
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Anisa Mitra | -- | 2690 | 2023-04-20 03:21:33 |
Video Upload Options
The continuing and rapid climate change will have an adverse effect on reproduction by jeopardising successful breeding and survival of fishes impacting on the viability of sustainability in both aquaculture systems and the ocean.
1. Introduction
Undisputed evidence of climate change calls for sustainable solutions [1]. Although the oceans were protected by the high specific heat capacity of water in the recent geological past, they were still subject to the consequences of climate change and eco-evolutionary effects [2]. Fundamental ecological processes are altered by climate change, which has a significant impact on oceans and aquaculture systems. Increasing sea levels and temperatures change monsoon patterns, cause extreme weather events, and stress the water supply [3]. Aquatic organisms' physiologies, patterns of growth, and behaviours are impacted by climate change, affects their geographic distribution and reproductive capacities [4][5]. This ultimately causes mortality, which alters the composition of the oceans and the productivity and functionality of aquatic ecosystems [6]. Since fish are poikilothermic, asymmetric climatic events can have an impact on a variety of aspects of their biology, including their metabolism, behaviour, and evolutionary process. Temperature change may result in biodiversity loss, have an impact on the world's fish stocks, have socioeconomic repercussions, and increase nutritional hunger [7][8].
The significance of "biocomplexity," or the constantly changing relationship between the biological systems and their environment," is perceived to be important in the future projections [9][10], where it is shown that the spatial and temporal changes may become more pronounced [11]. Diverse physical environments, the impact of changing environmental variables, intra-species concurrence, cycling of nutrients, and community dynamics are just a few of the biocomplexity factors that aquatic ecologists have addressed as affecting the efficiency of fish species [12].
The ongoing, rapid environmental changes as well as the disruption of breeding grounds may put the capacity to maintain viable sustainability in jeopardy. Sexual reproduction is a vital and energy-demanding process that is heavily reliant on the environment for fish species to survive and evolve [12]. These environmental cues trigger and regulate sexual development, reproduction, and child survival. Regardless of whether environmental stochasticity is brought on by genetic (evolutionary) or non-genetic (plastic) processes, an organism's response to it results in within-generation phenotypic plasticity, transgenerational plasticity, and genetic adaptation [13][14]. Certain adaptations may result in fecundity trade-offs [15], or they may interact with various species or habitats in ways that ultimately reduce survival rates [16].
2. Climatic and Aquatic System Changes
Issues like climate change and resource sustainability became universal in recent decades. The simultaneous change in air and water temperatures puts marine animals at great risk from climate change. The change could apply only locally or globally [1]. Oceanic and aquaculture system climate changes have a direct impact on the physical characteristics, biological growth patterns, and fertility rates of aquatic organisms, including fish [17]. Indirectly, this alters the composition and functioning of aquatic ecosystems, having an impact on both organisms and entire populations of species [18]. Fish and other aquatic organisms are poikilothermic by nature. They consequently react very quickly to changes in the ambient temperature. The warming of the ocean caused on by climate change slows fish growth because it increases their metabolic rate. solely environmental spawners, who are spawning grounds change according to changes in environmental characteristics like temperature, can relocate to locations in which their inner regulatory systems can restore internal homeostasis if the surrounding temperature surpasses their temperature tolerance [6]. This movement enables environmental spawners to leave slightly enclosed regions with shallow water and shallow coastlines [19]. The effects of climate change on marine ecosystems include sea level rise, an increase in tide frequency, a decrease in pH, a rise in carbonate ion concentration in seawater, and an increase disease in marine biota (Table 1).
The entire aquatic food chain is impacted by climate change, which influences fish production. The resources available for fish productivity and sustainability are influenced by the amount of sunlight and the availability of nutrients in water. Ground flow of water will be reduced because of decreased rainfall brought on by climate change, starving wetlands and mangroves and harming regional fisheries. Most of the small fishing grounds in the lowlands are significantly impacted by climate change. Increased rainfall in some regions raises the nutrient content of water bodies, leading to eutrophication and fish hypoxia [20]. Changes in temperature and light levels affect the availability of nutrients, which in turn affects the primary source of aquatic resources for fish productivity and sustainability. It is difficult to forecast the way climate change will impact freshwater aquaculture in tropical and subtropical regions, as stated by De Silva and Soto [5]. Eutrophication may increase the development rates and productivity of cultured species because it causes elevated temperatures and more plankton growth [4].
Table 1. Potential impacts of climate change on fisheries and aquaculture. Edited by Water, MDPI (https://doi.org/10.3390/w15040725)
|
Drivers of Change |
Impacts |
|---|---|
|
Variation of the surface temperature of the waters |
Expansion of harmful algae Lowering of dissolved O2 levels Spread of disease and parasites Extension of growth stations Changes of position and ranges of suitable species Mortality decreases during the winter season Enhanced growth and food conversion rates Alteration of local ecosystems induced by competition, parasitism and predation of competitors and exotic species |
|
Variation of oceanographic variables |
Decreased flushing rates and food availability to shellfish Alteration in the richness of edible species and fishmeal |
|
Sea level rise |
Reduction of areas destined for aquaculture Reduction of areas that provide physical protection Increased risks of flooding Salt infiltration into groundwater |
|
Increased frequency of storms |
Larger waves More frequent storms Floods caused by rainfall Variation of salinity parameters Structure damage |
|
Drought and water stress |
Variation of salinity parameters Decrease in water quality Increased disease Uncertain water supplies |
Changes in water availability, severe weather, vertical stratification, and nutrient availability may have a negative impact on freshwater aquaculture production depending on local conditions. Salinity changes may have an impact on aquaculture operations in brackish waters, again depending on regional runoff, marine circulation, etc. conditions. Additionally, as temperatures warm faster than in low latitude regions, an increased prevalence of pathogens could have a negative impact on aquaculture in temperate regions [3].
3. Climatic Change Effects on Fish Reproduction
Recent years have seen changes in reproductive phenology, the key processes in the reproduction of organisms, especially those with cold blood or incapable of controlling their own body temperature autonomously from that of their environment [4][6]. These asynchronous climate changes may have an impact on ecosystem stability, community organisation, ecosystem processes, and population dynamics, just as they do in other vertebrates and invertebrates [4][5]. They might also affect the inconsistencies between fish breeding phenology and phytoplankton blooms. Changes in species abundance were primarily brought on by precipitation, critical temperatures, and the fish mortality rate, according to a long-term assessment of the effects of climate [3][21].
Numerous studies have shown how the changing climate affects sexual behaviour, gonadal differentiation, gametogenesis, the quality of gametes, the reproductive cycle, and recruitment success [6][22][23][24].
Therefore, under the scenario of a changing climate, understanding the interactions between various environmental factors that control the brain-pituitary-gonad axis's functioning is essential for forecasting ecological event relating to wild fish populations and managing captive fish breeding. The central nervous system is recognised as having a crucial part in the fusion of internal (hormonal) and external signals that control reproduction by having an impact on the rate at which hormones are produced and released as well as the structure of hormones [25] (see Figure 1). The hypothalamus, is the most crucial brain region for regulating vertebrate reproductive functions and behaviours, is influenced by the sensory system [26][27][28].
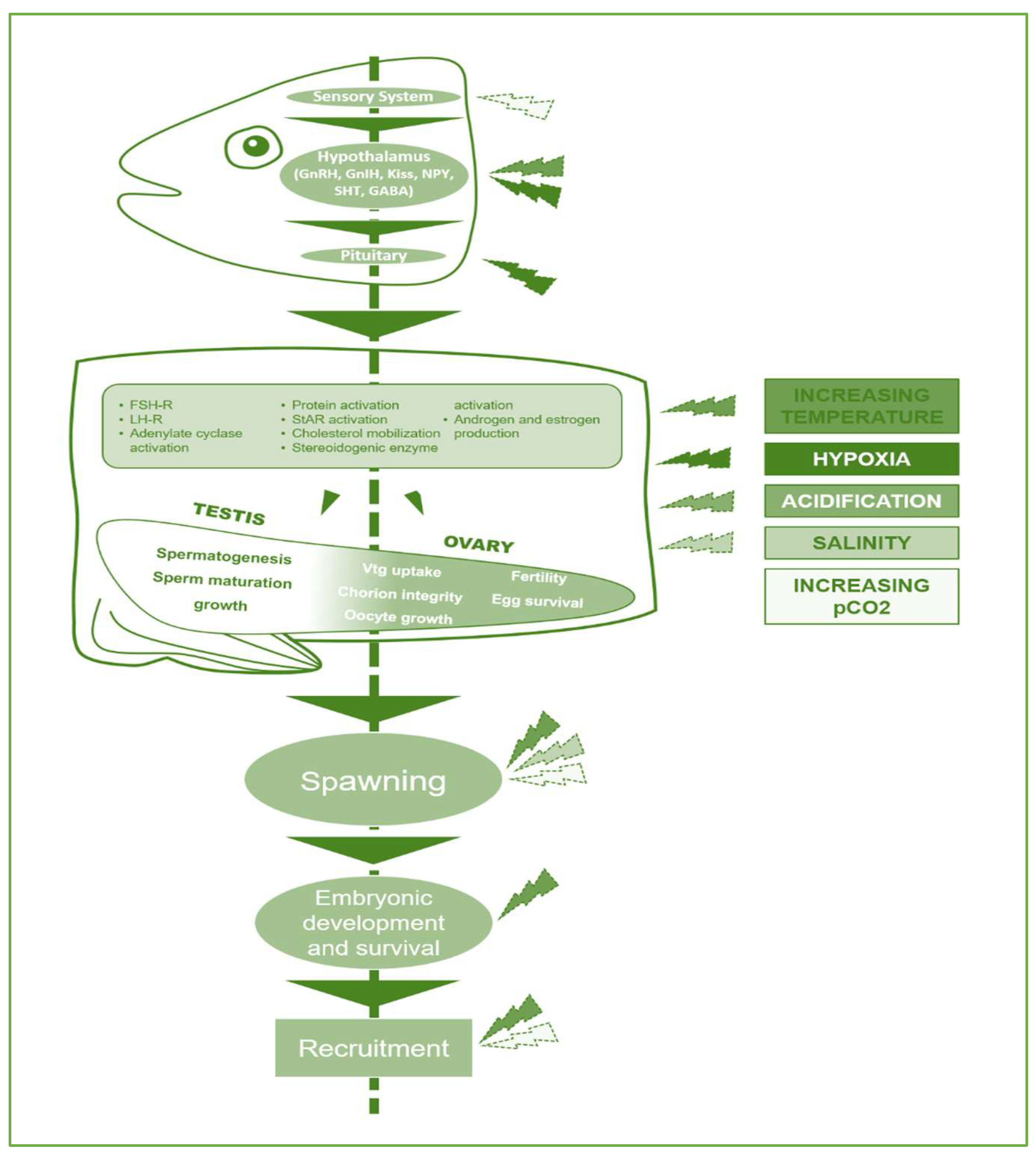
Figure 1. Signaling regulation: hypothalamus-pituitary-gonad-spawning-embryonic development altered by abiotic factors. Abbreviations: GnRH, gonadotropin releasing hormone; GnIH, Gonadotropin-inhibitory hormone; Kiss, kisspeptin; NPY, neuropeptide Y; SHT, somatotropin, GABA, gamma-aminobutyric acid. Edited by Water, MDPI (https://doi.org/10.3390/w15040725).
The endocrine regulation of reproduction is significantly influenced by the hormones dopamine, neuropeptide Y (NPY), gamma-aminobutyric acid (GABA), and kisspeptin (Kiss), which activate enzymes that produce sex hormones and regulate gametogenesis [29]. Free radical levels and balance, which are managed by the body's natural antioxidant defence mechanisms, have an impact on all of these [6][30]. Since their geographic ranges conceal an extensive spectrum of temperatures, many fish species are possibly able to adapt to changing temperatures [31].
The expression of genes is inhibited, proteins undergo conformational changes, and steroid hormones have a greater propensity to form water-soluble conjugates, all of which contribute to the development of the gonads being compromised by high water temperature [32]. By reducing stream flows and increasing water temperatures, climate change has been shown to primarily impact the reproductive performance of geo-graphic spawners, whose spawning grounds are determined by geographic features [33]. An intriguing earth system model [5] reports the impact of a high-emission, climate change situation on the future spawning season of the two classes of temperate, epipelagic fishes, "geographic spawners" and "environmental spawners." Predictions of the combined impacts of appear phenology and temperature on fish life cycles may lead to an improved assessment of the impact of climate change on present fish populations as well as more informed management for species conservation. Turbidity, channel geomorphology, and the suitability of spawning habitats are all impacted by water flow. Thus, these variables might start migration or spawning processes [34].
According to Fraser and colleagues' [35] hypothesis, habitat loss caused by reduced flow due to climate change would undoubtedly reduce population abundance, though this might be mitigated by upstream expansion of the habitats with better thermal conditions. Greater pCO2, or increased acidification, may limit mature fish's capacity to function aerobically, which may influence their reproductive productivity, claim Pottner and Farrell [36]. Hypoxia and high-water temperature have been related by researchers to unsuccessful migration and spawning in migratory species [37]. Due to a reduced ability to detect chemical cues and sexual pheromones, increased pCO2 in fish has been shown to impair mating as well as other reproductive processes.
According to Biswal and colleagues [38], both ocean acidification and hypoxia endanger larval survival by affecting their behaviour and sensory abilities. Additionally, according to research, a change in salinity may have an impact on fish behaviour, breeding, and reproduction success [39]. Changes in salinity can affect gonadal cell proliferation, apoptosis, and male nesting behaviour in marine fish, which can all influence fish spermatogenesis and testicular homeostasis. The mutually reinforcing effects of freshwater salinization and rising temperatures result in complex interactions that have significant consequences for the physiological responses of freshwater fishes, according to a growing body of research on complex multiple-stressor interactions [40]. The ability of fish to adapt genetically to rapid climate change is affected by a number of variables, including adaptive genetic variation, effective population numbers, generation times, and interaction within populations that may potentially promote the spread of tolerant genotypes [41].
4. Climate Change and Adaptive Strategies towards Sustainability
Global temperature increases of 0.74 °C have been recorded in the last 100 years [42]. To limit the unavoidable impact on fish biocomplexity, researchers are using an integrated spatio-temporal approach in both the fisheries and aquaculture sectors [10][11].
Evaluating the behaviors resulting from dynamic and often non-linear interactions of biological agents with control and feedback processes will allow for a greater understanding of how to intervene in favor of management and sustainability. Biocomplex elements include knowledge of the fishing area, of the biology and ecology of the species, of ethno taxonomy [10] .
Additionally, understanding how environmental factors interact by influencing the hypothalamic-pituitary-gonad axis will be fundamental for the prediction of ecological phenomena and for potential in captive fish management to reduce their impacts [28]. The study of the management of climatic invalidity is a useful condition for guaranteeing future fishing activity [43].
The implementation of the number of species and their response to temperature changes studies allows to improve the sustainable production of fish [44]. Yang and colleagues [45] proposed to evaluate the species-specific relationships related to oviposition with respect to abiotic factors. Simulations in marine protected areas will allow for an increase in stocks useful for preventing environmental damage and allowing for restocking [46].
To support the sustainability, particularly of migratory species, it may be helpful to implement fishing bans during the breeding season, establish Marine Protected Areas (MPAs) and restrict overall catches in these significant areas, or restrict the use of certain kinds of gear (such as bottom trawling species in sensitive habitats). Recruitment of larvae from offshore areas would also aid in the improvement of coastal stock [47]. To accomplish long-term objectives, we must adopt new economic principles [48]. Local markets must be established to fully realize the financial benefits of locally caught fresh fish to lessen their impact on migratory species [49].
The rate of climate change frequently outpaces the rate of evolution on average [50]. Recent meta-analyses confirm the possibility that species with adaptive traits are evolving inadequately to keep up with climate change [51]. Thus, it is crucial for resource management and the conservation of endangered species to keep track of various responses and comprehend their limitations [16]. The biomass of spawning organisms has decreased due to ongoing overfishing and inadequate size selection of major fishing gears [8]. Aquaculture research utilising Virtual Population Analysis (VPA) is only utilised to forecast fish stock sizes [52] and predict the most commercially exploited stocks due to the difficulty in calculating the size of fish populations [53].
As the human population grows, there is a greater need for high-quality protein, which fish provides. The River Ranching programme promotes the environmentally and financially beneficial sustainable use of fish resources. Additionally, it will guarantee the advancement of conventional fishing, trade, social protection of populations in the hinterland, and environmental sustainability by using a cluster- or area-based approach. Predictive modelling research and supercomputing tools could be useful in the development of adaptation strategies, such as breeding species that are resistant to the effects of the climate change or saline [54].
Improved nutrition and selective breeding can be used to reduce temperature rises above the limit of tolerance [55][56]. When selecting an oviposition induction procedure, it is important to consider the stress that hormonal manipulation may cause as well as the time frame between hormonal stimulation and stripping for inexpensive IVF [57]. According to earlier research, parental fitness affects egg survival and embryo growth [58].
A biostatistical approach using fry survival rate has shown to be an effective measure for estimating fish production rather than relying on fertilisation success and embryo survival [59]. Predictions could be made with greater accuracy using a more thorough approach that included analyses of multiple species and ecosystems [60]. In order to accomplish sustainable development in fisheries and aquaculture, an ecosystem approach to fisheries places an emphasis on the relationships between ecosystem elements and the human system [9]. Community-based periodic training, awareness-raising, and knowledge dissemination on trend adaptation measures [61] is advised as a pilot programme that can be used on a large scale and can help stakeholders improve output and livelihoods as a process to prevent ecological damage [62]. Successful management necessitates a database of ecological data and biological characteristics of different fish species, particularly diet and development and their correlation [63].
Aquaculture emerged as the best option to meet the current and higher consumption rates of growing populations and to withstand the effects of climate change on the potential fisheries production. Aquaculturists must implement innovative biotechnological techniques for preserving productivity in containment in order to oversee the fisheries resources sustainably (see Figure 2).
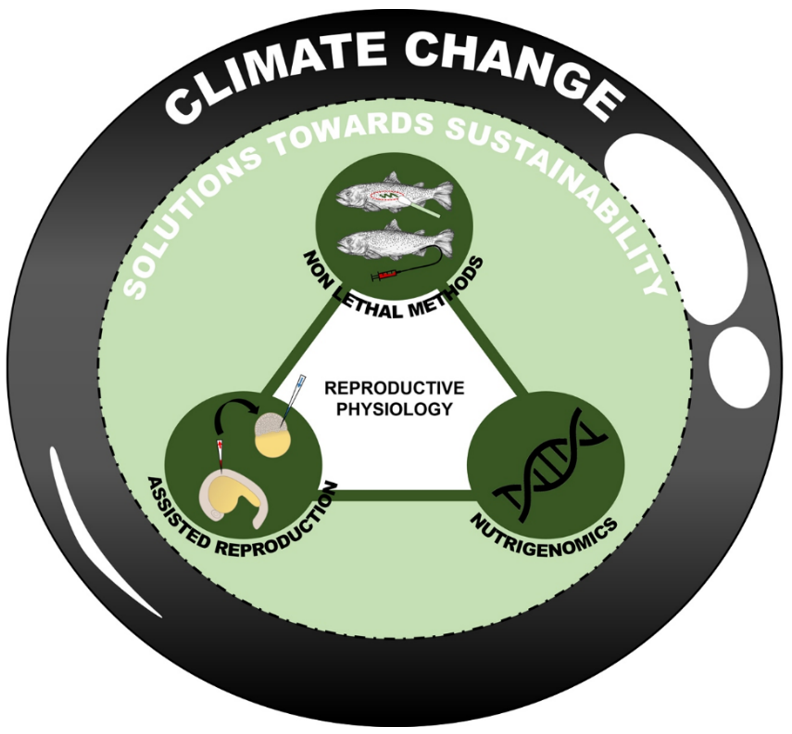
Figure 2 . Innovative biotechnological solutions towards sustainability proposed in Mitra et al., [64]. Edited by Water, MDPI (https://doi.org/10.3390/w15040725).
The biotechnological detection of antioxidants and fertility rate, according to the study by Mitra and colleagues [64], serves as a tool for evaluating as well as understanding of reprotoxicity risk through a non-invasive and non-lethal approach [6][65][66]; the application of germ cell transplantation through xenotransplantation and surrogacy [67][68][69] and the nutrigenomics programmes [70][71][72] could help efficiently to contrast the fish reproductive risk posed by the climate change.
5. Conclusions
Temperature is such a crucial element in aquatic environments; hence climate change has stoked a lot of research interest in thermal ecophysiology. Being poikilothermic, fish cannot control their body temperature. In fish, sexual maturation, breeding, and offspring survival are strongly regulated by and triggered by specific environmental cues. Effective management calls for an extensive inventory of ecological data and biological characteristics of different fish species, particularly nutrition and reproduction as well as their correlation. Additionally, technological advancements ought to offer a quick and creative evaluation approach to the creation of practical techniques for the sustainability of the fish resources.
References
- Pörtner, H.-O.; Roberts, D.C.; Adams, H.; Adler, C.; Aldunce, P.; Ali, E.; Begum, R.A.; Betts, R.; Kerr, R.B.; Biesbroek, R. Cli-mate Change 2022: Impacts, Adaptation and Vulnerability; IPCC Sixth Assessment Report; Cambridge University Press: Cam-bridge, UK; New York, NY, USA, 2022; Available online: https://www.ipcc.ch/report/ar6/wg2/downloads/report/IPCC_AR6_WGII_SummaryVolume.pdf (accessed on 1 January 2023).
- Walther, G.-R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.; Fromentin, J.-M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological Responses to Recent Climate Change. Nature 2002, 416, 389–395.
- 3. Barange, M.; Perry, R.I. Physical and Ecological Impacts of Climate Change Relevant to Marine and Inland Capture Fisheries and Aquaculture. In Climate Change Implications for Fisheries and Aquaculture: Overview of Current Scientific Knowledge; Cochrane, K., de Young, C., Soto, D., Bahri, T., Eds.; FAO: Rome, Italy, 2009; pp. 7–106. Available online: https://www.cakex.org/sites/default/files/documents/i0994e02a.pdf (accessed on 1 January 2023).
- De Silva, S.S.; Soto, D. Climate Change and Aquaculture: Potential Impacts, Adaptation and Mitigation. In Climate Change Implications for Fisheries and Aquaculture: Overview of Current Scientific Knowledge; FAO Fisheries and Aquaculture Technical Paper; FAO: Rome, Italy, 2009; Volume 530, pp. 151–212.
- Asch, R.G.; Stock, C.A.; Sarmiento, J.L. Climate Change Impacts on Mismatches between Phytoplankton Blooms and Fish Spawning Phenology. Glob. Chang. Biol. 2019, 25, 2544–2559.
- Parisi, C.; Guerriero, G. Antioxidative Defense and Fertility Rate in the Assessment of Reprotoxicity Risk Posed by Global Warming. Antioxidants 2019, 8, 622.
- Muhala, V.; Chicombo, T.F.; Macate, I.E.; Guimarães-Costa, A.; Gundana, H.; Malichocho, C.; Hasimuna, O.J.; Remédio, A.; Maulu, S.; Cuamba, L.; et al. Climate Change in Fisheries and Aquaculture: Analysis of the Impact Caused by Idai and Ken-neth Cyclones in Mozambique. Front. Sustain. Food Syst. 2021, 5, 714187.
- Brander, K. Effects of Environmental Variability on Growth and Recruitment in Cod (Gadus morhua) Using a Comparative Approach. Oceanol. Acta 2000, 23, 485–496.
- Froese, R.; Papaioannou, E.; Scotti, M. Climate Change or Mismanagement? Environ. Biol. Fish. 2022, 105, 1363–1380.
- FAO. The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation; FAO: Rome, Italy, 2022; ISBN 978-92-5-136364-5.
- Volpedo, A. Environmental Changes on Freshwater Fish Communities in South America in the Last Five Decades: A Case Study in Northeast Argentina. Sust. Agric. Food Environ. Res. 2016, 4, 47–61.
- Oyinlola, M.A.; Reygondeau, G.; Wabnitz, C.C.C.; Frölicher, T.L.; Lam, V.W.Y.; Cheung, W.W.L. Projecting Global Maricul-ture Production and Adaptation Pathways under Climate Change. Glob. Chang. Biol. 2022, 28, 1315–1331.
- Pankhurst, N.W.; Porter, M.J.R. Cold and Dark or Warm and Light: Variations on the Theme of Environmental Control of Reproduction. Fish Physiol. Biochem. 2003, 28, 385–389.
- Kingsolver, J.G.; Buckley, L.B. Quantifying Thermal Extremes and Biological Variation to Predict Evolutionary Responses to Changing Climate. Phil. Trans. R. Soc. B 2017, 372, 20160147.
- Shama, L.N.S.; Wegner, K.M. Grandparental Effects in Marine Sticklebacks: Transgenerational Plasticity across Multiple Generations. J. Evol. Biol. 2014, 27, 2297–2307.
- Audzijonyte, A.; Fulton, E.; Haddon, M.; Helidoniotis, F.; Hobday, A.J.; Kuparinen, A.; Morrongiello, J.; Smith, A.D.; Upston, J.; Waples, R.S. Trends and Management Implications of Human-Influenced Life-History Changes in Marine Ectotherms. Fish Fish. 2016, 17, 1005–1028.
- Weiskopf, S.R.; Rubenstein, M.A.; Crozier, L.G.; Gaichas, S.; Griffis, R.; Halofsky, J.E.; Hyde, K.J.W.; Morelli, T.L.; Morisette, J.T.; Muñoz, R.C.; et al. Climate Change Effects on Biodiversity, Ecosystems, Ecosystem Services, and Natural Resource Management in the United States. Sci. Total Environ. 2020, 733, 137782.
- Gentilucci, M.; Parisi, C.; Coppola, M.R.; Majdoubi, F.-Z.; Madonna, A.; Guerriero, G. Influence of Mediterranean Sea Tem-perature Increase on Gaeta Gulf (Tyrrhenian Sea) Biodiversity. Proc. Zool. Soc. 2021, 74, 91–103.
- Hiddink, J.G.; Ter Hofstede, R. Climate Induced Increases in Species Richness of Marine Fishes: Climate change and fish spe-cies richness. Glob. Chang. Biol. 2008, 14, 453–460.
- Lin, Y.-J.; Rabaoui, L.; Basali, A.U.; Lopez, M.; Lindo, R.; Krishnakumar, P.K.; Qurban, M.A.; Prihartato, P.K.; Cortes, D.L.; Qasem, A.; et al. Long-Term Ecological Changes in Fishes and Macro-Invertebrates in the World’s Warmest Coral Reefs. Sci. Total Environ. 2021, 750, 142254.
- Colautti, D.; Baigún, C.; Llompart, F.; Maiztegui, T.; Garcia de Souza, J.; Solimano, P.; Balboni, L.; Berasain, G. Fish Assem-blage of a Pampean Shallow Lake, a Story of Instability. Hydrobiologia 2015, 752, 175–186.
- Alix, M.; Kjesbu, O.S.; Anderson, K.C. From Gametogenesis to Spawning: How Climate-driven Warming Affects Teleost Re-productive Biology. J. Fish. Biol. 2020, 97, 607–632.
- Feugere, L.; Scott, V.F.; Rodriguez-Barucg, Q.; Beltran-Alvarez, P.; Wollenberg Valero, K.C. Thermal Stress Induces a Positive Phenotypic and Molecular Feedback Loop in Zebrafish Embryos. J. Therm. Biol. 2021, 102, 103114.
- Yu, Y.; Chen, M.; Lu, Z.-Y.; Liu, Y.; Li, B.; Gao, Z.-X.; Shen, Z.-G. High-Temperature Stress Will Put the Thermo-Sensitive Teleost Yellow Catfish (Tachysurus Fulvidraco) in Danger through Reducing Reproductivity. Ecotoxicol. Environ. Saf. 2022, 239, 113638.
- Guerriero, G. Vertebrate Sex Steroid Receptors: Evolution, Ligands, and Neurodistribution. Ann. N. Y. Acad. Sci. 2009, 1163, 154–168.
- Guerriero, G.; Roselli, C.E.; Paolucci, M.; Botte, V.; Ciarcia, G. Estrogen Receptors and Aromatase Activity in the Hypothal-amus of the Female Frog, Rana Esculenta. Fluctuations throughout the Reproductive Cycle. Brain Res. 2000, 880, 92–101.
- Guerriero, G.; Roselli, C.E.; Ciarcia, G. The Amphibian (Rana esculenta) Brain Progesterone Receptor: Relationship to Plasma Steroids and Vitellogenic Cycle during the Gonadal Recovery Phase. Ann. N. Y. Acad. Sci. 2009, 1163, 407–409.
- Miranda, L.A.; Chalde, T.; Elisio, M.; Strüssmann, C.A. Effects of Global Warming on Fish Reproductive Endocrine Axis, with Special Emphasis in Pejerrey Odontesthes Bonariensis. Gen. Comp. Endocrinol. 2013, 192, 45–54.
- Muñoz-Cueto, J.A.; Zmora, N.; Paullada-Salmerón, J.A.; Marvel, M.; Mañanos, E.; Zohar, Y. The Gonadotropin-Releasing Hormones: Lessons from Fish. Gen. Comp. Endocrinol. 2020, 291, 113422.
- Guerriero, G.; Trocchia, S.; Abdel-Gawad, F.K.; Ciarcia, G. Roles of Reactive Oxygen Species in the Spermatogenesis Regula-tion. Front. Endocrinol. 2014, 5, 56.
- Luis Val, A.; Wood, C.M. Global Change and Physiological Challenges for Fish of the Amazon Today and in the near Future. J. Exp. Biol. 2022, 225, jeb216440.
- Lema, S.C.; Chow, M.I.; Dittman, A.H.; May, D.; Housh, M.J. Accustomed to the Heat: Temperature and Thyroid Hormone Influences on Oogenesis and Gonadal Steroidogenesis Pathways Vary among Populations of Amargosa Pupfish (Cyprinodon Nevadensis Amargosae). Comp. Biochem. Physiol. Part A 2022, 272, 111280.
- Morales-Marín, L.A.; Rokaya, P.; Sanyal, P.R.; Sereda, J.; Lindenschmidt, K.E. Changes in Streamflow and Water Tempera-ture Affect Fish Habitat in the Athabasca River Basin in the Context of Climate Change. Ecol. Model. 2019, 407, 108718.
- Fisher, M.C.; Helser, T.E.; Kang, S.; Gwak, W.; Canino, M.F.; Hauser, L. Genetic Structure and Dispersal in Peripheral Popu-lations of a Marine Fish (Pacific Cod, Gadus macrocephalus) and Their Importance for Adaptation to Climate Change. Ecol. Evol. 2022, 12, e8474.
- Fraser, G.S.; Bestgen, K.R.; Winkelman, D.L.; Thompson, K.G. Temperature—Not Flow—Predicts Native Fish Reproduction with Implications for Climate Change. Trans. Am. Fish. Soc. 2019, 148, 509–527.
- Pörtner, H.O.; Farrell, A.P. Physiology and Climate Change. Science 2008, 322, 690–692.
- Frommel, A.Y.; Lye, S.L.R.; Brauner, C.J.; Hunt, B.P.V. Air Exposure Moderates Ocean Acidification Effects during Embryon-ic Development of Intertidally Spawning Fish. Sci. Rep. 2022, 12, 12270.
- Biswal, A.; Srivastava, P.P.; Krishna, G.; Paul, T.; Pal, P.; Gupta, S.; Varghese, T.; Jayant, M. An Integrated Biomarker Ap-proach for Explaining the Potency of Exogenous Glucose on Transportation Induced Stress in Labeo Rohita Fingerlings. Sci. Rep. 2021, 11, 5713.
- Lehtonen, T.K.; Wong, B.B.M.; Kvarnemo, C. Effects of Salinity on Nest-Building Behaviour in a Marine Fish. BMC Ecol. 2016, 16, 7.
- Iglesias, M.C.-A. A Review of Recent Advances and Future Challenges in Freshwater Salinization. Limnetica 2020, 39, 185–211.
- Valdivieso, A.; Wilson, C.A.; Amores, A.; da Silva Rodrigues, M.; Nóbrega, R.H.; Ribas, L.; Postlethwait, J.H.; Piferrer, F. En-vironmentally-Induced Sex Reversal in Fish with Chromosomal vs. Polygenic Sex Determination. Environ. Res. 2022, 213, 113549.
- Gentilucci, M.; Moustafa, A.A.; Abdel-Gawad, F.K.; Mansour, S.R.; Coppola, M.R.; Caserta, L.; Inglese, S.; Pambianchi, G.; Guerriero, G. Advances in Egyptian Mediterranean Coast Climate Change Monitoring. Water 2021, 13, 1870.
- Spencer, P.D.; Hollowed, A.B.; Sigler, M.F.; Hermann, A.J.; Nelson, M.W. Trait-based Climate Vulnerability Assessments in Data-rich Systems: An Application to Eastern Bering Sea Fish and Invertebrate Stocks. Glob. Chang. Biol. 2019, 25, 3954–3971.
- Karr, J.R.; Larson, E.R.; Chu, E.W. Ecological Integrity Is Both Real and Valuable. Conserv. Sci. Pract. 2022, 4, e583.
- Yang, Z.; Zhu, Q.; Cao, J.; Jin, Y.; Zhao, N.; Xu, W.; Liu, H.; Tang, H.; Qiao, Y.; Chen, X. Using a Hierarchical Model Framework to Investigate the Relationships between Fish Spawning and Abiotic Factors for Environmental Flow Manage-ment. Sci. Total Environ. 2021, 787, 147618.
- Cornejo-Donoso, J.; Einarsson, B.; Birnir, B.; Gaines, S.D. Effects of Fish Movement Assumptions on the Design of a Marine Protected Area to Protect an Overfished Stock. PLoS ONE 2017, 12, e0186309.
- Bell, J.D.; Cisneros-Montemayor, A.; Hanich, Q.; Johnson, J.E.; Lehodey, P.; Moore, B.R.; Pratchett, M.S.; Reygondeau, G.; Se-nina, I.; Virdin, J.; et al. Adaptations to Maintain the Contributions of Small-Scale Fisheries to Food Security in the Pacific Is-lands. Mar. Policy 2018, 88, 303–314.
- Raworth, K. Doughnut Economics: Seven Ways to Think Like a 21st-Century Economist; Chelsea Green Publishing: White River Junction, VT, USA, 2017.
- Loring, P.A.; Fazzino, D.V.; Agapito, M.; Chuenpagdee, R.; Gannon, G.; Isaacs, M. Fish and Food Security in Small-Scale Fisheries. In Transdisciplinarity for Small-Scale Fisheries Governance; Chuenpagdee, R., Jentoft, S., Eds.; MARE Publication Se-ries; Springer International Publishing: Cham, Switzerland, 2019; Volume 21, pp. 55–73. ISBN 978-3-319-94937-6.
- Meester, L.D.; Stoks, R.; Brans, K.I. Genetic Adaptation as a Biological Buffer against Climate Change: Potential and Limita-tions. Integr. Zool. 2018, 13, 372–391.
- Radchuk, V.; Reed, T.; Teplitsky, C.; van de Pol, M.; Charmantier, A.; Hassall, C.; Adamík, P.; Adriaensen, F.; Ahola, M.P.; Arcese, P.; et al. Adaptive Responses of Animals to Climate Change Are Most Likely Insufficient. Nat. Commun. 2019, 10, 3109.
- Denechaud, C.; Smoliński, S.; Geffen, A.J.; Godiksen, J.A.; Campana, S.E. A Century of Fish Growth in Relation to Climate Change, Population Dynamics and Exploitation. Glob. Chang. Biol. 2020, 26, 5661–5678.
- Lavin, C.P.; Jones, G.P.; Williamson, D.H.; Harrison, H.B. Minimum Size Limits and the Reproductive Value of Numerous, Young, Mature Female Fish. Proc. R. Soc. B 2021, 288, 20202714.
- Becker, L.A.; Crichigno, S.A.; Cussac, V.E. Climate Change Impacts on Freshwater Fishes: A Patagonian Perspective. Hydro-biologia 2018, 816, 21–38.
- Ouizgane, A.; Farid, S.; Majdoubi, F.Z.; Droussi, M.; Guerriero, G.; Hasnaoui, M. Assessment of Climate Change Effects on Predation Activity and Growth of Largemouth Bass, Micropterus Salmoides (Lacepède, 1802) by Water Temperature Varia-tions. Emir. J. Food Agric. 2018, 30, 515–521.
- Gibson, D.; Riecke, T.V.; Catlin, D.H.; Hunt, K.L.; Weithman, C.E.; Koons, D.N.; Karpanty, S.M.; Fraser, J.D. Climate Change and Commercial Fishing Practices Codetermine Survival of a Long-lived Seabird. Glob. Chang. Biol. 2022, 29, 324–340.
- Knowles, J.; Vysloužil, J.; Policar, T.; Milla, S.; Holická, M.; Podhorec, P. Spawning Performance and Sex Steroid Levels in Female Pikeperch Sander Lucioperca Treated with Poly(Lactic-Co-Glycolic Acid) Microparticles. Animals 2022, 12, 208
- Shima, J.S.; Osenberg, C.W.; Alonzo, S.H.; Noonburg, E.G.; Mitterwallner, P.; Swearer, S.E. Reproductive Phenology across the Lunar Cycle: Parental Decisions, Offspring Responses, and Consequences for Reef Fish. Ecology 2020, 101, e03086.
- Majdoubi, F.-Z.; Ouizgane, A.; Farid, S.; Mossetti, S.L.; Droussi, M.; Guerriero, G.; Hasnaoui, M. Fry Survival Rate as a Pre-dictive Marker of Optimal Production of Silver Carp (Hypophthalmichthys molitrix, Valenciennes 1844): A Biostatistical Study in Deroua Fish Farm, Morocco. Proc. Zool. Soc. 2022, 75, 152–160.
- Wootton, H.F.; Audzijonyte, A.; Morrongiello, J. Multigenerational Exposure to Warming and Fishing Causes Recruitment Collapse, but Size Diversity and Periodic Cooling Can Aid Recovery. Proc. Natl. Acad. Sci. USA 2021, 118, e2100300118.
- Brosset, P.; Smith, A.D.; Plourde, S.; Castonguay, M.; Lehoux, C.; Van Beveren, E. A Fine-Scale Multi-Step Approach to Un-derstand Fish Recruitment Variability. Sci. Rep. 2020, 10, 16064.
- Oyebola, O.O.; Efitre, J.; Musinguzi, L.; Falaye, A.E. Potential Adaptation Strategies for Climate Change Impact among Flood-Prone Fish Farmers in Climate Hotspot Uganda. Environ. Dev. Sustain. 2021, 23, 12761–12790.
- Mitra, A.; Mukhopadhyay, P.K.; Homechaudhuri, S. An Overview of Biology And Culture Potentials of Humped Feather-back Chitala chitala (Hamilton, 1822)—A New Candidate for Aquaculture Diversification. Rev. Fish. Sci. Aquac. 2018, 26, 371–380.
- Mitra, A.; Abdel-Gawad, F.K.; Bassem, S.; Barua, P.; Assisi, L.; Parisi, C.; Temraz, T.A.; Vangone, R.; Kajbaf, K.; Kumar, V.; Guerriero, G. Climate Change and Reproductive Biocomplexity in Fishes: Innovative Management Approaches towards Sus-tainability of Fisheries and Aquaculture. Water 2023, 15, 725. https://doi.org/10.3390/w15040725
- D’Errico, G.; Vitiello, G.; De Tommaso, G.; Abdel-Gawad, F.K.; Brundo, M.V.; Ferrante, M.; De Maio, A.; Trocchia, S.; Bianchi, A.R.; Ciarcia, G.; et al. Electron Spin Resonance (ESR) for the Study of Reactive Oxygen Species (ROS) on the Isolat-ed Frog Skin (Pelophylax Bergeri): A Non-Invasive Method for Environmental Monitoring. Environ. Res. 2018, 165, 11–18.
- Guerriero, G.; D’Errico, G. Effect of Oxidative Stress on Reproduction and Development. Antioxidants 2022, 11, 312.
- Margules, C.R.; Pressey, R.L. Systematic Conservation Planning. Nature 2000, 405, 243–253, doi:10.1038/35012251
- Pukazhenthi, B.; Comizzoli, P.; Travis, A.J.; Wildt, D.E. Applications of Emerging Technologies to the Study and Conserva-tion of Threatened and Endangered Species. Reprod. Fertil. Dev. 2006, 18, 77, doi:10.1071/RD05117.
- De Siqueira-Silva, D.H.; Saito, T.; dos Santos-Silva, A.P.; da Silva Costa, R.; Psenicka, M.; Yasui, G.S. Biotechnology Applied to Fish Reproduction: Tools for Conservation. Fish Physiol. Biochem. 2018, 44, 1469–1485.
- Mohanty, B.P.; Ganguly, S.; Mahanty, A.; Mitra, T.; Mohanty, S. Nutrigenomics and Fish. CABI Reviews 2020, 2020, PAVSNNR202015048, doi:10.1079/PAVSNNR202015048.
- Dawson, K.A. Nutrigenomics: Feeding the Genes for Improved Fertility. Animal Reproduction Science 2006, 96, 312–322, doi:10.1016/j.anireprosci.2006.08.009.
- Tincy, V.; Mishal, P.; Akhtar, M.S.; Pal, A.K. Turning Challenges into Opportunities. World Aquaculture 2014, 67.




