Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Claudia Giuliani | -- | 3719 | 2023-04-28 08:35:08 | | | |
| 2 | Jason Zhu | -15 word(s) | 3704 | 2023-05-05 03:56:56 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Bottoni, M.; Baron, G.; Gado, F.; Milani, F.; Santagostini, L.; Colombo, L.; Colombo, P.S.; Caporali, E.; Spada, A.; Biagi, M.; et al. Achillea moschata Wulfen. Encyclopedia. Available online: https://encyclopedia.pub/entry/43605 (accessed on 08 February 2026).
Bottoni M, Baron G, Gado F, Milani F, Santagostini L, Colombo L, et al. Achillea moschata Wulfen. Encyclopedia. Available at: https://encyclopedia.pub/entry/43605. Accessed February 08, 2026.
Bottoni, Martina, Giovanna Baron, Francesca Gado, Fabrizia Milani, Laura Santagostini, Lorenzo Colombo, Paola Sira Colombo, Elisabetta Caporali, Alberto Spada, Marco Biagi, et al. "Achillea moschata Wulfen" Encyclopedia, https://encyclopedia.pub/entry/43605 (accessed February 08, 2026).
Bottoni, M., Baron, G., Gado, F., Milani, F., Santagostini, L., Colombo, L., Colombo, P.S., Caporali, E., Spada, A., Biagi, M., Giuliani, C., Bruschi, P., Aldini, G., & Fico, G. (2023, April 28). Achillea moschata Wulfen. In Encyclopedia. https://encyclopedia.pub/entry/43605
Bottoni, Martina, et al. "Achillea moschata Wulfen." Encyclopedia. Web. 28 April, 2023.
Copy Citation
Achillea moschata Wulfen (Asteraceae) is a suffruticose chamaephyte. It is endemic to the Alpine region and grows between 1400 m and over 3000 m a.s.l. on cliffs, stony ground, and moraines, exclusively on silica. It is known by various common and vernacular names, including erba iva, taneda, daneda, and aneda. The decoction of its flower heads, which represents the drug, is traditionally used to treat digestive tract disorders. The balsamic time coincides with anthesis (July–August).
primary data
traditional decoction
flower heads
aqueous and methanolic extracts
mass spectrometry
1. Introduction
Achillea moschata Wulfen (Asteraceae) is a suffruticose chamaephyte. It is endemic to the Alpine region and grows between 1400 m and over 3000 m a.s.l. on cliffs, stony ground, and moraines, exclusively on silica. It is known by various common and vernacular names, including erba iva, taneda, daneda, and aneda. The decoction of its flower heads, which represents the drug, is traditionally used to treat digestive tract disorders [1]. The balsamic time coincides with anthesis (July–August) [1].
This entry is derived from an ethnobotanical survey related to the European Interreg Italy–Switzerland B-ICE project. The main purpose of this project is to create a management model useful in dealing with emerging issues in Valmalenco (Sondrio, Lombardy, Northern Italy) due to climate change. Glacial retreat and diminished snowfall are adversely affecting tourism in the area. Therefore, the B-ICE management model includes strategies aimed at looking for new tourism avenues, such as the revitalization of traditional practices linked to medicinal and food wild plants [1]. Specifically, the work focuses on Chiesa in Valmalenco, which is the main village of the Valley. It has about 2400 inhabitants, is located at approximately 1000 m a.s.l., and is enclosed by the Bernina Alps.
Previous ethnobotanical works that mentioned A. moschata referred to the Alpine and Pre-alpine regions, with special focus on the Italian territory [1][2][3][4][5][6]. Common aspects regarding the traditions of use emerged: from the employed plant parts (flower heads, flowered aerial parts, and flowered epigeal organs) to the most popular preparations (decoctions/infusions and digestive liqueurs). Folk uses in the Alpine and Pre-alpine regions mainly referred to a digestive, antispasmodic, and carminative action. They also mentioned antiseptic, emollient, anti-inflammatory, and painkiller properties, as well as hypotensive characteristics [1][2][3][4][5][6].
Concerning previous phytochemical studies, the literature proposed various works on the essential oil composition [7][8][9] and the polar extracts [9][10][11][12], as well as the non-polar and aqueous profiles [9][13], obtained from the dried flowered aerial parts. The acetonic extracts were also analyzed for the identification of the epicuticular components [14]. Finally, Apel et al. (2021) evaluated and compared the hydroalcoholic extracts (methanol/water) of three congeneric species: A. millefolium, A. moschata, and A. atrata [15].
The characterization of the aqueous extract proposed in the literature deserves further investigation, with morphological studies on the target species aimed at investigating the secreting structures responsible for productivity in secondary metabolites lacking. Indeed, previous micromorphological contributions focused on European and Western Asian congeneric species [16][17][18][19][20][21]. Regarding the biological activity, A. moschata extracts were generally screened for their radical scavenger properties with spectrophotometric assays such as the DPPH test [11][12]. Moreover, studies never reported the aqueous extract, which is significant, being the traditional decoction used in folk medicine in the Alpine and Pre-alpine regions. Only recently, Vitalini et al. [9] tested several extracts, including the aqueous one, for their anti-inflammatory activity, evaluating the modulation of interleukins 8 and 1β on CaCo-2 cells. Concerning congeneric species, the antioxidant potential of different extracts was evaluated as radical scavenger activity, but no in vitro studies were reported to date in the literature [10][22][23][24].
2. Phytochemical Investigation
Regarding the phytochemical investigation, the qualitative profile of the aqueous and methanolic extract of the dried flowered aerial parts of A. moschata was evaluated. One of the elements of novelty offered was the faithful reproduction of the traditional aqueous preparation, which was directly documented in the form of decoction through the ethnobotanical survey conducted in the study area. Another original aspect concerned the characterization of the polyphenolic content of the traditional preparation, with the aim to better understand its potential in terms of bioactive compounds. Furthermore, this analysis was enriched with the characterization of the methanolic extract obtained by extraction with solvents of increasing polarity. This, in turn, facilitated a comparison with the literature data currently available about the target species, which mainly refer to this type of extract [10][11][12][15].
The LC-MS/MS analyses allowed for the identification of all the main compounds, detecting a qualitative overlap between aqueous and methanolic extracts. A total of 31 compounds were identified, 28 of which were detected in both extracts. To this end, luteolin C-glucoside (4), desmethoxycentaureidin (27), and isorhamnetin (28) were found only in the methanolic extract, even if only in trace amounts. Among the 31 total compounds (31 in negative ion mode, 27 in positive ion mode), 5 were phenolic acids, 13 were flavonols, and 13 were flavones. In detail, the phenolic acids were represented by caffeoylquinic acids or dicaffeoylquinic acids isomers (1, 2, 11, 13, 19), with all present in both extracts. Flavonols were detected as glucosides and aglycones, and they were quercetin (6, 29), kaempferol (7, 30), isorhamnetin (12, 14, 16, 28), mearnsetin (9, 10), syringetin (17), eupatolin (21), and chrysosplenol-D (or jaceidin) (31) derivatives. These were also detected in both extracts, except for isorhamnetin aglycone (28), which was found only in the methanolic extract. Analogously, flavones were detected as glucosides and aglycones of apigenin (3, 15, 25), apparently the most abundant component, luteolin (4, 8, 23), isoorientin (18), axillarin (24), chrysoeriol (or hispidulin) (26), and desmethoxycentaureidin (27). Finally, malonyl derivatives of luteolin (luteolin 7-malonyl-glucoside, 20) and apigenin (apigenin 7-malonyl-glucoside, 22) were identified in the two extracts. Special attention was paid to apigenin 7-malonyl-glucoside (22), whose peak has a higher relative abundance in respect to the other as opposed to the methanolic one, thus revealing an important feature of the traditional preparation, considering that the decoction extraction process adopted for the analysis was derived from the folk medicine.
Regarding the phytochemical profiling of the phenolic content of A. moschata, the literature proposed only five works. Two of these highlighted the quali-quantitative characterization of the methanolic extract [11][12]; one proposed the quali-quantitative analysis of the acetone/water extract, later solubilized in methanol/water [15]; one focused on the quantification of the total polyphenolic content of the methanolic fraction [10]; and, finally, a more recent work, proposed a quantitative comparison between the methanolic extract and the aqueous preparation, without presenting any chromatogram and fragmentation pattern of the listed compounds [9].
From a comparison with these papers [9][11][12][15], the phytochemical characterization identified the highest total number of compounds. The phenolic classes were found to be the same, including simple phenols (phenolic acids) and flavonoids (flavones and flavonols). Moreover, the identification exhibited 14 exclusive compounds, not detected in the other profiles: vicenin-2 (3), schaftoside (5), eupatolin (21), axillarin (24), chrysoeriol/hispidulin (26), isorhamnetin (28), quercetin-3,3′-dimethylether (29), 6-hydroxykaempferol-3,6-dimethylether (30), and chrysosplenol/jaceidin (31), as well as glycosides of syringetin (17), luteolin (4), and isoorientin 7- methyl ether (18). In addition, the detection of two malonic acid derivatives, luteolin 7-malonyl-glucoside (20) and apigenin 7-malonyl-glucoside (22), highlighted another unique feature of the profiles, both in the methanolic extract and the decoction. Specifically, the latter compound seems to be highly concentrated in the aqueous extract and, both molecules were previously identified only in congeneric species such as A. millefolium, A. alpestris, and A. ceretanum [25].
These phytochemical data deepened the field work conducted by the research group on the traditional uses of A. moschata in the very distribution area of the species. This aspect further distinguishes the survey from previous works, which based their phytochemical investigation on ethnobotanical data obtained in the scientific literature [9][11][12][15], or sometimes analyzing commercial forms of the herbal drug, instead of spontaneous ones [15].
3. Morphological Investigation
3.1. Botanical Description
Achillea moschata presents creeping woody stems from which ascending sterile shoots and erect flowering branches start. The latter are sub-glabrous or with short hairs. The leaves are alternate, deeply pinnate with a lanceolate–spatulate outline and 7–10 segments on each side. The basal leaves are pedicellate, while the cauline ones are sessile, toothed, and progressively linear. The leaves are strongly aromatic, hairless, or with scattered hairs [26]. The flowers are grouped in small flower heads (capitula) with a diameter of 2–6 mm, in turn gathered in loose corymbs, with each composed of about 10 flower heads. The peduncles of the flower heads are cylindrical, striated, sub-glabrous, or with scattered hairs [27]. The capitula are composed of both ray (6–9) and disk (10–15) florets. The receptacle is conic or convex, enclosed by various layers of imbricate involucral bracts. The bracts are obovate-oblong, sub-glabrous [27], with narrow transparent membranous margin, yellowish-to-brownish, and whole or notched only towards the apex with shallow denticles. The disk florets vary in number, from 10 up to 15 per head and are hermaphrodite. Each floret is subtended by an interfloral scale called “palea”. The corolla tube is yellow with the top split in 5 apical laciniae overhanging the tube. The stamens are 5 with free filaments and connate anthers, welded in a sleeve surrounding the stylus with deeply bifid stigma; the carpels are 2 forming a unilocular inferior ovary. The ray florets are 6–9 per head, pistillate, fertile, 3–4 mm long. The corollas are white, with a tubular lower portion surmounted by a ribbon-like extension, the ligule, ending with sub-round denticles, with scattered golden glands on the lower side [26]. The stigma is 2-lobed, with a papillose surface.
3.2. Indumentum
The indumentum of the examined organs in all the replicates showed a general consistency both in terms of trichome morphotypes and distribution pattern. Both covering and glandular hairs were observed (Figure 1). The formers were simple, multicellular, uniseriate, and distinguished in two subtypes: short and 5–7 celled with isodiametric cells of uniform diameter from the base to the apex, so as to appear digitiform (Figure 1a); long and filamentous with a pointed apex which may be straight or slightly twisted (Figure 1b). Although the cell number could not be defined based on LM and SEM analyses, the literature data indicated the occurrence of 5-celled filamentous hairs in some congeneric species [15][17]. The basal cells of both subtypes were usually protruding from the epidermal surface of the organs bearing them, and sporadically appeared swollen. The short digitiform hairs were distributed on flowering stems and on both leaf sides (Figure 2a–f), while the long filamentous ones were on the leaf abaxial sides, on the abaxial side of the involucral bracts, and on peduncles of the capitulum (Figure 2c and Figure 3a–d). The glandular trichomes belonged to a single morphotype (Figure 1b–g) that was a 10-celled biseriate, composed of two rows of five cells each (Figure 1c,f,g). There are two basal cells, two stalk cells, and six secretory cells, as already described in some congeneric species [19][20]. At full growth, the mature glands presented a peculiar bilobed, heart-like shape, resulting from the expansion of the cuticle covering the apical cells. In fact, during development, the subcuticular space at the apex grew into a sac-like structure, although it was unclear whether the sac embraced four or six apical cells (Figure 1f,g). These hairs may be sunken or may protrude to varying degrees from the epidermal surface because of the different elongation of the basal cells (Figure 1d–g). They appeared sunken on the flowering stems, leaves, involucral bracts, and interfloral scale of the disk florets (Figure 2a–f and Figure 3a–d,g–j), but protruded from the epidermis of the corolla of both disk and ray florets and on their ovaries (Figure 3k–m and Figure 4a–f). On the abaxial surfaces of involucral bracts, trichomes presented a peculiar distribution pattern: filamentous glands and trichomes were arranged in two rows in troughs along either side of the leaf midrib (Figure 3b–d). Most of the secretion was discharged externally following the rupture of the cuticle, along a predetermined line of weakness, visible on the apical cuticular layer (Figure 1e and Figure 2e–f). The cuticular envelope appeared broken to several degrees in many of the mature glands. Fractured cuticles also revealed the flocculent appearance of the secreted materials within the subcuticular chamber (Figure 1e).
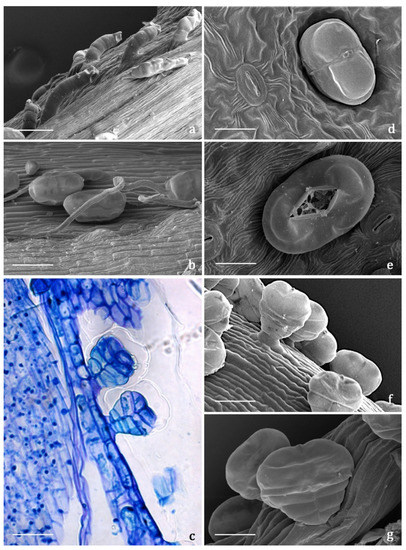
Figure 1. SEM and LM micrographs showing the covering and glandular trichomes observed on the vegetative and reproductive organs of Achillea moschata. (a) Short digitiform trichomes. (b) Long filamentous trichome and biseriate glandular trichomes. (c) Longitudinal section of biseriate 10-celled glandular trichomes stained with Toluidine Blue. (d) Sunken biseriate glandular trichome with intact cuticle. (e) Sunken glandular trichome showing the rupture of the cuticle along a line of weakness. (f,g) Protruding biseriate 10-celled glandular trichomes. Scale bars: 50 μm (a–c,f); 20 μm (d,e,g).
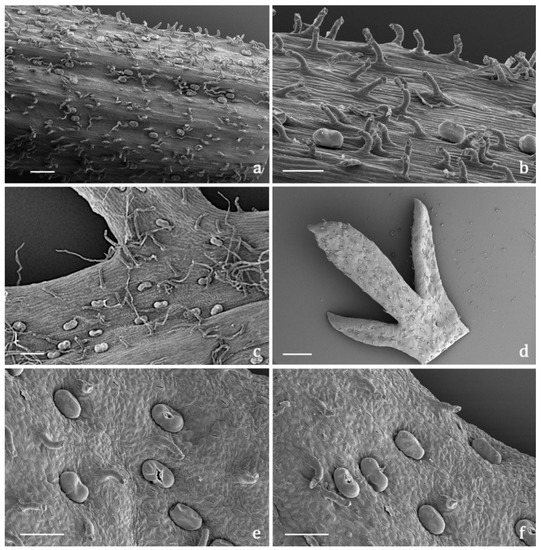
Figure 2. SEM micrographs showing the flowering stem and leaf morphology of Achillea moschata. (a,b) General view (a) and detail (b) of a flowering stem with biseriate glandular trichomes and digitiform covering trichomes. (c) Leaf abaxial surface with the two types of covering trichomes (short digitiform and long filamentous) and the biseriate glandular hairs. (d) General view of the apical portion of a deeply pinnate leaf (adaxial side). (e,f) Details of the leaf adaxial side with sunken biseriate glandular trichomes and digitiform hairs; notice the broken cuticle of several glandular trichomes for the release of the secreted materials. Scale bars: 500 μm (a,d); 100 μm (b,c,e,f).
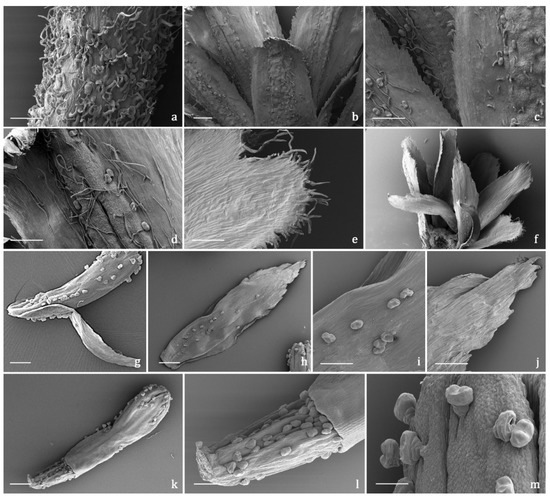
Figure 3. SEM micrographs showing the peduncle of the capitulum and the involucral bracts (a–f) and the disk florets (g–m) of Achillea moschata. (a) Peduncle of the capitulum. (b) General view of the involucral bracts (abaxial sides). (c,d) Details of the abaxial side of the involucral bracts with biseriate glandular trichomes and long filamentous hairs. (e) General view of the involucral bracts (adaxial sides). (f) Details of the apical portion of the abaxial side of an involucral bract. (g) General view of a disk floret subtended by an interfloral scale. (h–j) Interfloral scale: general view (h), details of the median portion (i), and of the apical portion (j) of the abaxial side. (k–m) Disk floret: general view (k), details of the ovary (l), and of the apical portion (m) of the corolla. Scale bars: 200 μm (a–f,g,h,k); 100 μm (i,j,l); 50 μm (m).
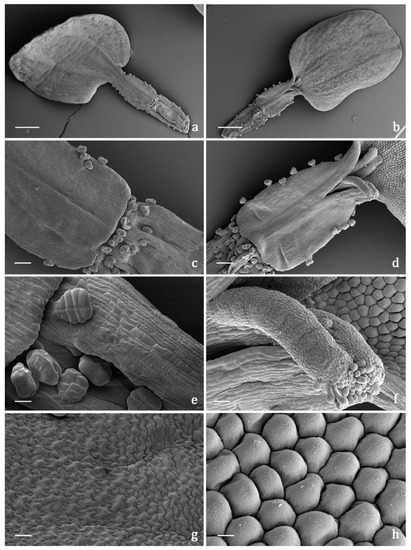
Figure 4. SEM micrographs showing the pistillate ray florets of the capitulum of Achillea moschata. (a,b) General view of a ray floret: abaxial (a) and adaxial (b) sides. (c,d) Details of the proximal portion of the corolla tube and of the distal portion of the ovary: abaxial (c) and adaxial (d) sides. (e) Biseriate, 10-celled glandular trichomes on the distal portion of the ovary. (f) Details of the 2-lobed stigma with a papillose surface. (g) Epidermis of the ligule adaxial side with elongated puzzle-like striated cells. (h) Epidermis of the ligule adaxial side with isodiametric conical striated cells. Scale bars: 500 μm (a,b); 100 μm (c,d); 20 μm (e,f); 10 μm (g,h).
3.3. Histochemistry
The secretion products of the biseriate glandular trichomes exhibited positive responses both to lipophilic tests, such as Fluoral Yellow-088 and Nadi reagent (Figure 5a,b), and to the FeCl3 test (Figure 5c,d), specific to polyphenols, and to the AlCl3 test (Figure 5e) and Naturstoff reagent A specific to flavonoids. They showed weak coloration or negative responses to the hydrophilic dyes Alcian Blue (Figure 5f) and Ruthenium Red. Concerning internal tissues, the histochemical observations turned out to be uneven, since the results were generally negative for all the employed dyes, with sporadic weak positive responses only for polyphenols. Only one previous histochemical investigation was performed on a congeneric species, A. millefolium, and the production of monoterpenes was ascertained [17]. The secretory products are released externally after cuticle rupture: the volatile terpenoids emitted in the air are responsible for the typical aroma of A. moschata, while the polyphenolic/flavonoidic components presumably cover the epidermis constituting epicuticular exudates. Considering the overall micromorphological results, researchers exclude the presence of internal secretory structures, as well as the massive accumulation at tissue level of the polyphenols and flavonoids detected in the aqueous and methanolic extracts. The main sites of synthesis, accumulation, and release of such substances, along with volatile terpenoids, were represented by the biseriate glandular trichomes, distributed on the whole plant epidermal surfaces, being particularly abundant on the inflorescences (involucral bracts, interfloral scales, corollas, and ovaries of both disk and ray florets).
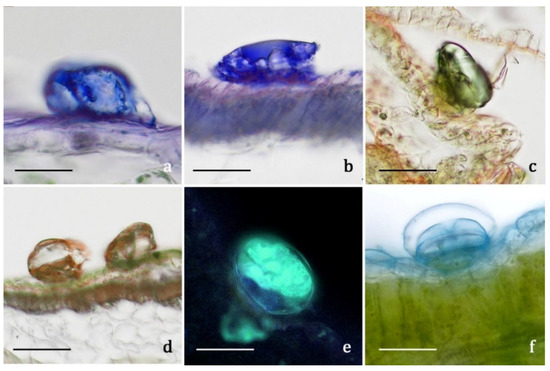
Figure 5. LM and FM micrographs showing the histochemical results on the secretory materials of the biseriate glandular trichomes of Achillea moschata. (a,b) Nadi reagent. (c,d) Ferric Trichloride test. (e) Aluminum Trichloride test. (f) Alcian Blue test. Scale bars: 30 μm (a,b,e,f); 50 μm (c,d).
4. Biological Activity
The aqueous extract was evaluated for its antioxidant and anti-inflammatory potential in two different cell lines. Regarding the antioxidant activity, researchers tested the ability of the extract to activate the response of NRF2, a transcription factor associated with antioxidant enzymes, and which plays a master role in redox homeostasis in the cells. Data reported in Figure 6A show significant increase of NRF2 activation compared to the control. The activity is already significant at 10 μg/mL, while at the highest tested dose, 100 μg/mL, the increment is even higher than the one registered with BDX (Bardoxolone), a synthetic NRF2 activator used as a positive control. Concerning the anti-inflammatory activity, Figure 6B highlights some remarkable results. Indeed, the extract was able to significantly decrease the inflammatory response due to the stimulation of TNFα already at 1 μg/mL, while at 100 μg/mL, the response was comparable to control values. Cell viability was assessed and found to be comparable to the control values in both cell lines for all the tested concentrations (data not shown).
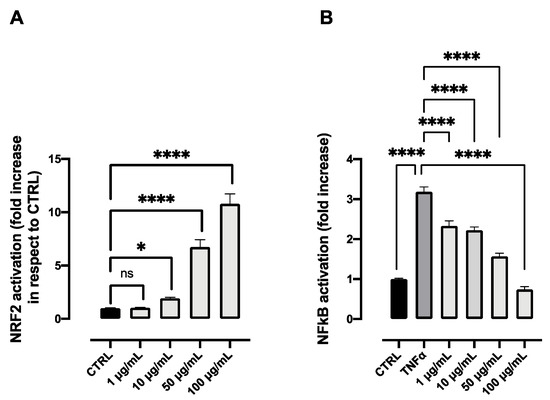
Figure 6. NRF2 activation (A) and anti-inflammatory activity (B) of different concentrations of the aqueous extract obtained from the flower head of Achillea moschata. Data are reported as mean ± SD. The statistical significance difference of each concentration in respect to the control was analyzed by one-way ANOVA followed by the Bonferroni post-test. * p < 0.05, **** p < 0.0001, ns = not significant.
Notably, it was the first time that these biological activities were reported in vitro for the aqueous extract of A. moschata. The antioxidant activity of A. moschata was previously reported using the DPPH assay, demonstrating, in in vitro conditions, a direct radical scavenging activity of the polyphenolic constituents of the species [9]. However, it is now well established that in in vivo conditions, the scavenging of radicals by polyphenols, and in general by antioxidants, cannot occur due to kinetic constraints (with the exception of vitamin E as a scavenger of lipid peroxyl radicals) [28]. Hence, the in vivo antioxidant defense of small antioxidants, such as polyphenols, is not due to a direct radical scavenging mechanism but rather to the activation of the NRF2 (NF-E2-related factor 2) signaling pathway which upregulates the enzymatic removal of the precursors of radical species, such as hydroperoxides, in two-electron redox reactions. researchers demonstrated herein the ability of polyphenols from A. moschata to activate the NRF2 pathway, a mechanism which could result in an in vivo antioxidant defense mechanism.
For the anti-inflammatory activity, an in vitro study was conducted on the aqueous extract by Vitalini et al. [9] on CaCo-2 cells focusing on the modulation of the release of interleukins. However, the novelty of the work regarding the study of the anti-inflammatory mechanism, which once activated, led to an anti-inflammatory response of which the release of inflammatory interleukins is one of the effects. The activation of NFKB is only partially related to the transcription of interleukins; it is responsible for numerous other mediators [29]. A strict crosstalk between inflammation and oxidative stress and between the two transcriptional factors involved, NFKB and NRF2, is now well established. When inflammation occurs as a primary disorder, it induces oxidative stress as a secondary disorder, which can further enhance inflammation. On the other hand, when oxidative stress is induced, inflammation develops as a secondary disorder and further enhances oxidative stress [30][31]. Based on this mechanism, the anti-inflammatory activity of the aqueous extract could be related not only to its action on the NFKB pathway, but also to the activation of NRF2. Because of the striking importance these two pathways have in the regulation of anti-inflammatory and antioxidant processes, it is notable that the aqueous extract is able to significantly work even at low concentrations on both processes.
Regarding the previous studies on the potential biological activity of A. moschata extracts [9][11][12][15], the phenolic profile was generally investigated concerning antioxidant and anti-bacterial properties. Once again, the tested activities rarely considered the traditional uses, or they did not describe them properly [9][15]. Moreover, even in such cases as these, the tested extracts deviated from the traditional remedies documented in the considered ethnobotanical studies.
With reference to the composition of the hot aqueous extract, which faithfully reproduces the decoction traditionally used and which was firstly characterized, researchers proposed hereafter some considerations on the available literature data. Regarding the biological activity, researchers performed a survey with special focus on the gastrointestinal tract. The scientific literature documented an interesting in vitro anti-inflammatory ability ascribable to some of the main compounds found in the aqueous and methanolic extracts. As for the mechanisms, a few studies conducted on apigenin 7-glucoside (15) and chlorogenic acid (2) recorded important antioxidant and radical scavenging activities. The inhibition of the expression and translocation of Nuclear factor-kappa B (NF-κB) and of the production of pro-inflammatory cytokines, such as Tumor necrosis factor-α (TNF-α) and Interleukin-8 (IL-8), was also detected [32][33][34][35][36][37]. Apigenin 7-glucoside was also considered as a potential candidate against gastric cancer progression [38], due to the positive modulation of intracellular Reactive Oxygen Species (ROS) production. Moreover, some authors attributed to chlorogenic acid the healing properties of Taraxacum spp. on gastrointestinal disorders such as dyspepsia and gastritis [39]. The inhibition of the nuclear translocation of NF-κB, with the subsequent dwindling of COX-2 gene expression and of Prostaglandin E2 (PGE2) production, could be the mechanism underlying the anti-inflammatory activity of dicaffeoylquinic acids (13). In addition, another mechanism can be considered, namely, the inhibition of the expression of Inducible Nitric Oxide Synthase (iNOS) and the production of Nitric Oxide (NO) [40][41]. Karbab et al. [42] ascribed to isorhamnetin 3-O-beta-glucoside, which is one of the potential compounds for peak number 16, anti-inflammatory properties related to the inhibition of protein denaturation. Little has been reported on compound number 22, apigenin 7-malonyl-glucoside, which characterizes the aqueous extract. Han et al. [43] showed that it is one of the potential NF-κB inhibitor activity components of Flos Chrysanthemi, while Martínez-Vázquez et al. [44] described a sedative effect of the Dracocephalum moldavica L. aqueous extract with a high content of apigenin- and luteolin 7-malonyl-glucosides. Malonyl-glucosides can either be hydrolyzed in the gastrointestinal tract up to their relative glucosides [45], or absorbed in their intact form [46], thus exerting their activity in both conditions.
References
- Bottoni, M.; Milani, F.; Colombo, L.; Nallio, K.; Colombo, P.S.; Giuliani, C.; Bruschi, P.; Fico, G. Using Medicinal Plants in Valmalenco (Italian Alps): From Tradition to Scientific Approaches. Molecules 2020, 25, 4144.
- Dei Cas, L.; Pugni, F.; Fico, G. Tradition of use on medicinal species in Valfurva (Sondrio, Italy). J. Ethnopharmacol. 2015, 163, 113–134.
- Abbet, C.; Mayor, R.; Roguet, D.; Spichiger, R.; Hamburger, M.; Potterat, O. Ethnobotanical survey on wild alpine food plants in Lower and Central Valais (Switzerland). J. Ethnopharmacol. 2014, 151, 624–634.
- Mattalia, G.; Quave, C.L.; Pieroni, A. Traditional uses of wild food and medicinal plants among Brigasc, Kyé, and Provençal communities on the Western Italian Alps. Genet. Resour. Crop Evol. 2013, 60, 587–603.
- Vitalini, S.; Iriti, M.; Puricelli, C.; Ciuchi, D.; Segale, A.; Fico, G. Traditional knowledge on medicinal and food plants used in Val San Giacomo (Sondrio, Italy)—An alpine ethnobotanical study. J. Ethnopharmacol. 2013, 145, 517–529.
- Pieroni, A.; Giusti, M.E. Alpine ethnobotany in Italy: Traditional knowledge of gastronomic and medicinal plants among the Occitans of the upper Varaita valley, Piedmont. J. Ethnobiol. Ethnomed. 2009, 5, 32.
- Maffei, M.; Chialva, F.; Codignola, A. Essential oils and chromosome numbers from Italian Achillea species. J. Essent. Oil Res. 1989, 1, 57–64.
- Tava, A.; Iriti, M.; Vitalini, S. Composition and Antioxidant Activity of the Essential Oil from Achillea moschata Wulfen Growing in Valchiavenna and Valmalenco (Italian Central Alps) Introduction. Int. J. Hortic. Sci. Technol. 2020, 7, 335–341.
- Vitalini, S.; Garzoli, S.; Sisto, F.; Pezzani, R.; Argentieri, M.P.; Scarafoni, A.; Ciappellano, S.; Zorzan, M.; Capraro, J.; Collazuol, D.; et al. Digestive and gastroprotective effects of Achillea erba-rotta subsp. moschata (Wulfen) I. Richardson (syn. A. moschata Wulfen) (Asteraceae): From traditional uses to preclinical studies. J. Ethnopharmacol. 2022, 298, 115670.
- Vitalini, S.; Grande, S.; Visioli, F.; Agradi, E.; Fico, G.; Tome, F. Antioxidant activity of wild plants collected in Valsesia, an Alpine Region of Northern Italy. Phyther. Res. 2006, 20, 576–580.
- Vitalini, S.; Madeo, M.; Tava, A.; Iriti, M.; Vallone, L.; Avato, P.; Cocuzza, C.E.; Simonetti, P.; Argentieri, M.P. Chemical profile, antioxidant and antibacterial activities of Achillea moschata Wulfen, an endemic species from the alps. Molecules 2016, 21, 830.
- Argentieri, M.P.; Madeo, M.; Avato, P.; Iriti, M.; Vitalini, S. Polyphenol content and bioactivity of Achillea moschata from the Italian and Swiss Alps. Zeitschrift fur Naturforsch. Sect. C J. Biosci. 2020, 75, 57–64.
- Bottoni, M.; Baron, G.; Milani, F.; Colombo, L.; Colombo, P.S.; Santagostini, L.; Giuliani, C.; Bruschi, P.; Aldini, G.; Fico, G. Achillea moschata Wulfen: Traditional uses and chemical characterization. In Proceedings of the XVII Italian Phytochemical Society Congress jointly with Third ICEMAP Congress 2022, Bari, Italy, 22–24 June 2022.
- Valant-Vetschera, K.M.; Wollenweber, E. Exudate flavonoid aglycones in the alpine species of Achillea sect. Ptarmica: Chemosystematics of A. moschata and related species (Compositae-Anthemideae). Biochem. Syst. Ecol. 2001, 29, 149–159.
- Apel, L.; Lorenz, P.; Urban, S.; Sauer, S.; Spring, O.; Stintzing, F.C.; Kammerer, D.R. Phytochemical characterization of different yarrow species (Achillea sp.) and investigations into their antimicrobial activity. Zeitschrift fur Naturforsch. Sect. C J. Biosci. 2021, 76, 55–65.
- Afshari, M.; Rahimmalek, M. Variation in essential oil composition, anatomical, and antioxidant characteristics of Achillea filipendulina Lam. as affected by different phenological stages. J. Essent. Oil Res. 2021, 33, 283–298.
- Fathi, E.; Majdi, M.; Dastan, D.; Maroufi, A. The spatio-temporal expression of some genes involved in the biosynthetic pathways of terpenes/phenylpropanoids in yarrow (Achillea millefolium). Plant Physiol. Biochem. 2019, 142, 43–52.
- Afshari, M.; Rahimmalek, M. Variation in Essential Oil Composition, Bioactive Compounds, Anatomical and Antioxidant Activity of Achillea aucheri, an Endemic Species of Iran, at Different Phenological Stages. Chem. Biodivers. 2018, 15, e1800319.
- Nedelcheva, A. Micro-morphology of Achillea clypeolata: Contribution to the pharmacognostical profile. J. Appl. Pharm. Sci. 2012, 2, 165–170.
- Ciccarelli, D.; Garbari, F.; Pagni, A.M. Glandular hairs of the ovary: A helpful character for Asteroideae (Asteraceae) taxonomy? Ann. Bot. Fenn. 2007, 44, 1–7.
- Figueiredo, A.C.; Pais, M.S.S. Ultrastructural Aspects of the Glandular Cells from the Secretory Trichomes and from the Cell Suspension Cultures of Achillea millefolium L. ssp. millefolium. Ann. Bot. 1994, 74, 179–190.
- Gharibi, S.; Tabatabaei, B.E.S.; Saeidi, G.; Goli, S.A.H. Effect of drought stress on total phenolic, lipid peroxidation, and antioxidant activity of Achillea species. Appl. Biochem. Biotechnol. 2016, 178, 796–809.
- Raudone, L.; Radušiene, J.; Seyis, F.; Yayla, F.; Vilkickyte, G.; Marksa, M.; Ivanauskas, L.; Cırak, C. Distribution of phenolic compounds and antioxidant activity in plant parts and populations of seven underutilized wild Achillea species. Plants 2022, 11, 447.
- Afshari, M.; Rahimmalek, M.; Miroliaei, M. Variation in polyphenolic profiles, antioxidant and antimicrobial activity of different Achillea species as natural sources of antiglycative compounds. Chem. Biodivers. 2018, 15, e1800075.
- Si, X.T.; Zhang, M.L.; Shi, Q.W.; Kiyota, H. Chemical constituents of the plants in the genus Achillea. Chem. Biodivers. 2006, 3, 1163–1180.
- Pignatti, S.; Guarino, R.; La Rosa, M. Flora d’Italia, 2nd ed.; Edagricole—New Business Media: Bologna, Italy, 2017.
- Pignatti, S. Flora d’Italia; Edagricole: Bologna, Italy, 1982.
- Forman, H.J.; Davies, K.J.; Ursini, F. How do nutritional antioxidants really work: Nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic. Biol. Med. 2014, 66, 24–35.
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023.
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626.
- Ahmed, S.; Luo, L.; Namani, A.; Wang, X.; Tang, X. Nrf2 Signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta. Mol. Basis. Dis. 2017, 1863, 585–597.
- Alcázar Magaña, A.; Kamimura, N.; Soumyanath, A.; Stevens, J.F.; Maier, C.S. Caffeoylquinic acids: Chemistry, biosynthesis, occurrence, analytical challenges, and bioactivity. Plant J. 2021, 107, 1299–1319.
- Bensouici, C.; Kabouche, A.; Karioti, A.; Öztürk, M.; Duru, M.E.; Bilia, A.R.; Kabouche, Z. Compounds from Sedum caeruleum with antioxidant, anticholinesterase, and antibacterial activities. Pharm. Biol. 2016, 54, 174–179.
- Villalva, M.; Jaime, L.; Villanueva-Bermejo, D.; Lara, B.; Fornari, T.; Reglero, G.; Santoyo, S. Supercritical anti-solvent fractionation for improving antioxidant and anti-inflammatory activities of an Achillea millefolium L. extract. Food Res. Int. 2019, 115, 128–134.
- Vitalini, S.; Beretta, G.; Iriti, M.; Orsenigo, S.; Basilico, N.; Dall’Acqua, S.; Iorizzi, M.; Fico, G. Phenolic compounds from Achillea millefolium L. and their bioactivity. Acta Biochim. Pol. 2011, 58, 203–209.
- Wang, W.; Yue, R.F.; Jin, Z.; He, L.M.; Shen, R.; Du, D.; Tang, Y.Z. Efficiency comparison of apigenin-7-O-glucoside and trolox in antioxidative stress and anti-inflammatory properties. J. Pharm. Pharmacol. 2020, 72, 1645–1656.
- Witkowska-Banaszczak, E.; Krajka-Kuźniak, V.; Papierska, K. The effect of luteolin 7-glucoside, apigenin 7-glucoside and Succisa pratensis extracts on NF-κB activation and α-amylase activity in HepG2 cells. Acta Biochim. Pol. 2020, 67, 41–47.
- Sun, Q.; Lu, N.N.; Feng, L. Apigetrin inhibits gastric cancer progression through inducing apoptosis and regulating ROS-modulated STAT3/JAK2 pathway. Biochem. Biophys. Res. Commun. 2018, 498, 164–170.
- Li, Y.; Chen, Y.; Sun-Waterhouse, D. The potential of dandelion in the fight against gastrointestinal diseases: A review. J. Ethnopharmacol. 2022, 293, 115272.
- Puangpraphant, S.; Berhow, M.A.; Vermillion, K.; Potts, G.; Gonzalez de Mejia, E. Dicaffeoylquinic acids in Yerba mate (Ilex paraguariensis St. Hilaire) inhibit NF-κB nucleus translocation in macrophages and induce apoptosis by activating caspases-8 and -3 in human colon cancer cells. Mol. Nutr. Food Res. 2011, 55, 1509–1522.
- Wan, P.; Xie, M.; Chen, G.; Dai, Z.; Hu, B.; Zeng, X.; Sun, Y. Anti-inflammatory effects of dicaffeoylquinic acids from Ilex kudingcha on lipopolysaccharide-treated RAW264.7 macrophages and potential mechanisms. Food Chem. Toxicol. 2019, 126, 332–342.
- Karbab, A.; Charef, N.; Abu Zarga, M.H.; Qadri, M.I.; Mubarak, M.S. Ethnomedicinal documentation and anti-inflammatory effects of n-butanol extract and of four compounds isolated from the stems of Pituranthos scoparius: An in vitro and in vivo investigation. J. Ethnopharmacol. 2021, 267, 113488.
- Han, Y.; Zhou, M.; Wang, L.; Ying, X.; Peng, J.; Jiang, M.; Bai, G.; Luo, G. Comparative evaluation of different cultivars of Flos Chrysanthemi by an anti-inflammatory-based NF-κB reporter gene assay coupled to UPLC-Q/TOF MS with PCA and ANN. J. Ethnopharmacol. 2015, 174, 387–395.
- Martínez-Vázquez, M.; Estrada-Reyes, R.; Martínez-Laurrabaquio, A.; López-Rubalcava, C.; Heinze, G. Neuropharmacological study of Dracocephalum moldavica L. (Lamiaceae) in mice: Sedative effect and chemical analysis of an aqueous extract. J. Ethnopharmacol. 2012, 141, 908–917.
- Cheng, D.M.; Roopchand, D.E.; Poulev, A.; Kuhn, P.; Armas, I.; Johnson, W.D.; Oren, A.; Ribnicky, D.; Zelzion, E.; Bhattacharya, D.; et al. High phenolics Rutgers Scarlet Lettuce improves glucose metabolism in high fat diet-induced obese mice. Mol. Nutr. Food Res. 2016, 60, 2367–2378.
- Jiang, Q.; Zhang, H.; Yang, R.; Hui, Q.; Chen, Y.; Mats, L.; Tsao, R.; Yang, C. Red-osier dogwood extracts prevent inflammatory responses in Caco-2 cells and a Caco-2 BBe1/EA.hy926 cell co-culture model. Antioxidants 2019, 8, 428.
More
Information
Subjects:
Plant Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
988
Revisions:
2 times
(View History)
Update Date:
05 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




