution. Thus, for all other factors remaining the same, chitosan solubility is directly impacted to the degree of deacetylation since this determines the extent of glucosamine units and modifies its crystal structure [23].
The molecular weight of chitosan and its viscosity in aqueous media also have a determinative effect in the biochemical, nanomedicinal, and pharmacological applications of the polymer. Additional determinative factors include the degree of crystallinity, crystal size, ash content, moisture content, and the presence of heavy metals [24]. An additional benefit of its industrial use is that chitosan harvesting leads to ameliorating the pollution of the environment caused by the disposal of crustacians’ shells by the seafood industry [7]. Every year millions of tons of crustacean shells are produced as waste, which can degrade slowly and pollute the environment. The conversion of these shells into chitin and chitosan is one of the best solutions to combat this pollution, as chitin and chitosan have many applications in a variety of fields.
References
- Nagpal, K.; Singh, S.K.; Mishra, D.N. Chitosan Nanoparticles: A Promising System in Novel Drug Delivery. Chem. Pharm. Bull. 2010, 58, 1423–1430.
- Alshahrani, A. The Advantages of Nanotechnology in Medical Field. Int. J. Innovat. Res. Electr. Electron. Instrum. Contr. Eng. 2016, 4, 1–4.
- Bowman, K.; Leong, K.W. Chitosan Nanoparticles for Oral Drug and Gene Delivery. Int. J. Nanomed. 2006, 1, 117–128.
- Jelfs, S.; Ertl, P.; Selzer, P. Estimation of Ionization Constants (pKa) Using Semiempirical and Information-Based Descriptors, J. Chem. Inf. Model. 2007, 47, 450–459.
- Peniston, Q.P.; Johnson, E.L. Process for the Manufacture of Chitosan. U.S. Patent 4,195,175A, 25 March 1980.
- Cheng, Y.-L.; Lee, C.-Y.; Huang, Y.-L.; Buckner, C.A.; Lafrenie, R.M.; Dénommée, J.A.; Caswell, J.M.; Want, D.A.; Gan, G.G.; Leong, Y.C.; et al. We Are IntechOpen, the World’s Leading Publisher of Open Access Books Built by Scientists, for Scientists TOP 1%. Intech 2016, 11, 13.
- Divya, K.; Jisha, M.S. Chitosan Nanoparticles Preparation and Applications. Environ. Chem. Lett. 2018, 16, 101–112.
- Henri, B. Sur la nature des champibnons. Ann. Chim. Phys. 1811, 79, 265–304.
- Badawy, M.E.I.; Rabea, E.I. A Biopolymer Chitosan and Its Derivatives as Promising Antimicrobial Agents against Plant Pathogens and Their Applications in Crop Protection. Int. J. Carbohydr. Chem. 2011, 2011, 460381.
- Wang, X.; Xing, B. Importance of Structural Makeup of Biopolymers for Organic Contaminant Sorption. Environ. Sci. Technol. 2007, 41, 3559–3565.
- Einbu, A.; Vårum, K.M. Characterization of Chitin and Its Hydrolysis to GlcNAc and GlcN. Biomacromolecules 2008, 9, 1870–1875.
- Muzzarelli, R.; Jeuniau, C.; Gooday, G.W. (Eds.) Chitin in Nature and Technology; Plenum Press: New York, NY, USA, 1986.
- Sakurai, K. Structure of Chitin and Chitosan. Sen’i Gakkaishi 1990, 46, P-553–P-557.
- Franca, E.F.; Lins, R.D.; Freitas, L.C.G.; Straatsma, T.P. Characterization of Chitin and Chitosan Molecular Structure in Aqueous Solution. J. Chem. Theory Comput. 2008, 4, 2141–2149.
- Kumirska, J.; Weinhold, M.X.; Thöming, J.; Stepnowski, P. Biomedical Activity of Chitin/Chitosan Based Materials- Influence of Physicochemical Properties Apart from Molecular Weight and Degree of N-Acetylation. Polymers 2011, 3, 1875–1901.
- Aranaz, I.; Mengibar, M.; Harris, R.; Panos, I.; Miralles, B.; Acosta, N.; Galed, G.; Heras, A. Functional Characterization of Chitin and Chitosan. Curr. Chem. Biol. 2009, 3, 203–230.
- Kumirska, J.; Czerwicka, M.; Kaczyński, Z.; Bychowska, A.; Brzozowski, K.; Thöming, J.; Stepnowski, P. Application of Spectroscopic Methods for Structural Analysis of Chitin and Chitosan. Mar. Drugs 2010, 8, 1567–1636.
- Hussain, R.; Maji, T.K.; Maji, T.K. Determination of Degree of Deacetylation of Chitosan and Their Effect on the Release Behavior of Essential Oil from Chitosan and Chitosan-Gelatin Complex Microcapsules. Int. J. Adv. Eng. Appl. 2013, 2, 4–12.
- Kasaai, M.R. Various Methods for Determination of the Degree of N-Acetylation of Chitin and Chitosan: A Review. J. Agric. Food Chem. 2009, 57, 1667–1676.
- Zhang, Y.; Zhang, X.; Ding, R.; Zhang, J.; Liu, J. Determination of the Degree of Deacetylation of Chitosan by Potentiometric Titration Preceded by Enzymatic Pretreatment. Carbohydr. Polym. 2011, 83, 813–817.
- Ravi Kumar, M.N.V. Chitin and Chitosan Fibres: A Review. Bull. Mater. Sci. 1999, 22, 905–915.
- Verbeeik, R.M.H.; Haiben, M.; Thin, H.P.; Verbeek, F. Solubility and Solution Behaviour of Strontiumhydroxyapatite. Z. Phys. Chem. 1977, 108, 203–215.
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256.
- Rinaudo, M. Chitin and Chitosan: Properties and Applications. Prog. Polym. Sci. 2006, 31, 603–632.
More
Information
Subjects:
Physics, Applied
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.2K
Revisions:
2 times
(View History)
Update Date:
04 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





