Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rebecca Wingert | -- | 3252 | 2023-04-25 23:23:43 | | | |
| 2 | Rita Xu | Meta information modification | 3252 | 2023-04-26 04:42:13 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Hawkins, M.R.; Wingert, R.A. Retinoic Acid in Development and Disease. Encyclopedia. Available online: https://encyclopedia.pub/entry/43487 (accessed on 07 February 2026).
Hawkins MR, Wingert RA. Retinoic Acid in Development and Disease. Encyclopedia. Available at: https://encyclopedia.pub/entry/43487. Accessed February 07, 2026.
Hawkins, Matthew R., Rebecca A. Wingert. "Retinoic Acid in Development and Disease" Encyclopedia, https://encyclopedia.pub/entry/43487 (accessed February 07, 2026).
Hawkins, M.R., & Wingert, R.A. (2023, April 25). Retinoic Acid in Development and Disease. In Encyclopedia. https://encyclopedia.pub/entry/43487
Hawkins, Matthew R. and Rebecca A. Wingert. "Retinoic Acid in Development and Disease." Encyclopedia. Web. 25 April, 2023.
Copy Citation
Retinoic acid (RA) is a metabolite of vitamin A (retinol) that plays various roles in development to influence differentiation, patterning, and organogenesis. RA also serves as a crucial homeostatic regulator in adult tissues. The role of RA and its associated pathways are well conserved from zebrafish to humans in both development and disease.
retinoic acid
vitamin A
development
zebrafish
1. Introduction
For nearly 100 years, vitamin A in the maternal diet has been linked to normal embryonic ontogeny in vertebrates. Initially, this was based on observations in female pigs and rats that subsistence on nourishments lacking vitamin A during pregnancy was associated with a plethora of birth defects in newborns ranging from eye abnormalities to genitourinary defects [1][2][3][4][5][6][7][8]. Continued nutrition research uncovered a complex spectrum of congenital malformations that occurred consequent to a maternal vitamin A deficient (VAD) diet, which came to be known as the VAD syndrome [9]. The malformations included defects in the central nervous system, eyes, ears, heart, lungs, limbs, skin, and urogenital system [9]. These and subsequent studies helped to stimulate continued research on how retinoids, the biologically active metabolites of vitamin A, modulate development. Many fundamental insights were uncovered by investigating the teratogenic effects of RA—how global or local exposure impacted normal processes [10][11][12][13][14][15][16]. RA signaling is now appreciated as being essential for the genesis of nearly every vertebrate tissue and organ [10][11][12][13][14][15][16]. The wide range of RA functions include patterning of the body axis, regional patterning of the central nervous system, neurogenesis, limb development, and pleiotropic roles in organogenesis [10][11][12][13][14][15][16].
2. RA in Development
2.1. Neural Plate Progenitors
Early hindbrain development is reliant on proper RA signaling. During this process, RA synthesis is thought to be acted on by pbx2 and pbx4 to regulate early hindbrain fate decisions [17]. In aldh1a2-deficient zebrafish neckless (nls) mutants [18], the down regulation of RARɑ leads to imprecise regulation of hoxb4. The neuroectoderm of 14-somite stage nls mutants lacks hoxb4 expression, despite ubiquitous expression in mesodermal tissues [18]. However, by the 16-somite stage (roughly one hour later), hoxb4 expression is restored to neural tube progenitors within nls mutants, thus implying a potential compensatory mechanism of RA-mediated hoxb4 expression. Both RA and FGF gradients work synergistically to develop positional identities within the rhombomeres, a group of vital cell populations necessary for early neural crest migration [19][20][21][22].
Through the exogenous treatment of embryos with the aldehyde dehydrogenase inhibitor molecule N,N-diethylaminobenzaldehyde (DEAB) (Figure 2) starting at the 4 hpf stage, rhombomere 5 and 6 identities were found to be lost at 16 hpf, implying the importance of RA in signaling fates in early hindbrain populations [22]. Other work performed in zebrafish showed that loss of RAR signaling via treatment with a pan-RAR antagonist led to not only a lack of posterior hindbrain identity, but also an increase in the domain of anterior rhombomeres two, three, and four [23]. This implies that the two tailed gradient of RA is opposed by Cyp26 enzymatic activity at both the far anterior and posterior ends of the developing fish. In work performed by Qiu et al., RA, FGF, and other morphogenic gradients were wonderfully computationally modeled to highlight the precision necessary for essential fates within the developing hindbrain and the associated rhombomeres within zebrafish [24].
In later points of development within the forebrain, loss of both aldh1a2 and aldh1a3 results in altered yet intact expression of fgf8 and shh, implying RA is not solely responsible for early forebrain organization in mice [25]. In zebrafish, depletion of hmx4, a homeobox gene and ortholog of the human HMX1, results in a significant decrease in aldh1a2 expression which leads to lack of neural tube closure [26]. In these same studies conducted by Gongal et al., the addition of exogenous RA to hmx4 morphants rescues gli3 expression and allows for regular forebrain development, suggesting that hmx4 works via RA to mediate Shh activation [26]. This work opens exciting new avenues for investigation into RA and its role in forebrain development.
Eye development in zebrafish as well as other vertebrates originates within the eye field, a primordial section of the neural plate. Transcriptionally, a grouping of factors known as eye field transcription factors (EFTF) are credited with this specification. These factors include homeodomain genes such as rx3, otx2, and lhx2, and pax6 [27]. In RNA-sequencing analysis of rx3-/- fish at 13 hpf, select RA-related orphan receptors were found to be transcriptionally downregulated [28]. Loss of this EFTF and the resulting down regulation of RA-associated machinery may imply transcriptional priming within these early eye field cells for RA that is used in later regulation of optic development.
2.2. Kidney
The nephron is the functional unit in the kidney, and it is tasked with several critical physiological roles: the filtering of the blood through the glomerulus, the facilitation of solutes in and out of circulation via the tubules, and the passage of waste for dismissal in the collecting duct [29]. In higher vertebrates, the pronephros is the first transient form of the kidney, followed by the mesonephros, and the final formation of the kidney is named the metanephros [29]. In zebrafish, only the first two, the pronephros and mesonephros, are formed [30]. Pronephros development in zebrafish begins during the process of intermediate mesoderm formation through the action of signals derived from Bone Morphogenic Proteins (BMPs) [31]. As the intermediate mesoderm forms, RA signals originating from the paraxial mesoderm orchestrate the genesis of the bilateral nephrons [32][33]. These movements can be visualized through the use of transgenic lines expressing renal progenitor field markers such as lh1xa, pax2a, and pax8 [34].
In the presence of exogenous (10−6 M) ATRA exposure from 60% epiboly/6 hpf stage to the six-somite stage, two of these same markers, pax2a and pax8, are found to have the non-discrete spatial expression pattern seen in regular development [33]. In this same line of experiments, use of the RA synthesis inhibitor DEAB at the same six-somite stage results in rostralization of evi1 (mecom) [33] a downstream mediator of Fgf signaling [35] and a marker for more distal tubule fates at the 28-somite/24 hpf stage [32][36]. Further analysis of the inhibition of RA synthesis through the comparison of aldh1a2 knockdown via morpholino oligonucleotide and chemical inhibition via DEAB shows that the time of RA synthesis is crucial to the tubule phenotype within the nephron; and it further confirms the redundancy within ALDH enzymatic expression patterns [33]. In aldh1a2 knockdown fish, all of the tubules form by 48 hpf; however, distal tubule fates expand rostrally at the cost of anterior segments [33]. In the chemical inhibition of RA synthesis via DEAB, time of addition experiments concluded that RA is most needed in tubule fate decisions between 60% epiboly and 15-somite stages, or approximately 6–16 hpf [33]. Lack of sufficient RA synthesis during this period of gastrulation and somitogenesis leads to the complete loss of proximal cell fates (Figure 1) [33]. This work concluded that RA works to posteriorize proximal cell fates in juxtaposition to Fgf signaling. Though loss of RA is associated with inefficient pronephros formation, overproduction or addition of exogenous RA can be equally detrimental to the organization of the tubules. At the same ATRA concentration (10−6 M) used to determine that intermediate mesoderm patterning is sensitive to opposing RA and FGF-inducing gradients, exposure to such high levels of ATRA from 90% epiboly to the 5-somite stage (5–10 hpf) leads to complete loss of distal segment fates and highly increased proximal convoluted tubule (PCT) and proximal straight tubule (PST) populations at the 24 hpf/28-somite stage (Figure 1) [33]. This work, and others involving the nephron and the associated tubule dependence on RA for regulation of segmentation, is well characterized, and the investigation of the downstream gene regulatory network has revealed a number of transcription factors crucial for fashioning segment fates, such as sim1a, tbx2a/b, and emx1, among others [32][37][38][39][40][41][42][43]. However, openings emerge for further interrogation due to the ever-evolving field of RA biology, the ease of performing chemical/drug/biologic screens in the zebrafish model, and the prevalence of next generation sequencing approaches in development.
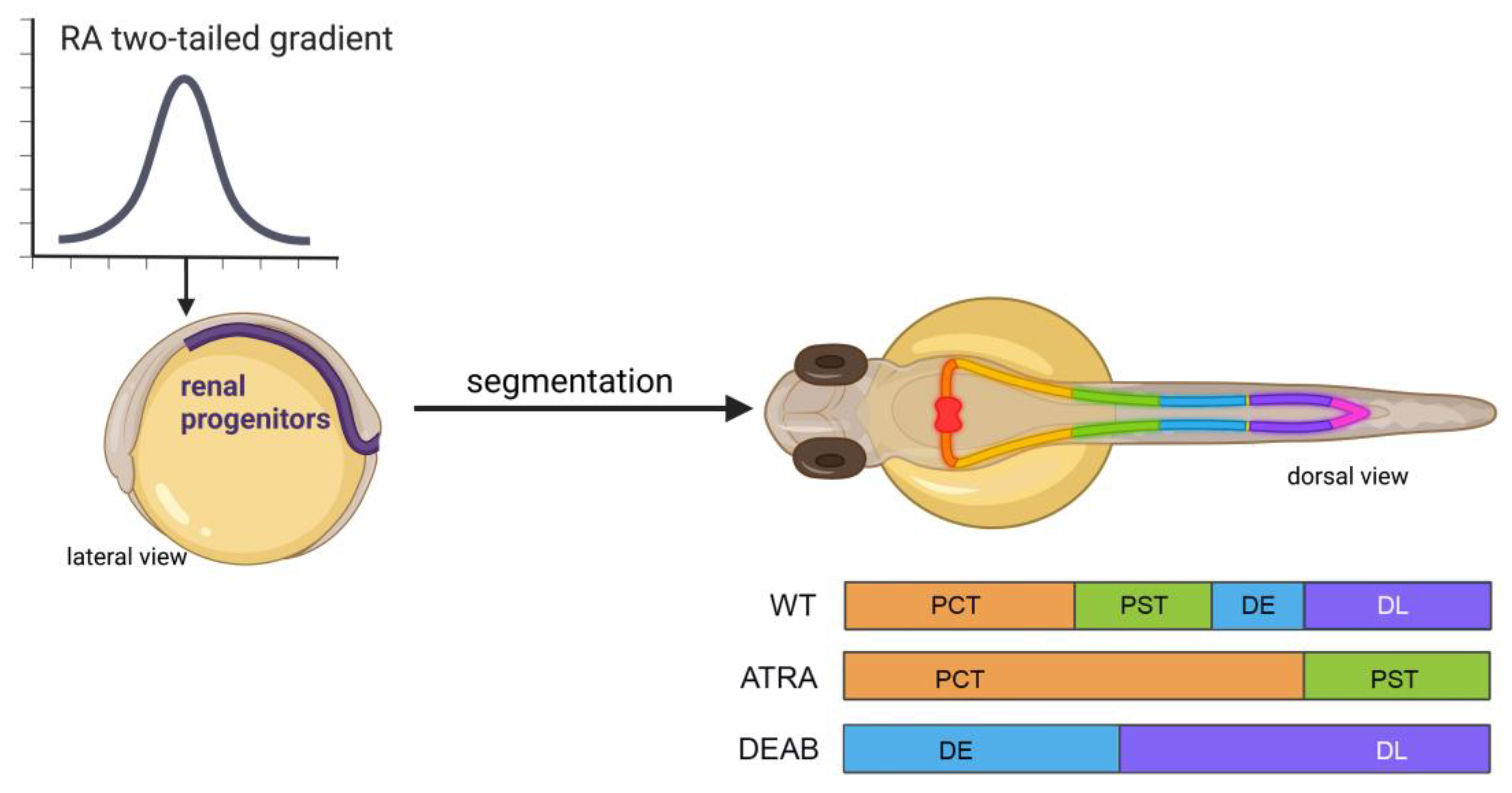
Figure 1. RA levels influence proximo-distal nephron fates during embryonic kidney development in the zebrafish. (Left) Renal progenitors are exposed to a gradient of RA during gastrulation and axis formation, with the highest level at the rostral-most locations (arrow). (Right, top) As development proceeds, these RA levels exert an influence on the proximo-distal segmentation patterning. The zebrafish embryonic kidney comprises two bilateral nephrons with several unique cell populations, and these undergo morphogenesis events by the 48 stage to connect rostrally at a single midline blood filter and caudally at the cloaca where waste exits the body. (Right, bottom) The effects of RA are best understood for the 4 main tubule populations: the proximal convoluted tubule (PCT), proximal straight tubule (PST), distal early (DE), and distal late (DL). Embryos exposed to a high dosage of ATRA starting at 60% epiboly form nephrons with only proximal segments. Conversely, embryos exposed to the RA biosynthesis inhibitor DEAB starting at 60% epiboly form nephrons with only distal segments.
Within the nephron, a subpopulation of aptly named multiciliated cells (MCCs) project clusters of motile cilia into the tubule lumen and govern fluid flow [44]. MCCs are detectable via WISH as early as the 10-somite stage [44]. Much like the nephron tubule populations, MCCs are dependent on RA signaling for differentiation from the renal progenitors [36]. In the proposed MCC regulatory network, RA works upstream to downregulate mecom, a positive regulator of Notch, and a factor for distal cell fates [36]. In knockdown of mecom and the subsequent addition of DEAB from late gastrulation to 24 hpf, MCC formation was almost completely rescued in terms of the domain they occupied within the nephrons and the position of this domain in the developing fish [36]. These experiments involving RA signaling and MCC fate determinants highlight exciting avenues of future interrogation into the potential regenerative capacities of MCCs after injury, which may lend potential insights into human disease.
2.3. Heart
From its genesis, the zebrafish heart is reliant on RA for correct spatial patterning of progenitors. Work performed by Keegan et al. delicately described both RA addition and the time in which RA is necessary for restricting progenitor fates [45]. When they treated embryos with a pan-RAR antagonist and then assessed cmlc2 and nkx2.5 expression, which marking cardiomyocytes precardiac mesoderm, respectively, they observed increased domains of expression at the 16-somite stage, suggesting RA works in restricting cardiac cell fates in post gastrulation fish [45]. This hypothesis was then confirmed through the use of exogenous ATRA treatments spanning a time point straddling gastrulation initiation leads to lessened cmlc2 expression at the 18-somite stage [45]. The mechanism of RA restricting the cardiac field was further characterized as being mediated by cdx4 and cdx1a expression. In this process, much like in hindbrain development, RA works through downregulating cdx4 expression, thereby activating cyp26a1 expression to metabolize RA within the cell [46][47]. This hypothesis further postulates the intricate nature of RA self-regulation, as RAREs exist as regulatory units of cyp26/Cyp26/CYP26 family expression [48][49]. Other work involving regulators of cardiac cell fate such as NR2F have been found to have RARES within these factors’ promoter regions, which in the presence of DEAB leads to a decreased nr2f1a domain. The loss of nr2f1a/b contributes to a relative expansion of the nkx2.5 domain, a commonality seen with RAR antagonism.
Further investigation into RA and its role in early heart field regulation revealed that RA works indirectly through the homeobox gene hoxb5b to limit cardiac progenitor populations [50]. Other work established that RA and FGF signaling worked in opposing manners to regulate heart field establishment, as the heat shock resulting in overexpression of FGF machinery in late gastrulation led to increased cardiac cell count, a phenotype also seen in DEAB-treated embryos [51][52]. Similarly, Aldh2 null mouse embryos bear a posteriorization and general domain increase of Fgf8 and its target Isl1 within the developing cardiac field, thus leading to improper chamber development [53][54].
RA signaling-associated machinery has also been a topic that has provided insights into RA and its role in heart development. One avenue of investigation included that of Cyp26 metabolic action for its role in maintaining RA equilibria. Opposite of a pan-RAR antagonist, Cyp26-deficient embryos result in a decreased heart field domain marked by nkx2.5, in course leading to decreased ventricular fates later in development [55]. Interestingly, the use of DEAB on these same cyp26 morphants results in a restoration of expression, concluding that loss of RA in early cardiac development results in increased heart field size, while increases in RA restrict these early progenitor populations and can alter later fate decisions [55]. These insights into RA’s role in cardiac development not only provide an understanding of base developmental processes, but also present a translational model for in utero VAD and accompanying congenital defects of the heart.
In work performed at later stages in development, Cyp26-deficient embryos were found to have increased mmp9 expression, leading to improper outflow tract morphogenesis due to the diminished addition from second heart field progenitors [56]. This work proposed a potential mechanism for RA-mediated outflow tract defects, and adds to the body of knowledge involving the relationship between vitamin A and mmp9. Further avenues of interrogation into processes associated with retinoid transport and synthesis within cardiac/cardiac progenitor cells remain open for insights, and make for exciting new lines of investigation.
One of the many reasons the zebrafish is such an attractive model is due to its regenerative capacity within the heart and other tissues [57]. In the ventricular amputation model [58], one hour after ventricular amputation of the adult zebrafish heart, aldh1a2 expression was remarkably abundant within the atrial endocardium, and within 3 days post amputation (dpa), expression of the RA-synthesizing enzyme was also localized to the epicardium, then to the epicardial cells that surround the wound clot by 7 dpa [59][60].
In this same line of experiments performed by Kikuchi et al., overexpression of cyp26a1, the enzyme responsible for RA degradation, led to dramatic decreases in cardiomyocyte proliferation at seven days post injury [60]. However, in the case of retinoid agonist supplementation, no increase in cardiomyocyte proliferation was induced, thus inferring that RA does not promote the regenerative process, but rather plays a permissive role [60]. Within the mouse model of acute damage to the heart, conflicting reports surround RA, and the protective effects it may or may not have in the case of myocardial infarction-induced cellular death [61][62].
2.4. Hematopoiesis
Research with the zebrafish has provided many fundamental insights into the genetic mechanisms of primitive and definitive hematopoiesis [63], from events involving hematopoietic stem cell (HSC) patterning [64] to fate choice [65][66] and differentiation [67][68][69][70]. A number of studies have contributed new insights into the effects of RA signaling on both the primitive and definitive hematopoietic waves, which are distinct in the cells they produce; namely, red blood cells and macrophages during the initial wave, followed by the production of all lineages in the subsequent wave, respectively [63].
Exogenous ATRA treatment of embryos from the late gastrula to the 5-somite stage elicits a dose-dependent inhibition of primitive erythropoiesis, based on reduced expression of the erythroid-specific transcription factor gata1 [71], and inhibition of primitive myelopoiesis, based on reduced expression of several markers [72]. Conversely, DEAB treatment increased specification of hematopoietic stem and progenitor cells, leading to elevated primitive erythropoiesis [73]. Further, interference in RA signaling with DEAB or though aldh1a2 knockdown has been associated with reductions in definitive blood cell production based on reduced expression of stem cell markers such as cmyb and thymic rag1/ikaros expression [74]. These findings parallel the ability of RA to enhance progenitor formation in culture [75], and the requirement for Aldh1a2 expression in the endothelium to produce definitive HSCs [76].
3. RA in Disease and Dysfunction
3.1. RA in Deficiency and Surplus in Humans
In addition to studying development, zebrafish have been a powerful system to study human disease (Figure 2) [77][78]. In work utilizing zebrafish, loss of RA associated with exposure to teratogenic compounds such as alcohol has been well documented. Zebrafish as a model for fetal alcohol syndrome has been attractive for the many reasons previously described [79][80]. In fish, when treated with 150 mM of ethanol from 3–24 hpf, severe phenotypes were observed within tissues and organs such as the eyes, otic vesicle, facial cartilage, and pericardial edema, among others [81]. These malformations fall in line with the severe defects seen clinically, as eye, heart, and improper craniofacial development are all found. These maladies are thought to be due to acetaldehyde, the byproduct of ethanol metabolism further being metabolized by an ALDH, thus reducing retinaldehydes’ ability to bind to ALDHs. In order to bypass this inhibition of RA synthesis, exogenous ATRA treatments at low levels (10−9 M) have been found to rescue the effects of ethanol exposure [81][82].
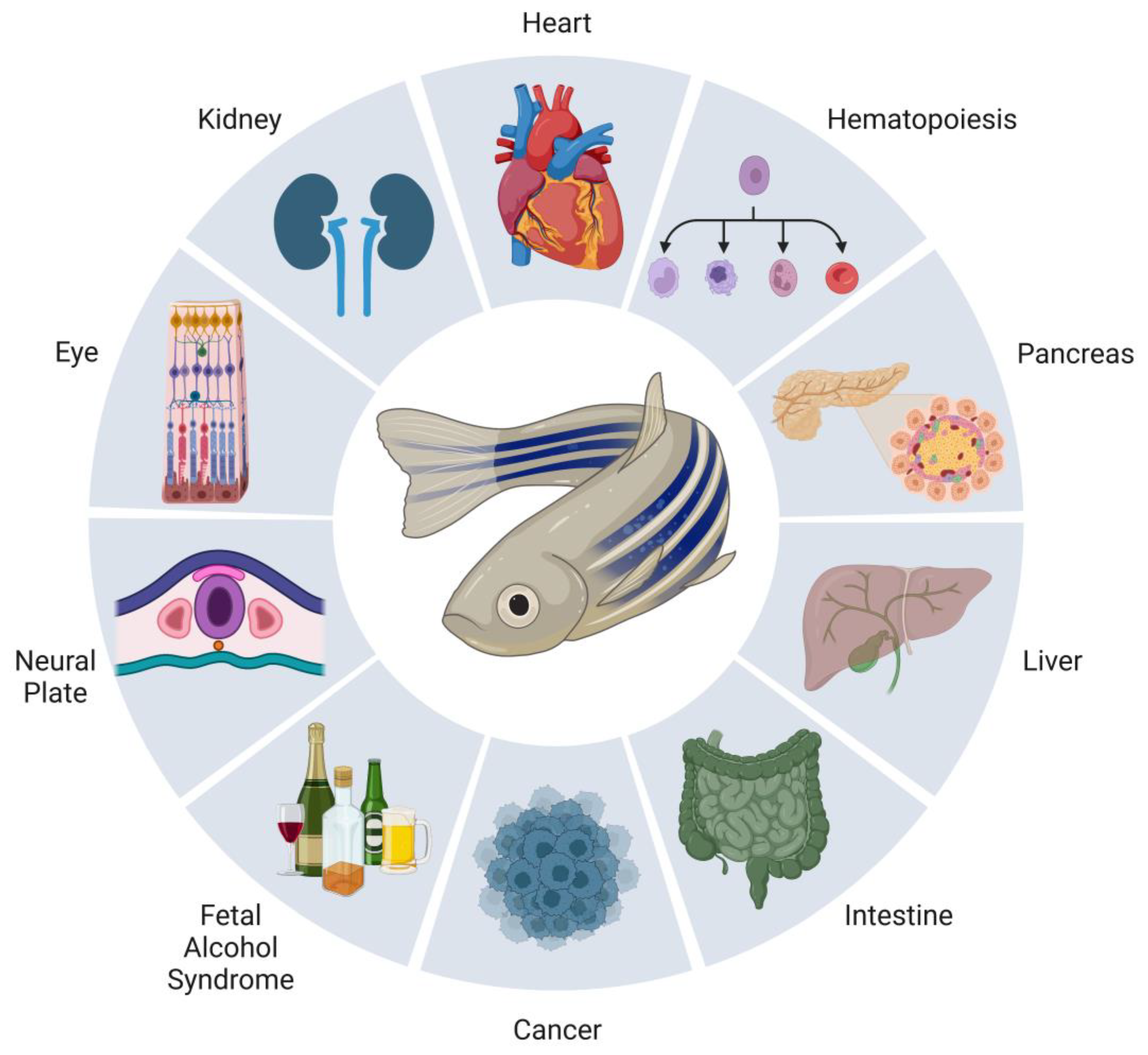
Figure 2. Summary of zebrafish models of RA signaling in development and disease. Research using the zebrafish has been a powerful good for realizing new insights into the development of many tissues and organs. Numbering among them, and discussed in the present work, are the neural plate, eye, kidney, heart, blood, pancreas, liver, and intestine. The understanding about the teratogenic effects of RA in conditions such as fetal alcohol syndrome and its role in cancer have also been expanded through the use of zebrafish.
Much like insufficient RA is detrimental for fetal development in humans, an excess can be equally as harmful. Perhaps most notably, the dermatological drug isotretinoin, an RA isomer, is well characterized for the teratologic role it can have in pregnant individuals and their offspring. In utero exposure to the drug is estimated to have a 20–35% chance of congenital defects to many of the same tissues researchers have described as being RA-dependent in development. In terms of immeasurable maladies, it is estimated that potentially over 50% of individuals exposed to isotretinoin in utero may have cognitive impairment [83][84]. In zebrafish, isotretinoin has been used in neuroblastoma work, and has been successfully shown to have the capacity for decreasing tumor size in larval fish [85].
3.2. RA Pathways in Cancer
Within the realm of cancer biology, the zebrafish model has emerged as an efficient in vivo model, much for the same reasons why it is so popular in development. Thankfully, due to high amounts of conservation between the human and zebrafish genome, many oncologic markers are subsequently retained, making the fish useful for studying many aspects of cancer biology (Figure 2) [86][87][88]. The zebrafish immune system allows for tissue xenografts from mammalian donors, allowing for not only large-scale drug and genetic screens to take place, but also holding promise for a future in precision medicine [89][90][91][92].
In a zebrafish pancreatic cancer model, antagonization of RARs were used to downregulate miR-10a expression, resulting in a loss of invasive and metastatic phenotype; interestingly, it was also found that microRNA-10a (miR-10a) is an intermediate regulator between RARs and hox1 and hoxb3 [93]. This same work also showed that miR-10a is also upregulated in pancreatitis patient samples; this postulates the questions of whether RA is mediating the micro-RNA in both disease states, and if RA regulation of miR-10a is associated with normal development of the pancreas [93].
In neuroblastoma, loss of chromatin assembly factor 1 subunit p150 (CHAF1A) promotes oncogenesis/malignancy [94]. Alongside a mouse model, zebrafish were used to investigate chaf1a expression within regular neural crest cell development as well as carcinogenesis [95]. The use of ectopic chaf1a expression revealed that chaf1a plays roles in the critical fate determinant stages of neural crest cells in development. This work was in step with mouse work that was then used to hypothesize that RA could be used in combinatorial therapies for those diagnosed with neuroblastomas [95]. In the future, researchers believe zebrafish will further emerge as an in vivo model for oncological work as a supplement for early genetic and/or pharmacological screening methods, as well as for targeting therapeutics for individual patients [96].
References
- Hale, F. The relation of vitamin A to anophthalmos in pigs. Am. J. Ophth. 1935, 18, 1087–1093.
- Warkany, J.; Schraffenberger, E. Congenital malformations of the eyes induced in rats by maternal A deficiency. V. Effects of a purified diet lacking riboflavin. Proc. Soc. Exp. Biol. Med. 1943, 54, 92–94.
- Warkany, J.; Schraffenberger, E. Congenital malformations of the eyes induced in rats by maternal A deficiency. Proc. Soc. Exp. Biol. Med. 1944, 57, 49–52.
- Warkany, J.; Schraffenberger, E. Congenital malformations induced in rats by maternal vitamin A deficiency: I. Defects of the eye. Arrh. Ophthal. 1946, 35, 150–169.
- Wilson, J.G.; Warkany, J. Epithelial keratinization as evidence of fetal vitamin A deficiency. Proc. Soc. Exp. Biol. Med. 1947, 64, 419–422.
- Wilson, J.G.; Warkany, J. Abnormalities of the genitourinary tract induced by maternal A deficiency in fetal rats. Anat. Rec. 1947, 97, 396.
- Warkany, J.; Roth, C.B. Congenital malformations induced in rats by maternal vitamin A deficiency: II. Effect of varying the preparatory diet upon the yield of abnormal young. J. Nutr. 1948, 35, 1–11.
- Wilson, J.G.; Warkany, J. Malformations in the genito-urinary tract induced by maternal vitamin A deficiency in the rat. Am. J. Anat. 1948, 83, 357–407.
- Wilson, J.G.; Roth, C.B.; Warkany, J. An analysis of the syndrome of malformations induced by maternal vitamin A deficiency. Effects of restoration of vitamin A at various times during gestation. Am. J. Anat. 1953, 92, 189–217.
- Clagett-Dame, M.; DeLuca, H.F. The role of vitamin A in mammalian reproduction and embryonic development. Annu. Rev. Nutr. 2002, 22, 347–381.
- Niederreither, K.; Dolle, P. Retinoic acid in development: Towards an integrated view. Nat. Rev. Genet. 2008, 9, 541–553.
- Duester, G. Retinoic acid synthesis and signaling during early organogenesis. Cell 2008, 134, 921–931.
- Rhinn, M.; Dollé, P. Retinoic acid signalling during development. Development 2012, 139, 843–858.
- Cunningham, T.J.; Duester, G. Mechanisms of retinoic acid signalling and its roles in organ and limb development. Nat. Rev. Mol. Cell. Biol. 2015, 16, 110–123.
- Ghyselinck, N.B.; Duester, G. Retinoic acid signaling pathways. Development 2019, 146, dev167502.
- Mezquita, B.; Mezquita, C. Two opposing faces of retinoic acid: Induction of stemness or induction of differentiation depending on cell-type. Biomolecules 2019, 9, 567.
- Selland, L.G.; Koch, S.; Laraque, M.; Waskiewicz, A.J. Coordinate regulation of retinoic acid synthesis by pbx genes and fibroblast growth factor signaling by hoxb1b is required for hindbrain patterning and development. Mech. Dev. 2018, 150, 28–41.
- Begemann, G.; Schilling, T.F.; Rauch, G.J.; Geisler, R.; Ingham, P.W. The zebrafish neckless mutation reveals a requirement for raldh2 in mesodermal signals that pattern the hindbrain. Development 2001, 128, 3081–3094.
- Moens, C.B.; Prince, V.E. Constructing the hindbrain: Insights from the zebrafish. Dev. Dyn. 2002, 224, 1–17.
- Maves, L.; Kimmel, C.B. Dynamic and sequential patterning of the zebrafish posterior hindbrain by retinoic acid. Dev. Biol. 2005, 285, 593–605.
- Hernandez, R.E.; Rikhof, H.A.; Bachmann, R.; Moens, C.B. vhnf1 integrates global RA patterning and local FGF signals to direct posterior hindbrain development in zebrafish. Development 2004, 131, 4511–4520.
- Ghosh, P.; Maurer, J.M.; Sagerström, C.G. Analysis of novel caudal hindbrain genes reveals different regulatory logic for gene expression in rhombomere 4 versus 5/6 in embryonic zebrafish. Neural Dev. 2018, 13, 13.
- Linville, A.; Gumusaneli, E.; Chandraratna, R.A.; Schilling, T.F. Independent roles for retinoic acid in segmentation and neuronal differentiation in the zebrafish hindbrain. Dev. Biol. 2004, 270, 186–199.
- Qiu, Y.; Fung, L.; Schilling, T.F.; Nie, Q. Multiple morphogens and rapid elongation promote segmental patterning during development. PLoS Comput. Biol. 2021, 17, e1009077.
- Molotkova, N.; Molotkov, A.; Duester, G. Role of retinoic acid during forebrain development begins late when Raldh3 generates retinoic acid in the ventral subventricular zone. Dev. Biol. 2007, 303, 601–610.
- Gongal, P.A.; March, L.D.; Holly, V.L.; Pillay, L.M.; Berry-Wynne, K.M.; Kagechika, H.; Waskiewicz, A.J. Hmx4 regulates Sonic hedgehog signaling through control of retinoic acid synthesis during forebrain patterning. Dev. Biol. 2011, 355, 55–64.
- Buono, L.; Corbacho, J.; Naranjo, S.; Almuedo-Castillo, M.; Moreno-Marmol, T.; de la Cerda, B.; Sanabria-Reinoso, E.; Polvillo, R.; Díaz-Corrales, F.J.; Bogdanovic, O.; et al. Analysis of gene network bifurcation during optic cup morphogenesis in zebrafish. Nat. Commun. 2021, 12, 3866.
- Yin, J.; Morrissey, M.E.; Shine, L.; Kennedy, C.; Higgins, D.G.; Kennedy, B.N. Genes and signaling networks regulated during zebrafish optic vesicle morphogenesis. BMC Genomics. 2014, 15, 825.
- Nguyen, T.K.; Petrikas, M.; Chambers, B.E.; Wingert, R.A. Principles of nephron segment development. J. Dev. Biol. 2023, 11, 14.
- Gerlach, G.F.; Wingert, R.A. Kidney organogenesis in the zebrafish: Insights into vertebrate nephrogenesis and regeneration. Wiley Interdiscip. Rev. Dev. Biol. 2013, 2, 559–585.
- Naylor, R.W.; Skvarca, L.B.; Thisse, C.; Thisse, B.; Hukriede, N.A.; Davidson, A.J. BMP and retinoic acid regulate anterior-posterior patterning of the non-axial mesoderm across the dorsal-ventral axis. Nat. Commun. 2016, 7, 12197.
- Wingert, R.A.; Davidson, A.J. Zebrafish nephrogenesis involves dynamic spatiotemporal expression changes in renal progenitors and essential signals from retinoic acid and irx3b. Dev. Dyn. 2011, 240, 2011–2027.
- Wingert, R.A.; Selleck, R.; Yu, J.; Song, H.; Chen, Z.; Song, A.; Zhou, Y.; Thisse, B.; Thisse, C.; McMahon, A.P.; et al. The cdx genes and retinoic acid control the positioning and segmentation of the zebrafish pronephros. PLoS Genet. 2007, 3, 1922–1938.
- de Groh, E.D.; Swanhart, L.M.; Cosentino, C.C.; Jackson, R.L.; Dai, W.; Kitchens, C.A.; Day, B.W.; Smithgall, T.E.; Hukriede, N.A. Inhibition of histone deacetylase expands the renal progenitor cell population. J. Am. Soc. Nephrol. 2010, 21, 794–802.
- Celá, P.; Balková, S.M.; Bryjová, A.; Horáková, D.; Míšek, I.; Richman, J.M.; Buchtová, M. Expression, function and regulation of Evi-1 during embryonic avian development. Gene Expr. Patterns 2013, 13, 343–353.
- Li, Y.; Cheng, C.N.; Verdun, V.A.; Wingert, R.A. Zebrafish nephrogenesis is regulated by interactions between retinoic acid, mecom, and Notch signaling. Dev. Biol. 2014, 386, 111–122.
- Cheng, C.N.; Wingert, R.A. Nephron proximal tubule patterning and corpuscles of Stannius formation are regulated by the sim1a transcription factor and retinoic acid in zebrafish. Dev. Biol. 2015, 399, 100–116.
- Marra, A.N.; Wingert, R.A. Epithelial cell fate in the nephron tubule is mediated by the ETS transcription factors etv5a and etv4 during zebrafish kidney development. Dev. Biol. 2016, 411, 231–245.
- Drummond, B.E.; Li, Y.; Marra, A.N.; Cheng, C.N.; Wingert, R.A. The tbx2a/b transcription factors direct pronephros segmentation and corpuscle of Stannius formation in zebrafish. Dev. Biol. 2017, 421, 52–66.
- Morales, E.E.; Handa, N.; Drummond, B.E.; Chambers, J.M.; Marra, A.N.; Addiego, A.; Wingert, R.A. Homeogene emx1 is required for nephron distal segment development in zebrafish. Sci. Rep. 2018, 8, 18038.
- Marra, A.N.; Cheng, C.N.; Adeeb, B.; Addiego, A.; Wesselman, H.M.; Chambers, B.E.; Chambers, J.M.; Wingert, R.A. Iroquois transcription factor irx2a is required for multiciliated and transporter cell fate decisions during zebrafish pronephros development. Sci. Rep. 2019, 9, 6454.
- Chambers, B.E.; Clark, E.G.; Gatz, A.E.; Wingert, R.A. Kctd15 regulates nephron segment development by repressing Tfap2a activity. Development 2020, 147, dev191973.
- Weaver, N.E.; Healy, A.; Wingert, R.A. gldc is essential for renal progenitor patterning during kidney development. Biomedicines 2022, 10, 3220.
- Wesselman, H.M.; Nguyen, T.K.; Chambers, J.M.; Drummond, B.E.; Wingert, R.A. Advances in understanding the genetic mechanisms of zebrafish renal multiciliated cell development. J. Dev. Biol. 2022, 11, 1.
- Keegan, B.R.; Feldman, J.L.; Begemann, G.; Ingham, P.W.; Yelon, D. Retinoic acid signaling restricts the cardiac progenitor pool. Science 2005, 307, 247–249.
- Lengerke, C.; Wingert, R.; Beeretz, M.; Grauer, M.; Schmidt, A.G.; Konantz, M.; Daley, G.Q.; Davidson, A.J. Interactions between Cdx genes and retinoic acid modulate early cardiogenesis. Dev. Biol. 2011, 354, 134–142.
- Chang, J.; Skromne, I.; Ho, R.K. CDX4 and retinoic acid interact to position the hindbrain-spinal cord transition. Dev. Biol. 2016, 410, 178–189.
- Loudig, O.; Babichuk, C.; White, J.; Abu-Abed, S.; Mueller, C.; Petkovich, M. Cytochrome P450RAI(CYP26) promoter: A distinct composite retinoic acid response element underlies the complex regulation of retinoic acid metabolism. Mol. Endocrinol. 2000, 14, 1483–1497.
- Loudig, O.; Maclean, G.A.; Dore, N.L.; Luu, L.; Petkovich, M. Transcriptional co-operativity between distant retinoic acid response elements in regulation of Cyp26A1 inducibility. Biochem. J. 2005, 392, 241–248.
- Waxman, J.S.; Keegan, B.R.; Roberts, R.W.; Poss, K.D.; Yelon, D. Hoxb5b acts downstream of retinoic acid signaling in the forelimb field to restrict heart field potential in zebrafish. Dev. Cell 2008, 15, 923–934.
- Marques, S.R.; Lee, Y.; Poss, K.D.; Yelon, D. Reiterative roles for FGF signaling in the establishment of size and proportion of the zebrafish heart. Dev. Biol. 2008, 321, 397–406.
- Sorrell, M.R.; Waxman, J.S. Restraint of Fgf8 signaling by retinoic acid signaling is required for proper heart and forelimb formation. Dev. Biol. 2011, 358, 44–55.
- Niederreither, K.; Vermot, J.; Messaddeq, N.; Schuhbaur, B.; Chambon, P.; Dollé, P. Embryonic retinoic acid synthesis is essential for heart morphogenesis in the mouse. Development 2001, 128, 1019–1031.
- Sirbu, I.O.; Zhao, X.; Duester, G. Retinoic acid controls heart anteroposterior patterning by down-regulating Isl1 through the Fgf8 pathway. Dev. Dyn. 2008, 237, 1627–1635.
- Rydeen, A.B.; Waxman, J.S. Cyp26 enzymes are required to balance the cardiac and vascular lineages within the anterior lateral plate mesoderm. Development 2014, 141, 1638–1648.
- Rydeen, A.B.; Waxman, J.S. Cyp26 enzymes facilitate second heart field progenitor addition and maintenance of ventricular integrity. PLoS Biol. 2016, 14, e2000504.
- Goldman, J.A.; Poss, K.D. Gene regulatory programmes of tissue regeneration. Nat. Rev. Genet. 2020, 21, 511–525.
- Poss, K.D.; Wilson, L.G.; Keating, M.T. Heart regeneration in zebrafish. Science 2002, 298, 2188–2190.
- Lepilina, A.; Coon, A.N.; Kikuchi, K.; Holdway, J.E.; Roberts, R.W.; Burns, C.G.; Poss, K.D. A dynamic epicardial injury response supports progenitor cell activity during zebrafish heart regeneration. Cell 2006, 127, 607–619.
- Kikuchi, K.; Holdway, J.E.; Major, R.J.; Blum, N.; Dahn, R.D.; Begemann, G.; Poss, K.D. Retinoic acid production by endocardium and epicardium is an injury response essential for zebrafish heart regeneration. Dev. Cell 2011, 20, 397–404.
- Danzl, K.; Messner, B.; Doppler, C.; Nebert, C.; Abfalterer, A.; Sakic, A.; Temml, V.; Heinz, K.; Streitwieser, R.; Edelmann, T.; et al. Early inhibition of endothelial retinoid uptake upon myocardial infarction restores cardiac function and prevents cell, tissue, and animal death. J. Mol. Cell Cardiol. 2019, 126, 105–117.
- Da Silva, F.; Jian Motamedi, F.; Weerasinghe Arachchige, L.C.; Tison, A.; Bradford, S.T.; Lefebvre, J.; Dolle, P.; Ghyselinck, N.B.; Wagner, K.D.; Schedl, A. Retinoic acid signaling is directly activated in cardiomyocytes and protects mouse hearts from apoptosis after myocardial infarction. Elife 2021, 10, e68280.
- Gore, A.V.; Pillay, L.M.; Venero Galanternik, M.; Weinstein, B.M. The zebrafish: A fintastic model for hematopoietic development and disease. Wiley Interdiscip. Rev. Dev. Biol. 2018, 7, e312.
- Ransom, D.G.; Bahary, N.; Niss, K.; Traver, D.; Burns, C.; Trede, N.S.; Paffett-Lugassy, N.; Saganic, W.J.; Lim, C.A.; Hersey, C.; et al. The zebrafish moonshine gene encodes transcriptional intermediary factor 1 gamma, an essential regulator of hematopoiesis. PLoS Biol. 2004, 2, E237.
- Rhodes, J.; Hagen, A.; Hsu, K.; Deng, M.; Liu, T.X.; Look, A.T.; Kanki, J.P. Interplay of pu.1 and gata1 determines myelo-erythroid progenitor cell fate in zebrafish. Dev. Cell 2005, 8, 97–108.
- Galloway, J.L.; Wingert, R.A.; Thisse, C.; Thisse, B.; Zon, L.I. Loss of gata1 but not gata2 converts erythropoiesis to myelopoiesis in zebrafish embryos. Dev. Cell 2005, 8, 109–116.
- Donovan, A.; Brownlie, A.; Zhou, Y.; Shepard, J.; Pratt, S.J.; Moynihan, J.; Paw, B.H.; Drejer, A.; Barut, B.; Zapata, A.; et al. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature 2000, 403, 776–781.
- Paw, B.H.; Davidson, A.J.; Zhou, Y.; Li, R.; Pratt, S.J.; Lee, C.; Trede, N.S.; Brownlie, A.; Donovan, A.; Liao, E.C.; et al. Cell-specific mitotic defect and dyserythropoiesis associated with erythroid band 3 deficiency. Nat. Genet. 2003, 34, 59–64.
- Wingert, R.A.; Galloway, J.L.; Barut, B.; Foott, H.; Fraenkel, P.; Axe, J.L.; Weber, G.J.; Dooley, K.; Davidson, A.J.; Schmidt, B.; et al. Deficiency of glutaredoxin 5 reveals Fe-S clusters are required for vertebrate haem synthesis. Nature 2005, 436, 1035–1039.
- Shaw, G.C.; Cope, J.J.; Li, L.; Corson, K.; Hersey, C.; Ackermann, G.E.; Gwynn, B.; Lambert, A.J.; Wingert, R.A.; Traver, D.; et al. Mitoferrin is essential for erythroid iron assimilation. Nature 2006, 440, 96–100.
- De Jong, J.L.O.; Davidson, A.J.; Wang, Y.; Palis, J.; Opara, P.; Pugach, E.; Daley, G.Q.; Zon, L.I. Interaction of retinoic acid and scl controls primitive blood development. Blood 2010, 116, 201–209.
- Liang, D.; Jia, W.; Li, J.; Li, K.; Zhao, Q. Retinoic acid signaling plays a restrictive role in zebrafish primitive myelopoiesis. PLoS ONE 2012, 7, e30865.
- Ma, A.C.H.; Chung, M.I.S.; Liang, R.; Leung, A.Y.H. A DEAB-sensitive aldehyde dehydrogenase regulates hematopoietic stem and progenitor cells development during primitive hematopoiesis in zebrafish embryos. Leukemia 2010, 24, 2090–2099.
- Pillay, L.M.; Mackowetzky, K.J.; Widen, S.A.; Waskiewicz, A.J. Somite-derived retinoic acid regulates zebrafish hematopoietic stem cell formation. PLoS ONE 2016, 11, e0166040.
- Yu, C.; Liu, Y.; Miao, Z.; Yin, M.; Lu, W.; Lv, Y.; Ding, M.; Deng, H. Retinoic acid enhances the generation of hematopoietic progenitors from human embryonic stem cell-derived hemato-vascular precursors. Blood 2010, 116, 4786–4794.
- Chanda, B.; Ditadi, A.; Iscove, N.N.; Keller, G. Retinoic acid signaling is essential for embryonic hematopoietic stem cell development. Cell 2013, 155, 215–227.
- Lieschke, G.J.; Currie, P.D. Animal models of human disease: Zebrafish swim into view. Nat. Rev. Genet. 2007, 8, 353–367.
- Patton, E.E.; Zon, L.I.; Langenau, D.M. Zebrafish disease models in drug discovery: From preclinical modelling to clinical trials. Nat. Rev. Drug Discov. 2021, 20, 611–628.
- Fernandes, Y.; Lovely, C.B. Zebrafish models of fetal alcohol spectrum disorders. Genesis 2021, 59, e23460.
- Manikandan, P.; Sarmah, S.; Marrs, J.A. Ethanol effects on early developmental stages studied using the zebrafish. Biomedicines 2022, 10, 2555.
- Marrs, J.A.; Clendenon, S.G.; Ratcliffe, D.R.; Fielding, S.M.; Liu, Q.; Bosron, W.F. Zebrafish fetal alcohol syndrome model: Effects of ethanol are rescued by retinoic acid supplement. Alcohol 2010, 44, 707–715.
- Muralidharan, P.; Sarmah, S.; Marrs, J.A. Zebrafish retinal defects induced by ethanol exposure are rescued by retinoic acid and folic acid supplement. Alcohol 2015, 49, 149–163.
- Lammer, E.J.; Chen, D.T.; Hoar, R.M.; Agnish, N.D.; Benke, P.J.; Braun, J.T.; Curry, C.J.; Fernhoff, P.M.; Grix, A.W., Jr.; Lott, I.T.; et al. Retinoic acid embryopathy. N. Engl. J. Med. 1985, 313, 837–841.
- Draghici, C.C.; Miulescu, R.G.; Petca, R.C.; Petca, A.; Dumitrașcu, M.C.; Șandru, F. Teratogenic effect of isotretinoin in both fertile females and males. Exp. Ther. Med. 2021, 21, 534.
- He, S.; Mansour, M.R.; Zimmerman, M.W.; Ki, D.H.; Layden, H.M.; Akahane, K.; Gjini, E.; de Groh, E.D.; Perez-Atayde, A.R.; Zhu, S.; et al. Synergy between loss of NF1 and overexpression of MYCN in neuroblastoma is mediated by the GAP-related domain. Elife 2016, 5, e14713.
- Howe, K.; Clark, M.; Torroja, C.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503.
- Lam, S.H.; Wu, Y.L.; Vega, V.B.; Miller, L.D.; Spitsbergen, J.; Tong, Y.; Zhan, H.; Govindarajan, K.R.; Lee, S.; Mathavan, S.; et al. Conservation of gene expression signatures between zebrafish and human liver tumors and tumor progression. Nat. Biotechnol. 2006, 24, 73–75.
- Kobar, K.; Collett, K.; Prykhozhij, S.V.; Berman, J.N. Zebrafish cancer predisposition models. Front. Cell Dev. Biol. 2021, 9, 660069.
- Haldi, M.; Ton, C.; Seng, W.L.; McGrath, P. Human melanoma cells transplanted into zebrafish proliferate, migrate, produce melanin, form masses and stimulate angiogenesis in zebrafish. Angiogenesis 2006, 9, 139–151.
- Nicoli, S.; Ribatti, D.; Cotelli, F.; Presta, M. Mammalian tumor xenografts induce neovascularization in zebrafish embryos. Cancer Res. 2007, 67, 2927–2931.
- Fazio, M.; Ablain, J.; Chuan, Y.; Langenau, D.M.; Zon, L.I. Zebrafish patient avatars in cancer biology and precision cancer therapy. Nat. Rev. Cancer 2020, 20, 263–273.
- Mandelbaum, J.; Shestopalov, I.A.; Henderson, R.E.; Chau, N.G.; Knoechel, B.; Wick, M.J.; Zon, L.I. Zebrafish blastomere screen identifies retinoic acid suppression of MYB in adenoid cystic carcinoma. J. Exp. Med. 2018, 215, 2673–2685.
- Weiss, F.U.; Marques, I.J.; Woltering, J.M.; Vlecken, D.H.; Aghdassi, A.; Partecke, L.I.; Heidecke, C.D.; Lerch, M.M.; Bagowski, C.P. Retinoic acid receptor antagonists inhibit miR-10a expression and block metastatic behavior of pancreatic cancer. Gastroenterology 2009, 137, 2136–2145.e1-e7.
- Barbieri, E.; De Preter, K.; Capasso, M.; Chen, Z.; Hsu, D.M.; Tonini, G.P.; Lefever, S.; Hicks, J.; Versteeg, R.; Pession, A.; et al. Histone chaperone CHAF1A inhibits differentiation and promotes aggressive neuroblastoma. Cancer Res. 2014, 74, 765–774.
- Tao, L.; Moreno-Smith, M.; Ibarra-García-Padilla, R.; Milazzo, G.; Drolet, N.A.; Hernandez, B.E.; Oh, Y.S.; Patel, I.; Kim, J.J.; Zorman, B.; et al. CHAF1A blocks neuronal differentiation and promotes neuroblastoma oncogenesis via metabolic reprogramming. Adv. Sci. 2021, 8, e2005047.
- Xiao, J.; Glasgow, E.; Agarwal, S. Zebrafish xenografts for drug discovery and personalized medicine. Trends Cancer 2020, 6, 569–579.
More
Information
Subjects:
Developmental Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
737
Revisions:
2 times
(View History)
Update Date:
26 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




