
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mohd Imran | -- | 2664 | 2023-04-20 11:21:31 | | | |
| 2 | Lindsay Dong | + 6 word(s) | 2670 | 2023-04-21 03:57:40 | | |
Video Upload Options
Lung cancer is acknowledged to be the major driver of cancer death attributable to treatment challenges and poor prognosis. Classical cancer treatment regimens, such as chemotherapy or radiotherapy, can be used to treat lung cancer, but the appended adverse effects limit them. Because of the numerous side effects associated with these treatment modalities, it is crucial to strive to develop novel and better strategies for managing lung cancer. Attributes such as enhanced bioavailability, better in vivo stability, intestinal absorption pattern, solubility, prolonged and targeted distribution, and the superior therapeutic effectiveness of numerous anticancer drugs have all been boosted with the emergence of nano-based therapeutic systems. Nano-based approaches are pioneering the route for primary and metastatic lung cancer diagnosis and treatment.
1. Targeting of Nano-Formulations for Lung Cancer
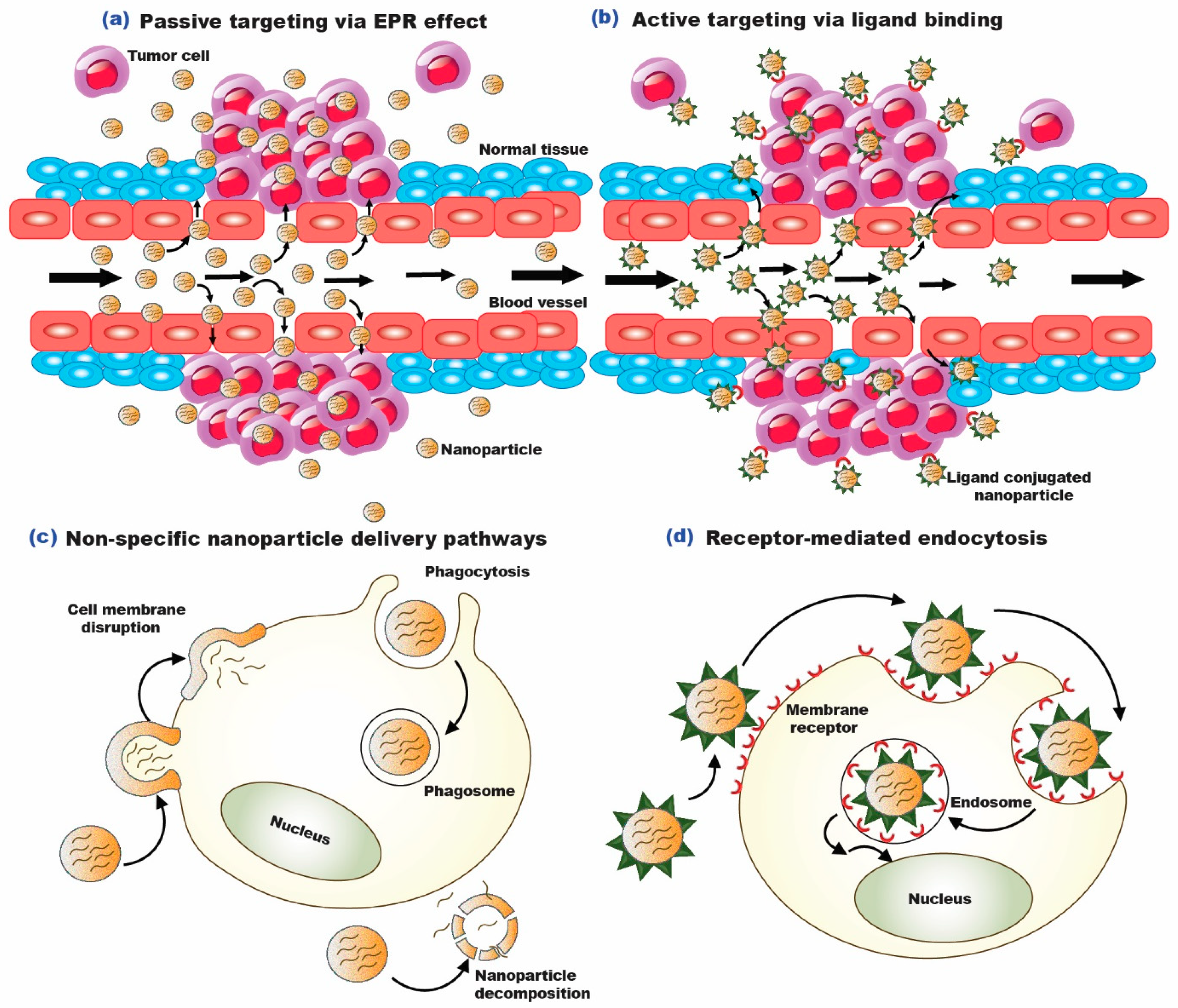
1.1. Passive Targeting of Nano-Formulations for Lung Cancer
1.2. Active Targeting of Nano-Formulations for Lung Cancer
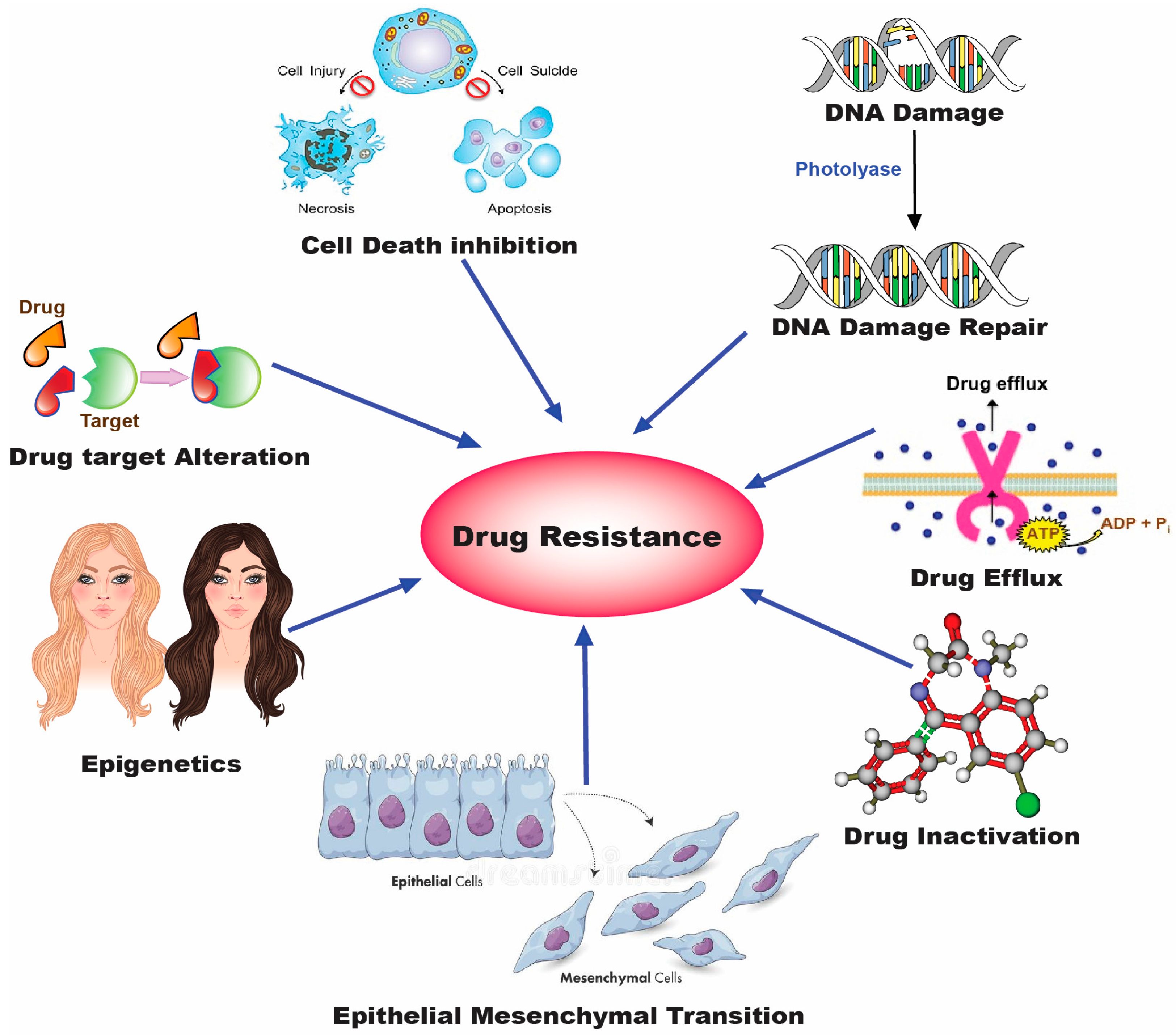
2. Drug Resistance in Lung Cancer
3. Combinatorial Therapy for Lung Cancer Treatment
4. Clinical Studies of Nanocarriers in Lung Cancer
5. Challenges Associated with the Use of Nanomedicine in Cancer
5.1. Challenges Associated with Non-Targeted (Passively Targeted) Nano-Formulations
5.2. Challenges Associated with Targeted (Ligand Anchored/Active Targeted) Nano-Formulations
References
- Padhi, S.; Behera, A. Cellular Internalization and Toxicity of Polymeric Nanoparticles. In Polymeric Nanoparticles for the Treatment of Solid Tumors; Padhi, S., Behera, A., Licht-fouse, E., Eds.; Springer Nature: Cham, Switzerland, 2022; Volume 71, pp. 473–488.
- Padhi, S.; Behera, A. Advanced Drug Delivery Systems in the Treatment of Ovarian Cancer. Adv. Drug Deliv. Syst. Manag. Cancer 2021, 127–139.
- Bazak, R.; Houri, M.; El Achy, S.; Hussein, W.; Refaat, T. Passive Targeting of Nanoparticles to Cancer: A Comprehensive Review of the Literature. Mol. Clin. Oncol. 2014, 2, 904–908.
- Kundu, A.; Padhi, S.; Behera, A.; Hasnain, M.S.; Nayak, A.K. Tumor Targeting Strategies by Chitosan-Based Nanocarriers. Chitosan Biomed. Appl. 2022, 163–188.
- Padhi, S.; Azharuddin, M.; Behera, A.; Zakir, F.; Mirza, M.A.; Chyad, A.A.; Iqbal, Z.; Mansoor, S. Nanocarriers as Delivery Tool for COVID-19 Drugs. Coronavirus Drug Discov. 2022, 2, 293–332.
- Behera, A.; Padhi, S. pH-Sensitive Polymeric Nanoparticles for Cancer Treatment. In Polymeric Nanoparticles for the Treatment of Solid Tumors; Padhi, S., Behera, A., Licht-fouse, E., Eds.; Springer Nature: Cham, Switzerland, 2022; Volume 71, pp. 401–425.
- Lee, H.Y.; Mohammed, K.A.; Nasreen, N. Nanoparticle-Based Targeted Gene Therapy for Lung Cancer. Am. J. Cancer Res. 2016, 6, 1118.
- Leighl, N.B.; Goss, G.D.; Lopez, P.G.; Burkes, R.L.; Dancey, J.E.; Rahim, Y.H.; Rudinskas, L.C.; Pouliot, J.F.; Rodgers, A.; Pond, G.R.; et al. Phase II Study of Pegylated Liposomal Doxorubicin HCl (Caelyx) in Combination with Cyclophosphamide and Vincristine as Second-Line Treatment of Patients with Small Cell Lung Cancer. Lung Cancer 2006, 52, 327–332.
- Yuan, H.; Guo, H.; Luan, X.; He, M.; Li, F.; Burnett, J.; Truchan, N.; Sun, D. Albumin nanoparticle of paclitaxel (Abraxane) decreases while taxol increases breast cancer stem cells in treatment of triple negative breast cancer. Mol. Pharm. 2020, 17, 2275–2286.
- Sun, T.; Shrike Zhang, Y.; Bo, P.; Hyun, D.C.; Yang, M.; Xia, Y. Engineered Nanoparticles for Drug Delivery in Cancer Therapy*. Nanomater. Neoplasms 2021, 31–142.
- Alphandéry, E. Biodistribution and Targeting Properties of Iron Oxide Nanoparticles for Treatments of Cancer and Iron Anemia Disease. Nanotoxicology 2019, 13, 573–596.
- Behera, A.; Padhi, S. Passive and Active Targeting Strategies for the Delivery of the Camptothecin Anticancer Drug: A Review. Environ. Chem. Lett. 2020, 18, 1557–1567.
- Adhipandito, C.F.; Cheung, S.H.; Lin, Y.H.; Wu, S.H. Atypical renal clearance of nanoparticles larger than the kidney filtration threshold. Int. J. Mol. Sci. 2021, 22, 11182.
- Roma-Rodrigues, C.; Rivas-García, L.; Baptista, P.V.; Fernandes, A.R. Gene therapy in cancer treatment: Why go nano? Pharmaceutics 2020, 12, 233.
- Amreddy, N.; Babu, A.; Muralidharan, R.; Munshi, A.; Ramesh, R. Polymeric nanoparticle-mediated gene delivery for lung cancer treatment. Top. Curr. Chem. 2018, 375, 35.
- Wang, Z.; Qiao, R.; Tang, N.; Lu, Z.; Wang, H.; Zhang, Z.; Xue, X.; Huang, Z.; Zhang, S.; Zhang, G.; et al. Active Targeting Theranostic Iron Oxide Nanoparticles for MRI and Magnetic Resonance-Guided Focused Ultrasound Ablation of Lung Cancer. Biomaterials 2017, 127, 25–35.
- Padhi, S.; Behera, A. Nanotechnology Based Targeting Strategies for the Delivery of Camptothecin. In Sustainable Agriculture Reviews 44. Sustainable Agriculture Reviews; Saneja, A., Panda, A., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2020; pp. 243–272.
- Ulbrich, K.; Hola, I.; Bakandritsos, A.; Tuc, Í.; Zbor, R. Targeted Drug Delivery with Polymers and Magnetic Nanoparticles: Covalent and Noncovalent Approaches, Release Control, and Clinical Studies. Chem. Rev. 2016, 116, 5338–5431.
- Babu, A.; Amreddy, N.; Muralidharan, R.; Pathuri, G.; Gali, H.; Chen, A.; Zhao, Y.D.; Munshi, A.; Ramesh, R. Chemodrug Delivery Using Integrin-Targeted PLGA-Chitosan Nanoparticle for Lung Cancer Therapy. Sci. Rep. 2017, 7, 14674.
- Singh, R.P.; Sharma, G.; Sonali; Singh, S.; Bharti, S.; Pandey, B.L.; Koch, B.; Muthu, M.S. Chitosan-Folate Decorated Carbon Nanotubes for Site Specific Lung Cancer Delivery. Mater. Sci. Eng. C 2017, 77, 446–458.
- Ramalingam, V.; Varunkumar, K.; Ravikumar, V.; Rajaram, R. Target Delivery of Doxorubicin Tethered with PVP Stabilized Gold Nanoparticles for Effective Treatment of Lung Cancer. Sci. Rep. 2018, 8, 3815.
- Singh, R.D.; Shandilya, R.; Bhargava, A.; Kumar, R.; Tiwari, R.; Chaudhury, K.; Srivastava, R.K.; Goryacheva, I.Y.; Mishra, P.K. Quantum Dot Based Nano-Biosensors for Detection of Circulating Cell Free MiRNAs in Lung Carcinogenesis: From Biology to Clinical Translation. Front. Genet. 2018, 9, 616.
- Mashinchian, O.; Johari-Ahar, M.; Ghaemi, B.; Rashidi, M.; Barar, J.; Omidi, Y. Impacts of Quantum Dots in Molecular Detection and Bioimaging of Cancer. Bioimpacts 2014, 4, 149.
- Ranjbar-Navazi, Z.; Eskandani, M.; Johari-Ahar, M.; Nemati, A.; Akbari, H.; Davaran, S.; Omidi, Y. Doxorubicin-Conjugated D-Glucosamine- and Folate- Bi-Functionalised InP/ZnS Quantum Dots for Cancer Cells Imaging and Therapy. J. Drug Target 2017, 26, 267–277.
- Zhao, T.; Qin, S.; Peng, L.; Li, P.; Feng, T.; Wan, J.; Yuan, P.; Zhang, L. Novel Hyaluronic Acid-Modified Temperature-Sensitive Nanoparticles for Synergistic Chemo-Photothermal Therapy. Carbohydr. Polym. 2019, 214, 221–233.
- Haider, M.; Elsherbeny, A.; Pittalà, V.; Consoli, V.; Alghamdi, M.A.; Hussain, Z.; Khoder, G.; Greish, K. Nanomedicine Strategies for Management of Drug Resistance in Lung Cancer. Int. J. Mol. Sci. 2022, 23, 1853.
- Sarkar, S.; Horn, G.; Moulton, K.; Oza, A.; Byler, S.; Kokolus, S.; Longacre, M. Cancer Development, Progression, and Therapy: An Epigenetic Overview. Int. J. Mol. Sci. 2013, 14, 21087–21113.
- Galluzzi, L.; Kepp, O.; Heiden, M.G.V.; Kroemer, G. Metabolic Targets for Cancer Therapy. Nat. Rev. Drug Discov. 2013, 12, 829–846.
- Wangpaichitr, M.; Wu, C.; Li, Y.Y.; Nguyen, D.J.M.; Kandemir, H.; Shah, S.; Chen, S.; Feun, L.G.; Prince, J.S.; Kuo, M.T.; et al. Exploiting ROS and Metabolic Differences to Kill Cisplatin Resistant Lung Cancer. Oncotarget 2017, 8, 49275–49292.
- Yabuki, N.; Sakata, K.; Yamasaki, T.; Terashima, H.; Mio, T.; Miyazaki, Y.; Fujii, T.; Kitada, K. Gene Amplification and Expression in Lung Cancer Cells with Acquired Paclitaxel Resistance. Cancer Genet. Cytogenet. 2007, 173, 1–9.
- Saad, M.; Garbuzenko, O.B.; Minko, T. Co-Delivery of SiRNA and an Anticancer Drug for Treatment of Multidrug-Resistant Cancer. Nanomedicine 2008, 3, 761–776.
- Lu, Z.; Su, J.; Li, Z.; Zhan, Y.; Ye, D. Hyaluronic Acid-Coated, Prodrug-Based Nanostructured Lipid Carriers for Enhanced Pancreatic Cancer Therapy. Drug Dev. Ind. Pharm. 2016, 43, 160–170.
- Sharma, A.; Shambhwani, D.; Pandey, S.; Singh, J.; Lalhlenmawia, H.; Kumarasamy, M.; Singh, S.K.; Chellappan, D.K.; Gupta, G.; Prasher, P.; et al. Advances in Lung Cancer Treatment Using Nanomedicines. ACS Omega 2023, 8, 10–41.
- Roma-Rodrigues, C.; Raposo, L.R.; Valente, R.; Fernandes, A.R.; Baptista, P.V. Combined cancer therapeutics-Tackling the complexity of the tumor microenvironment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1704.
- Guo, S.; Zhang, Y.; Wu, Z.; Zhang, L.; He, D.; Li, X.; Wang, Z. Synergistic Combination Therapy of Lung Cancer: Cetuximab Functionalized Nanostructured Lipid Carriers for the Co-Delivery of Paclitaxel and 5-Demethylnobiletin. Biomed. Pharmacother. 2019, 118, 109225.
- Wu, L.; Leng, D.; Cun, D.; Foged, C.; Yang, M. Advances in Combination Therapy of Lung Cancer: Rationales, Delivery Technologies and Dosage Regimens. J. Control. Release 2017, 260, 78–91.
- Reda, M.; Ngamcherdtrakul, W.; Nelson, M.A.; Siriwon, N.; Wang, R.; Zaidan, H.Y.; Bejan, D.S.; Reda, S.; Hoang, N.H.; Crumrine, N.A.; et al. Development of a nanoparticle-based immunotherapy targeting PD-L1 and PLK1 for lung cancer treatment. Nat. Commun. 2022, 13, 4261.
- Hrkach, J.; Von Hoff, D.; Ali, M.M.; Andrianova, E.; Auer, J.; Campbell, T.; De Witt, D.; Figa, M.; Figueiredo, M.; Horhota, A.; et al. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci. Transl. Med. 2012, 4, 128ra39.
- Rizvi, N.A.; Riely, G.J.; Azzoli, C.G.; Miller, V.A.; Ng, K.K.; Fiore, J.; Chia, G.; Brower, M.; Heelan, R.; Hawkins, M.J.; et al. Phase I/II trial of weekly intravenous 130-nm albumin-bound paclitaxel as initial chemotherapy in patients with stage IV non–small-cell lung cancer. J. Clin. Oncol. 2008, 26, 639–643.
- Landen, C.N.; Kinch, M.S.; Sood, A.K. EphA2 as a Target for Ovarian Cancer Therapy. Expert Opin Ther Targets 2005, 9, 1179–1187.
- Wang, J.; Tian, S.; Petros, R.A.; Napier, M.E.; Desimone, J.M. The Complex Role of Multivalency in Nanoparticles Targeting the Transferrin Receptor for Cancer Therapies. J. Am. Chem. Soc. 2010, 132, 11306–11313.
- Attia, M.F.; Anton, N.; Wallyn, J.; Omran, Z.; Vandamme, T.F. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J. Pharm. Pharmacol. 2019, 71, 1185–1198.
- Rosenblum, D.; Peer, D. Omics-Based Nanomedicine: The Future of Personalized Oncology. Cancer Lett. 2014, 352, 126–136.
- Meacham, C.E.; Morrison, S.J. Tumour Heterogeneity and Cancer Cell Plasticity. Nature 2013, 501, 328–337.
- Ryan, M.B.; Der, C.J.; Wang-Gillam, A.; Cox, A.D. Targeting RAS-Mutant Cancers: Is ERK the Key? Trends Cancer 2015, 1, 183–198.
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and Challenges towards Targeted Delivery of Cancer Therapeutics. Nat. Commun. 2018, 9, 1410.
- Rasool, M.; Malik, A.; Waquar, S.; Arooj, M.; Zahid, S.; Asif, M.; Shaheen, S.; Hussain, A.; Ullah, H.; Gan, S.H. New Challenges in the Use of Nanomedicine in Cancer Therapy. Bioengineered 2022, 13, 759–773.
- Keller, J.G.; Graham, U.M.; Koltermann-Jülly, J.; Gelein, R.; Ma-Hock, L.; Landsiedel, R.; Wiemann, M.; Oberdörster, G.; Elder, A.; Wohlleben, W. Predicting dissolution and transformation of inhaled nanoparticles in the lung using abiotic flow cells: The case of barium sulfate. Sci. Rep. 2020, 10, 458.
- Thai, L.P.A.; Mousseau, F.; Oikonomou, E.; Radiom, M.; Berret, J.F. Effect of Nanoparticles on the Bulk Shear Viscosity of a Lung Surfactant Fluid. ACS Nano 2019, 14, 466–475.
- Radiom, M.; Sarkis, M.; Brookes, O.; Oikonomou, E.K.; Baeza-Squiban, A.; Berret, J.F. Pulmonary surfactant inhibition of nanoparticle uptake by alveolar epithelial cells. Sci. Rep. 2020, 10, 19436.




