Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nives Pecina-Slaus | -- | 1798 | 2023-04-26 23:05:00 | | | |
| 2 | Catherine Yang | -1 word(s) | 1797 | 2023-04-27 03:59:48 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pećina-Šlaus, N.; Bukovac, A.; Aničić, S.; Kafka, A. Wnt Signaling Inhibitors. Encyclopedia. Available online: https://encyclopedia.pub/entry/43544 (accessed on 07 February 2026).
Pećina-Šlaus N, Bukovac A, Aničić S, Kafka A. Wnt Signaling Inhibitors. Encyclopedia. Available at: https://encyclopedia.pub/entry/43544. Accessed February 07, 2026.
Pećina-Šlaus, Nives, Anja Bukovac, Sara Aničić, Anja Kafka. "Wnt Signaling Inhibitors" Encyclopedia, https://encyclopedia.pub/entry/43544 (accessed February 07, 2026).
Pećina-Šlaus, N., Bukovac, A., Aničić, S., & Kafka, A. (2023, April 26). Wnt Signaling Inhibitors. In Encyclopedia. https://encyclopedia.pub/entry/43544
Pećina-Šlaus, Nives, et al. "Wnt Signaling Inhibitors." Encyclopedia. Web. 26 April, 2023.
Copy Citation
The inhibition of the Wnt signaling pathway has been recognized as a promising target in the continous search for antitumor therapies. The altered functioning of the Wnt signaling in human tumors points to the therapeutic strategy of the inhibition of its activity. Drugging the Wnt signaling pathway continues to be one of the promising approaches for future tumor treatment, both alone and in combination therapy that would impact the clinical outcomes and survival of patients.
Wnt signaling pathway
β-catenin
porcupine
Wnt inhibitors
1. Introduction
In the last thirty years, inhibitors of the Wnt signaling pathway have been identified and characterized along with the functional explanation of the pathway’s molecular targets [1][2].
Wnt signaling is a conserved cellular pathway in all multicellular organisms that has been studied for more than four decades. The name was coined from the names of two genes, mouse int-1 and Drosophila’s wingless (wg). The discovery of a novel cellular proto-oncogene int-1, which was later on mapped to the chromosomal position of Drosophila gene wg, launched the marvelous research on this essential pathway and its many important components.
It is generally accepted that Wnt signaling consists of canonical β-catenin and two non-canonical beta-catenin independent pathways—the planar cell polarity (PCP) and the Wnt/Ca2+ . Canonical or classical Wnt signaling is involved in processes of body axes formation during development and, in the morphogenesis of limbs, the central nervous system, and other organs. In adult organisms, its role mainly lies in stem cell regeneration, regulation of proliferation, and differentiation. Activation of the β-catenin Wnt pathway leads to the transcription of the Wnt target genes. The planar cell polarity regulates the shaping of the cytoskeleton and the polarization of cells along the apical–basal plane, whereas the Wnt/Ca2+ pathway regulates cytoplasmic concentration of calcium ions through their exit from the endoplasmic reticulum.
The aberrant canonical Wnt pathway is involved in the formation and evolution of various types of tumors [3]. In addition, the PCP and Wnt/Ca2+ pathways are thought to be important in the acquisition of metastatic properties, primarily because of their role in cytoskeletal reorganization. There are 19 different Wnt ligands in mammals and humans that can activate different Wnt signaling branches, depending on receptors, coreceptors, and other regulatory molecules at a given time. Thus, some Wnt ligands can activate both canonical and non-canonical pathways, whereas others act specifically [4][5].
2. WNT Pathway Inhibitors
The reason for searching for inhibitors of the Wnt pathway is the hope that the inhibition would have a therapeutic effect on tumors [3]. For example, it has been observed that the silencing of β-catenin by siRNA has an inhibitory effect on the growth of colorectal cancers in vitro and in vivo. When knocking down β-catenin in colon cancer cell lines carrying the APC mutation, significant growth inhibition, differentiation, and reduction of proliferation occurred. However, after the cessation of beta-catenin silencing, tumor growth rapidly recovered, suggesting that Wnt inhibitor therapy would require continuous administration. Another strategy for Wnt pathway downregulationis the inhibition of the β-catenin interaction with TCF, which has been shown to be antiproliferative and proapoptotic in adrenocortical tumor cell lines. Moreover, compared to the knockdown of β-catenin, the knockdown of TCF4 has been shown to be more efficient. The inhibition of the Wnt pathway by knocking down β-catenin has been successful in arresting tumor growth [6][7]. Similarly successful was the inhibition of the transcription factors and coactivators TCF, BCL9, and CBP. Molecules that meet these requirements have been found. Parallel studies have also focused on examining the effect of growth inhibition of other Wnt pathway components, for example, the FZD7 gene. A reduced ability of tumor formation in mice was found after the transplantation of triple-negative breast cancer cell lines with knocked down FZD7. A similar effect was shown in squamous cell carcinoma of the esophagus, where the lack of FZD7 inhibited cell growth, induced apoptosis, and suppressed migration. Inhibition of Wnt-1 and Wnt-2 ligands by siRNA or specific antibodies has also been reported to promote apoptosis in non-small cell lung cancer cells [8][9].
Based on all these findings, molecular targets to which Wnt inhibitors are directed have been recognized, and the inhibitors are usually divided according to their site of action [10].
In addition, inhibitors which enhance the activity of the so-called negative Wnt pathway regulators have also been developed [11]. Most of these molecules have been considered as inhibitors based on screening, i.e., testing thousands of molecules, both synthetic and natural, to identify the most potent ones. Various tests were used to evaluate the inhibition, most commonly the TOPFlash assay, which shows how much the examined molecules interfere with β-catenin-dependent transcription of dTF12 (top flash-like luciferase reporter). In other cases, protein levels of β-catenin or other proteins were measured using immunohistochemical or Western blot analyses or on mRNA level using the qRT-PCR method. Such approaches have identified a very large number of molecules that inhibit the Wnt pathway. In order to summarize all the inhibitors and to give the reader the immediate location of the target sites, an illustration is given in Figure 1.
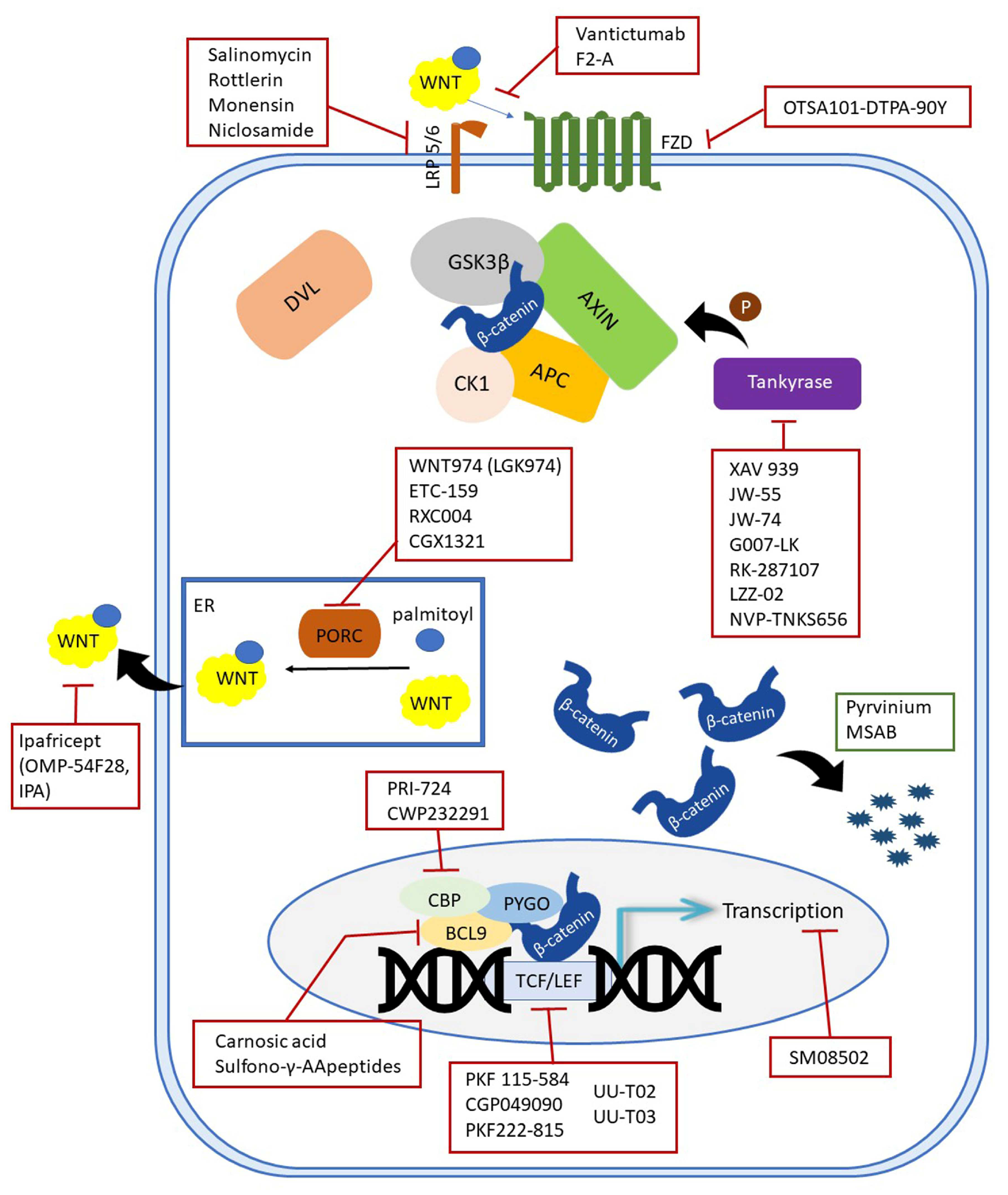
Figure 1. Schematic representation of inhibitors targeting the canonical Wnt components.
The beneficial effect of Wnt inhibitors on other modalities of antitumor therapy has also been established. The first interesting possibility was alleviating the resistance to checkpoint inhibitors by using Wnt inhibitors. It has been shown that the upregulation of Wnt signaling can cause resistance to immune checkpoint inhibitor therapy by modulating the tumor microenvironment through the interaction with tumor-associated macrophages, or by stimulating an acidic tumor environment that is immunosuppressive to cytotoxic T lymphocytes. Thus, Wnt signaling helps the so-called immune cell exclusion, preventing immune cells from reaching the tumor, and the tumor becomes resistant to checkpoint inhibitor therapy. Several preclinical studies have shown that inhibition of the canonical Wnt pathway in parallel with the use of checkpoint inhibitors can effectively overcome this resistance [12].
In line with these findings are the results of a phase 1 clinical trial using the porcupine inhibitor LGK974 in combination with spartalizumab, a monoclonal antibody to PD-1, that reported impressive results in patients with several types of solid tumors, including the stabilization of disease in 53% of urothelial carcinoma previously resistant to checkpoint inhibitors.
Besides influencing immunotherapy, the combination of Wnt inhibitors with taxanes also demonstrated superior clinical response. Taxanes, including paclitaxel, nab-paclitaxel, and docetaxel, block the M-phase of cell division by acting on microtubules. Wnt pathway components are involved in the cell cycle, too. β-catenin is necessary for the separation of centrosomes in the formation of the mitotic spindle, whereas APC and Dishevelled participate in the regulation of kinetochores binding and, together with FZD and LRP, affect the orientation of the spindle [5]. Thus, the inhibition of Wnt signaling leads to spindle defects. Nab-paclitaxel in mice xenografts of pancreatic cancer caused an increase in the number of cells in the G2-M phase and a three-fold increase in β-catenin levels in mitotic cells. The synergism of Wnt inhibitors with taxanes could be generally explained by a dual effect on the disruption of cell divisions and also the prevention of the Wnt pathway activation after taxane administration. The combination of ipafricept and vantictumab with taxanes was evaluated on mouse xenografts of breast, ovarian, and pancreatic tumors. Wnt inhibitors have been shown to potentiate the cytotoxic effect of taxanes by modulating Wnt pathway activity in mitotic cells but are less effective in combination with S-phase blockers or platinum-based drugs. The optimized protocol of vantictumab and ipafricept, 25 mg/kg every 2 or 3 weeks, proved to be more effective and less toxic to the bone. For the optimal effect of this combination, it is necessary to administer the Wnt inhibitor before taxane because reverse or concomitant use has been shown to be less effective. Clinical trials of this drug combination have been described in the section on ipafricept [12].
3. Side Effects of WNT Inhibitors
Because an optimally regulated Wnt pathway ensures cellular differentiation as well as the regeneration of many tissues, especially those with rapid cell turnover such as hematopoietic and gastrointestinal tissues, the inhibition of this pathway carries serious risks of side effects. Side effects can be expected when inhibiting such an essential cellular pathway. Nevertheless, early clinical trials have shown that the severity of side effects is not significant compared to the therapeutic benefits. They are usually associated with the malfunction of tissue regeneration and could drastically limit the use of Wnt pathway inhibitors in systemic antitumor therapy.
The most common side effects upon inhibition of Wnt signaling are gastrointestinal problems, hair loss, immunosuppression, fatigue, vitiligo, anemia, neutropenia, thrombocytopenia, bone fractures, and neurodegeneration. In addition to these adverse reactions, elevations in bilirubin and alkaline phosphatase have been observed, as well as hypophosphatemia. This could be explained by the change in bone remodeling. Because the Wnt pathway regulates bone remodeling in a complex way, one of the side effects reported in the early clinical stages is an increase in bone remodeling. However, it has been shown that this can be prevented with bisphosphonates, specifically zolendronic acid. It is known that decreased LRP5 expression in mouse osteoblasts leads to an osteopenic phenotype, whereas increased LRP5 expression results in increased bone mass The activation of the Wnt pathway promotes the differentiation of mesenchymal progenitor cells into osteoblasts, and β-catenin stimulates osteoprotegerin expression in differentiated osteoblasts, which by binding to the protein RANKL contribute to the inhibition of osteoclast differentiation. Thus, the activation of the Wnt pathway shifts the balance toward bone synthesis. Pathological fractures during the pharmacological inhibition of the Wnt pathway could be explained by these observations [10][11][12].
However, novel research indicated that a clinically approved anti-resorptive, alendronate, could mitigate the loss of bone mass and extend the beneficial antitumor effects of PORCN inhibitors. Needless to say, all Wnt pathway inhibitors are contraindicated in pregnancy.
In all phases of clinical trials, the question remains as to how the inhibition of the Wnt pathway would affect the cognitive abilities of patients. In the adult brain, the proper activation of the Wnt pathway [13][14] is crucial for adult neurogenesis and the survival of neurons in the supraventricular zone and hippocampus, but also for the maintenance of higher cognitive functions and dopaminergic pathways, and it appears to affect synapse formation.
All in all, the side effects caused by Wnt inhibitors are similar to those of chemotherapeutics that are already known and well established. However, it is important to distinguish whether the reported side effects are strictly due to Wnt inhibitors or whether they are the consequence of chemotherapeutics in combinational therapy [15]. Interactions with other molecules which can lead to the so-called off-target side effects have also been insufficiently investigated. To overcome the side effects of systemic Wnt inhibition, strategies have been proposed for delivering the inhibitors directly to tumor cells by using nanoparticles, liposomes, or binding the inhibitor to one of the molecules attracted by a particular tumor.
References
- Pećina-Šlaus, N.; Aničić, S.; Bukovac, A.; Kafka, A. Wnt Signaling Inhibitors and Their Promising Role in Tumor Treatment. Int. J. Mol. Sci. 2023, 24, 6733. https://doi.org/10.3390/ijms24076733
- Kahn, M. Can we safely target the WNT pathway? Nat. Rev. Drug Discov. 2014, 13, 513–532.
- Jung, Y.S.; Park, J.I. Wnt signaling in cancer: Therapeutic targeting of Wnt signaling beyond β-catenin and the destruction complex. Exp. Mol. Med. 2020, 52, 183–191.
- Tabatabai, R.; Linhares, Y.; Bolos, D.; Mita, M.; Mita, A. Targeting the Wnt Pathway in Cancer: A Review of Novel Therapeutics. Target. Oncol. 2017, 12, 623–641.
- Taciak, B.; Pruszynska, I.; Kiraga, L.; Bialasek, M.; Krol, M. Wnt signaling pathway in development and cancer. J. Physiol. Pharmacol. 2018, 69, 185–196.
- Kafka, A.; Bašić-Kinda, S.; Pećina-Šlaus, N. The cellular story of dishevelleds. Croat. Med. J. 2014, 55, 459–467.
- Duchartre, Y.; Kim, Y.M.; Kahn, M. The Wnt signaling pathway in cancer. Crit. Rev. Oncol. Hematol. 2016, 99, 141–149.
- Ghosh, N.; Hossain, U.; Mandal, A.; Sil, P.C. The Wnt signaling pathway: A potential therapeutic target against cancer. Ann. N. Y. Acad. Sci. 2019, 1443, 54–74.
- Voronkov, A.; Krauss, S. Wnt/beta-Catenin Signaling and Small Molecule Inhibitors. Curr. Pharm. Des. 2013, 19, 634–664.
- Krishnamurthy, N.; Kurzrock, R. Targeting the Wnt/beta-catenin pathway in cancer: Update on effectors and inhibitors. Cancer Treat. Rev. 2018, 62, 50–60.
- Le, P.N.; McDermott, J.D.; Jimeno, A. Targeting the Wnt pathway in human cancers: Therapeutic targeting with a focus on OMP-54F28. Pharmacol. Ther. 2015, 146, 1–11.
- Rim, E.Y.; Clevers, H.; Nusse, R. The Wnt Pathway: From Signaling Mechanisms to Synthetic Modulators. Annu. Rev. Biochem. 2022, 91, 571–598.
- Bukovac, A.; Kafka, A.; Raguž, M.; Brlek, P.; Dragičević, K.; Müller, D.; Pećina-Šlaus, N. Are We Benign? What Can Wnt Signaling Pathway and Epithelial to Mesenchymal Transition Tell Us about Intracranial Meningioma Progression. Cancers 2021, 13, 1633.
- Kafka, A.; Bukovac, A.; Brglez, E.; Jarmek, A.-M.; Poljak, K.; Brlek, P.; Žarković, K.; Njirić, N.; Pećina-Šlaus, N. Methylation Patterns of DKK1, DKK3 and GSK3β Are Accompanied with Different Expression Levels in Human Astrocytoma. Cancers 2021, 13, 2530.
- Fischer, M.M.; Cancilla, B.; Yeung, V.P.; Cattaruzza, F.; Chartier, C.; Murriel, C.L.; Cain, J.; Tam, R.; Cheng, C.-Y.; Evans, J.W.; et al. WNT antagonists exhibit unique combinatorial antitumor activity with taxanes by potentiating mitotic cell death. Sci. Adv. 2017, 3, e1700090.
More
Information
Subjects:
Medicine, Research & Experimental
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
924
Revisions:
2 times
(View History)
Update Date:
27 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




