Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Bhupendra G Prajapati | -- | 1508 | 2023-04-26 11:39:51 | | | |
| 2 | Camila Xu | Meta information modification | 1508 | 2023-04-27 03:17:22 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Alotaibi, G.; Alharthi, S.; Basu, B.; Ash, D.; Dutta, S.; Singh, S.; Prajapati, B.G.; Bhattacharya, S.; Chidrawar, V.R.; Chitme, H. Nano-Gels in Skin Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/43517 (accessed on 08 February 2026).
Alotaibi G, Alharthi S, Basu B, Ash D, Dutta S, Singh S, et al. Nano-Gels in Skin Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/43517. Accessed February 08, 2026.
Alotaibi, Ghallab, Sitah Alharthi, Biswajit Basu, Dipanjana Ash, Swarnali Dutta, Sudarshan Singh, Bhupendra G. Prajapati, Sankha Bhattacharya, Vijay R. Chidrawar, Havagiray Chitme. "Nano-Gels in Skin Cancer" Encyclopedia, https://encyclopedia.pub/entry/43517 (accessed February 08, 2026).
Alotaibi, G., Alharthi, S., Basu, B., Ash, D., Dutta, S., Singh, S., Prajapati, B.G., Bhattacharya, S., Chidrawar, V.R., & Chitme, H. (2023, April 26). Nano-Gels in Skin Cancer. In Encyclopedia. https://encyclopedia.pub/entry/43517
Alotaibi, Ghallab, et al. "Nano-Gels in Skin Cancer." Encyclopedia. Web. 26 April, 2023.
Copy Citation
Nano-gels, a dual combination of hydrogels and nanoparticles, have demonstrated significant promise as a targeted drug delivery system in the treatment of cancer, attributing to excellent drug loading capacity. Skin disorders, the 4th foremost source of non-fatal diseases, are frequently the outward manifestation of more serious systemic illnesses, such as HIV, and neglected tropical diseases, such as elephantiasis and other ailments causing lymphedema.
mitogen-activated protein kinase
nano-gel
polymeric cross linked
thermodynamic stability
1. Introduction
The concept “novel drug delivery system” (NDDS) refers to an innovative and rational approach based on physical (osmosis, diffusion, swelling, erosion, dissolution, and electron-chain transport) and bio-chemical (monoclonal antibodies, gene therapy, carrier loaded therapy, polymer-drug conjugates, liposome, and noisome) mechanisms that have been developed having optimized properties of drugs such as tunable particle size in micro and nano range, improved permeability parameters and specific site targeting via advancing our understanding of the pharmacokinetic and pharmacodynamic behavior of the drug. NDDS can be obtained via either of three ways the development of nano-carrier loaded, sustain released drug delivery systems, and application of micro-fluidics [1][2][3].
Nanoparticles have been revealed to be beneficial in gathering information at all phases of clinical trials due to their application in numerous innovative tests for the treatment and diagnosis of diseases. The advantageous characteristic of the nanoparticle is to allow multiple proteins to attach to their surface over conventional dosage forms [4].
In 2008, Alexander and Serguei first coined the phrase “nano-particle constructed gel” to describe cross-linked bi-functional scaffolds of a swellable non-ionic polymer and a polyion for delivery of polynucleotides. Extremely cross-linked, three-dimensional nano-ranged hydrogel systems that can be either co-polymerized or made of ionic or non-ionic monomers are known as nano-gels having a size range of 20–200 nm, offering several beneficial effects such as having prolonged serum half-life, absorbing large amounts of body fluids without altering the fundamental networked structure and avoiding renal clearance [5][6]. The desire for nano-gels as a delivery system is fueled by their well-known excellent properties, including higher thermodynamic stability, remarkable solubilization potential, comparatively lower viscosity, and resistance to aggressive sterilization procedures [7]. Nano-gels, a dual combination of hydrogels and nanoparticles, have demonstrated significant promise as a targeted drug delivery system in the treatment of cancer, attributing to excellent drug loading capacity. Other advantages of nano-gel over other nanoparticles are to achieve active targeting via making a response to internal or external stimuli such as pH, temperature, light, and reduction-oxidation reaction with improved drug deposition in a targeted area, minimizing adverse effects of a drug via preventing drug accumulation in non-targeted tissues [8]. Nano-gels can be differentiated into different categories such as physically-chemically cross-linked, hybrid nano-gel, polymeric, bio-mimetic nano-gel, pH, thermo, magnetic, hypoxia, ultrasound, enzyme, and reduction responsive nano-gel on the basis of synthesis procedure, nature of materials used and responsive towards stimuli respectively [8][9]. The swellable property of nano-gel makes them able to expand when in contact with physiological fluids providing flexibility to be near the targeted region drugs under particular physiological circumstances and increasing the dispersion of medicaments [10]. Nano-gels can be either synthetically modified to incorporate numerous ligands for targeted drug delivery or controlled drug release or primarily a transporter architecture for delivering pharmaceuticals and several bio-active compounds such as genes, proteins, etc. [11][12][13]. Depending on the type and properties of both drug and nano-gel being utilized as well as desired release pattern, there are many techniques to load drugs into them, such as physical entrapment (mixture of drug and nano-gel solution adsorb onto the surface or be encapsulated within the nano-gel matrix through physical entrapment), chemical conjugation (chemical reaction of drug molecules to nano-gel surface via covalent bonding), electrostatic interaction (electrostatic attraction between drug molecule and charged nano-gel surface) and stimuli (pH, temperature, and pressure) sensitive loading. However, the restriction in the entrapment of hydrophobic medications such as anticancer drugs in hydrophilic nano-gels can be overcome via modifying polymer structure, resulting in improved solubility and stability of poorly soluble drugs significantly [14]. Therefore, nano-gels are thought to be potential drug delivery carriers for the delivery and cellular uptake of proteins, peptides, and other biological compounds, attributing to relatively high affinity to aqueous solutions, remarkable thermal stability, bio-compatibility, fully or partially bio-degradability and suitability for molecular inclusion in bulk [15][16][17].
Skin disorders, the 4th foremost source of non-fatal diseases, are frequently the outward manifestation of more serious systemic illnesses, such as HIV, and neglected tropical diseases, such as elephantiasis and other ailments causing lymphedema [18]. Over a million cases of skin inflammation and cancer are discovered each year, making it by far the most prevalent form of cancer in people, especially in white people [19][20][21]. Skin malignancies are given names based on the cell from which they develop and their clinical characteristics. The three most frequent forms are cutaneous malignant melanoma, basal cell carcinomas, and squamous cell carcinomas. Basal cell carcinomas and squamous cell carcinomas are both also known as non-melanocytic skin cancers [19][22]. Similar to many other malignancies, Melanoma, the most combative skin cancer, greatly rises with age, likely due to the lengthy latency between carcinogen exposure and the development of cancer. In this case, UV exposure was a contributing factor [19][23]. In order to implement a coordinated and long-lasting global response to lowering the global burden, it is essential to comprehend the impact of dermatological disorders in wealthy countries around the world. Chemotherapeutic drugs cannot be used topically due to the physiological structure of the skin, poor affinity, and potency, whereas the drug-loaded targeted nano-gel formulations were found to be released more in slightly acidic melanoma micro-environment compared with other topical conventional formulations [24]. Therefore, nano-gels establish innovative architecture having improved pharmacological potential and maintained intracellular safety limits for the mitigation and treatment of targeted melanoma.
2. Nano-Gels in Skin Cancer
In order to overcome the limitations of traditional anticancer therapies and enhance the outcomes of cancer treatment, nano-gels have been demonstrated to be one of the best solutions. The fundamental drawback of traditional chemotherapy is that it targets both diseased and non-cancerous cells through a non-selective process. Increased toxicity and negative impacts result from this. In order to deliver chemotherapeutic medications to cancer cells with low side effects and reduced toxicity, stimuli-sensitive nano-gels (Figure 1) have been effectively developed and assessed [25]. Hormone therapies are effective for tumors linked to hormones [26] but several hormonal therapies have been linked to elevated risk factors for diabetes mellitus and thrombosis [27]. Nano-gels designed for targeted medication delivery in these cancer types are not linked to such risk factors. The most prevalent type of cancer in people is skin cancer.
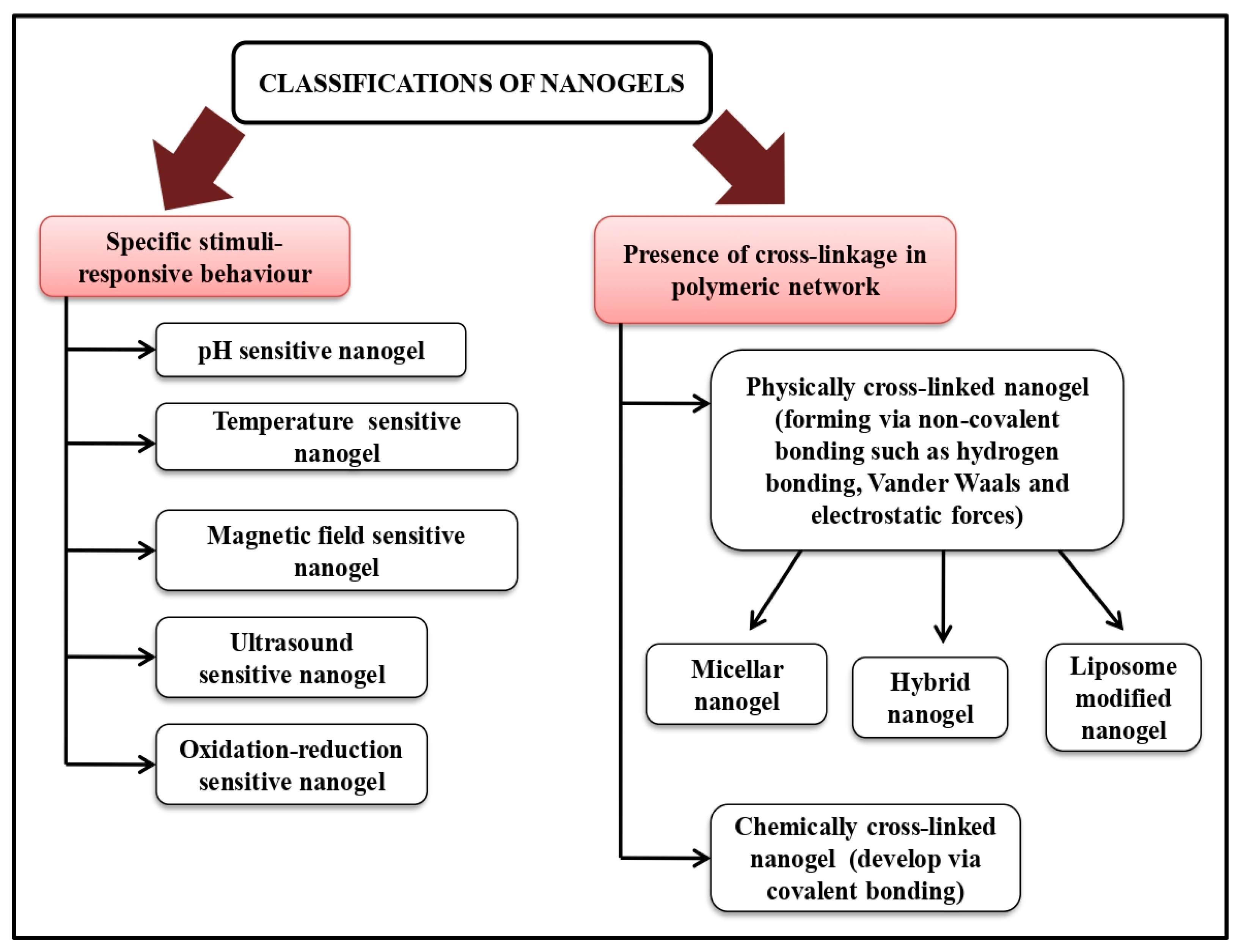
Figure 1. Mechanisms of polymeric nano-gels in the treatment of skin cancer. BCC: Basal cell carcinoma, SCC: Squamous cell carcinoma, SC: Superficial carcinoma, MCC: Merkel cell carcinoma.
“Skin cancer” refers to a variety of medical conditions that develop from distinct epidermal and dermal cells. Skin cancer has been successfully treated with loaded nano-gels of chitin-polymerized curcumin [28]. The therapeutic effectiveness of the B16-F10 melanoma tumor was improved by a thermo-sensitive nano-hydrogel co-loaded with DOX, IL-2, and IFN-γ, which boosted tumor cell death and enhanced replication of CD3+/CD4+ and CD3+/CD8+ T cells [25]. Drugs having high molecular weight have been formulated as nano-gels to enhance their solubility. For instance, tacrolimus, an immune-suppressive drug, was loaded in polyglycerol polymerized thermo-sensitive topical nano-gel and found to be improved adsorption of the drug through the layers of skin, attributing to the elevated body temperature for inflammation resulting in remarkable anti-proliferative activity [29]. pH-responsive biodegradable 5-fluorouracil-loaded chitosan nano-gel was developed to treat melanoma in the mild acidic cancer micro-environment, effectively preserving the integrity of the skin layer in comparison with other traditional melanoma formulations [30]. Transdermal curcumin nano-gels were developed to improve the solubility, transdermal permeability, and significant release of curcumin with lower toxicity attributed to their physicochemical characteristics when compared to traditional curcumin formulations [31]. In order to create hydrophilic and thermo-responsive three-dimensional crosslinked nano-gels that can improve the penetration of both smaller and larger molecules through the squamous cells and aggregate within the hair follicles, dendrimers made of dendritic polyglycerol (dPG) were utilized [15][32][33][34][35][36]. After interacting with the squamous cell, such nano-gels can be designed to go through a physical transition for improved dermal penetration and payload release in response to the ionic strength, temperature, or pH gradient of the skin [29][37]. For instance, chitosan or PLGA-chitosan nano-gels that are pH-responsive and biodegradable have demonstrated the capacity to release 5-FU in reaction to the tumor’s acidic environment to cure melanoma [30][38]. Nano-gels made of chitin have also demonstrated the capacity to carry medications deep within the skin, treating inflammatory conditions like psoriasis [39]. Another study reported that pH-sensitive biodegradable, bio-compatible, cytocompatible, self-assembled, and chemically cross-linked chitosan-Pluronic 127 loaded nano-gel exhibited pH-triggered bleomycin release in a sustained manner to the cutaneous area having significant entrapment efficiency (55%), providing a unique strategy against skin melanoma [38]. A chemo-preventive study revealed that the average integer of UVB-inducing tumor, tumor volume was less in a remarkable manner in the quercetin-titanium dioxide nano-gel treated animals with improved quercetin deposition on the skin via down-regulating COX-2, EP3, EP4, PCNA, and cyclin D1 expressions [40]. Therefore, for applications in targeted medication delivery, diagnostics, bio-sensing, and the separation of biological constituents, nano-gels have gained significant research interest.
References
- Bhagwat, R.; Vaidhya, I. Novel drug delivery systems: An overview. Int. J. Pharm. Sci. Res. 2013, 4, 970.
- Hirai, T.; Ogiwara, T.; Fujii, K.; Ueki, T.; Kinoshita, K.; Takasaki, M. Electrically Active Artificial Pupil Showing Amoeba-Like Pseudopodial Deformation. Adv. Mater. 2009, 21, 2886–2888.
- Kalaydina, R.-V.; Bajwa, K.; Qorri, B.; DeCarlo, A.; Szewczuk, M.R. Recent advances in “smart” delivery systems for extended drug release in cancer therapy. Int. J. Nanomed. 2018, 13, 4727–4745.
- Anooj, E.; Charumathy, M.; Sharma, V.; Vibala, B.; Gopukumar, S.; Jainab, S.B.; Vallinayagam, S. Nanogels: An overview of properties, biomedical applications, future research trends and developments. J. Mol. Struct. 2021, 1239, 130446.
- Vashist, A.; Atluri, V.; Raymond, A.; Kaushik, A.; Parira, T.; Huang, Z.; Durygin, A.; Tomitaka, A.; Nikkhah-Moshaie, R.; Vashist, A.; et al. Development of Multifunctional Biopolymeric Auto-Fluorescent Micro- and Nanogels as a Platform for Biomedical Applications. Front. Bioeng. Biotechnol. 2020, 8, 315.
- Ansari, S.; Karimi, M. Novel developments and trends of analytical methods for drug analysis in biological and environmental samples by molecularly imprinted polymers. TrAC Trends Anal. Chem. 2017, 89, 146–162.
- Vashist, A.; Kaushik, A.; Vashist, A.; Bala, J.; Nikkhah-Moshaie, R.; Sagar, V.; Nair, M. Nanogels as potential drug nanocarriers for CNS drug delivery. Drug Discov. Today 2018, 23, 1436–1443.
- Attama, A.A.; Nnamani, P.O.; Onokala, O.B.; Ugwu, A.A.; Onugwu, A.L. Nanogels as target drug delivery systems in cancer therapy: A review of the last decade. Front. Pharmacol. 2022, 13, 874510.
- Mauri, E.; Giannitelli, S.M.; Trombetta, M.; Rainer, A. Synthesis of Nanogels: Current Trends and Future Outlook. Gels 2021, 7, 36.
- Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. Design and engineering of nanogels for cancer treatment. Drug Discov. Today 2011, 16, 457–463.
- Sivaram, A.J.; Rajitha, P.; Maya, S.; Jayakumar, R.; Sabitha, M. Nanogels for delivery, imaging and therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 509–533.
- Ma, Y.; Ge, Y.; Li, L. Advancement of multifunctional hybrid nanogel systems: Construction and application in drug co-delivery and imaging technique. Mater. Sci. Eng. C 2017, 71, 1281–1292.
- Soni, K.S.; Desale, S.S.; Bronich, T.K. Nanogels: An overview of properties, biomedical applications and obstacles to clinical translation. J. Control. Release 2016, 240, 109–126.
- Khoee, S.; Asadi, H. Nanogels: Chemical approaches to preparation. Encycl. Biomed. Polym. Polym. Biomater. 2016, 27, 5266–5293.
- Asadian-Birjand, M.; Sousa-Herves, A.; Steinhilber, D.; Cuggino, J.C.; Calderon, M. Functional nanogels for biomedical applications. Curr. Med. Chem. 2012, 19, 5029–5043.
- Cui, W.; Liu, R.; Jin, H.; Lv, P.; Sun, Y.; Men, X.; Yang, S.; Qu, X.; Yang, Z.; Huang, Y. pH gradient difference around ischemic brain tissue can serve as a trigger for delivering polyethylene glycol-conjugated urokinase nanogels. J. Control. Release 2016, 225, 53–63.
- Li, Y.; Maciel, D.; Rodrigues, J.; Shi, X.; Tomás, H. Biodegradable Polymer Nanogels for Drug/Nucleic Acid Delivery. Chem. Rev. 2015, 115, 8564–8608.
- Engelman, D.; Fuller, L.C.; Solomon, A.W.; McCarthy, J.S.; Hay, R.J.; Lammie, P.J.; Steer, A.C. Opportunities for Integrated Control of Neglected Tropical Diseases That Affect the Skin. Trends Parasitol. 2016, 32, 843–854.
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248.
- Chittasupho, C.; Ditsri, S.; Singh, S.; Kanlayavattanakul, M.; Duangnin, N.; Ruksiriwanich, W.; Athikomkulchai, S. Ultraviolet Radiation Protective and Anti-Inflammatory Effects of Kaempferia galanga L. Rhizome Oil and Microemulsion: Formulation, Characterization, and Hydrogel Preparation. Gels 2022, 8, 639.
- Geller, A.C.; Annas, G.D. Epidemiology of melanoma and nonmelanoma skin cancer. Semin. Oncol. Nurs. 2003, 19, 2–11.
- Narayanan, D.L.; Saladi, R.N.; Fox, J.L. Review: Ultraviolet radiation and skin cancer. Int. J. Dermatol. 2010, 49, 978–986.
- Simões, M.C.F.; Sousa, J.J.S.; Pais, A. Skin cancer and new treatment perspectives: A review. Cancer Lett. 2015, 357, 8–42.
- Sahu, P.; Kashaw, S.K.; Sau, S.; Kushwah, V.; Jain, S.; Agrawal, R.K.; Iyer, A.K. pH Responsive 5-Fluorouracil Loaded Biocompatible Nanogels For Topical Chemotherapy of Aggressive Melanoma. Colloids Surf. B Biointerfaces 2018, 174, 232–245.
- Lv, Q.; He, C.; Quan, F.; Yu, S.; Chen, X. DOX/IL-2/IFN-γ co-loaded thermo-sensitive polypeptide hydrogel for efficient melanoma treatment. Bioact. Mater. 2018, 3, 118–128.
- Heit, J.A.; Silverstein, M.D.; Mohr, D.N.; Petterson, T.M.; O’Fallon, W.M.; Melton, L.J., 3rd. Risk factors for deep vein thrombosis and pulmonary embolism: A population-based case-control study. Arch. Intern. Med. 2000, 160, 809–815.
- Ye, F.; Wen, J.; Yang, A.; Wang, Y.; Li, N.; Yu, P.; Wei, W.; Tang, J. The Influence of Hormone Therapy on secondary diabetes mellitus in Breast Cancer: A Meta-analysis. Clin. Breast Cancer 2022, 22, e48–e58.
- Reeves, A.; Vinogradov, S.V.; Morrissey, P.; Chernin, M.; Ahmed, M.M. Curcumin-encapsulating Nanogels as an Effective Anticancer Formulation for Intracellular Uptake. Mol. Cell. Pharm. 2015, 7, 25–40.
- Rancan, F.; Asadian-Birjand, M.; Dogan, S.; Graf, C.; Cuellar, L.; Lommatzsch, S.; Blume-Peytavi, U.; Calderón, M.; Vogt, A. Effects of thermoresponsivity and softness on skin penetration and cellular uptake of polyglycerol-based nanogels. J. Control. Release 2016, 228, 159–169.
- Sahu, P.; Kashaw, S.K.; Sau, S.; Kushwah, V.; Jain, S.; Agrawal, R.K.; Iyer, A.K. pH triggered and charge attracted nanogel for simultaneous evaluation of penetration and toxicity against skin cancer: In-vitro and ex-vivo study. Int. J. Biol. Macromol. 2019, 128, 740–751.
- Ganesh, G.N.K.; Singh, M.K.; Datri, S.; Karri, V.V.S.R. Design and development of curcumin nanogel for squamous cell carcinoma. J. Pharm. Sci. Res. 2019, 11, 1683.
- Kabanov, A.V.; Vinogradov, S.V. Nanogels as Pharmaceutical Carriers: Finite Networks of Infinite Capabilities. Angew. Chem. Int. Ed. 2009, 48, 5418–5429.
- Abu Samah, N.H.; Williams, N.; Heard, C.M. Nanogel particulates located within diffusion cell receptor phases following topical application demonstrates uptake into and migration across skin. Int. J. Pharm. 2010, 401, 72–78.
- Asadian-Birjand, M.; Bergueiro, J.; Rancan, F.; Cuggino, J.C.; Mutihac, R.-C.; Achazi, K.; Dernedde, J.; Blume-Peytayi, U.; Vogt, A.; Calderón, M. Engineering thermoresponsive polyether-based nanogels for temperature dependent skin penetration. Polym. Chem. 2015, 6, 5827–5831.
- Witting, M.; Molina, M.; Obst, K.; Plank, R.; Eckl, K.M.; Hennies, H.C.; Calderon, M.; Frieß, W.; Hedtrich, S. Thermosensitive dendritic polyglycerol-based nanogels for cutaneous delivery of biomacromolecules. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1179–1187.
- Sahle, F.F.; Giulbudagian, M.; Bergueiro, J.; Lademann, J.; Calderón, M. Dendritic polyglycerol and N-isopropylacrylamide based thermoresponsive nanogels as smart carriers for controlled delivery of drugs through the hair follicle. Nanoscale 2016, 9, 172–182.
- Giulbudagian, M.; Yealland, G.; Hönzke, S.; Edlich, A.; Geisendörfer, B.; Kleuser, B.; Hedtrich, S.; Calderon, M. Breaking the Barrier-Potent Anti-Inflammatory Activity following Efficient Topical Delivery of Etanercept using Thermoresponsive Nanogels. Theranostics 2018, 8, 450–463.
- Sahu, P.; Kashaw, S.K.; Kushwah, V.; Sau, S.; Jain, S.; Iyer, A.K. pH responsive biodegradable nanogels for sustained release of bleomycin. Bioorganic Med. Chem. 2017, 25, 4595–4613.
- Divya, G.; Panonnummal, R.; Gupta, S.; Jayakumar, R.; Sabitha, M. Acitretin and aloe-emodin loaded chitin nanogel for the treatment of psoriasis. Eur. J. Pharm. Biopharm. 2016, 107, 97–109.
- Bagde, A.; Patel, K.; Mondal, A.; Kutlehria, S.; Chowdhury, N.; Gebeyehu, A.; Patel, N.; Kumar, N.; Singh, M. Combination of UVB Absorbing Titanium Dioxide and Quercetin Nanogel for Skin Cancer Chemoprevention. AAPS PharmSciTech 2019, 20, 240.
More
Information
Subjects:
Pharmacology & Pharmacy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Entry Collection:
Biopharmaceuticals Technology
Revisions:
2 times
(View History)
Update Date:
27 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




