Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Adeline KAROLKOWSKI | -- | 3481 | 2023-04-19 17:51:53 | | | |
| 2 | Beatrix Zheng | Meta information modification | 3481 | 2023-04-20 03:15:10 | | | | |
| 3 | Beatrix Zheng | Meta information modification | 3481 | 2023-04-20 03:15:52 | | | | |
| 4 | Adeline KAROLKOWSKI | Meta information modification | 3481 | 2023-04-20 09:23:55 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Karolkowski, A.; Belloir, C.; Briand, L.; Salles, C. Compounds Involved in Bitterness and Astringency of Pulses. Encyclopedia. Available online: https://encyclopedia.pub/entry/43255 (accessed on 15 January 2026).
Karolkowski A, Belloir C, Briand L, Salles C. Compounds Involved in Bitterness and Astringency of Pulses. Encyclopedia. Available at: https://encyclopedia.pub/entry/43255. Accessed January 15, 2026.
Karolkowski, Adeline, Christine Belloir, Loïc Briand, Christian Salles. "Compounds Involved in Bitterness and Astringency of Pulses" Encyclopedia, https://encyclopedia.pub/entry/43255 (accessed January 15, 2026).
Karolkowski, A., Belloir, C., Briand, L., & Salles, C. (2023, April 19). Compounds Involved in Bitterness and Astringency of Pulses. In Encyclopedia. https://encyclopedia.pub/entry/43255
Karolkowski, Adeline, et al. "Compounds Involved in Bitterness and Astringency of Pulses." Encyclopedia. Web. 19 April, 2023.
Copy Citation
Despite the many advantages of pulses, they are characterised by off-flavours that limit their consumption. Off-notes, bitterness and astringency contribute to negative perceptions of pulses. Several hypotheses have assumed that non-volatile compounds, including saponins, phenolic compounds, and alkaloids, are responsible for pulse bitterness and astringency.
pulses
off-flavours
bitterness
astringency
1. Saponins
Saponins are amphiphile molecules. They consist of a steroidal or triterpene hydrophobic aglycone and one to three sugars (hydrophilic part) attached by ester or ether linkage [1]. In legumes, triterpenoid saponins were the main saponins identified, although some steroidal saponins were also detected [2][3][4]. In soybeans, saponins were found in cotyledons and derived from soyasapogenols A, B, and E (Figure 1) [1]. The amount of saponins in legume seeds was very different according to their type. Chickpeas contained 2.6 to 60 g/kg (dry matter) of saponins against 0.1 to 3.7 g/kg (dry matter) for broad beans and 1.8 to 11 g/kg (dry matter) for green peas [5].
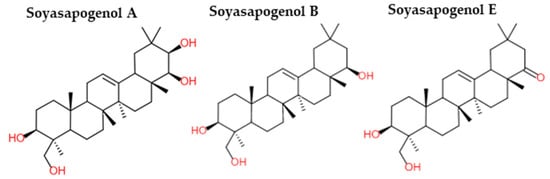
Figure 1. Chemical structures of soyasapogenol A, B and E (adapted from [6]).
In peas, an extract of isolated soyasaponin βb (soyasaponin I) is described as bitter and astringent. Moreover, the protein fraction obtained through air-classification should contain sufficient saponins to detect these off-flavours [7]. Indeed, saponins interact with proteins and are found more easily in the protein fraction than in the starch fraction [8][9]. Concerning another pea study, soyasaponin βb and DDMP soyasaponin (also called soyasaponin βg, soyasaponin VI or chromosaponin I) were extracted and sensorially evaluated by thirteen trained panellists. The bitterness DT of soyasaponin βb is approximately 8 mg/L in water, and a mixture of soyasaponin βb and DDMP soyasaponin in a ratio of 1:4 is less than 2 mg/L in water [10]. Indeed, DDMP soyasaponin is degraded into soyasaponin βb + maltol at temperatures greater than 30 °C, which makes it difficult to extract and purify [10][11][12][13]. Maltol has a caramel-like odour and a sweet taste that can modify the flavour. Moreover, DDMP soyasaponin is also degraded by lipoxygenase during grinding due to an important amount of dioxygen. The peroxidation of DDMP soyasaponin leads to soyasaponin βb, whereas dehydrogenation leads to dehydrosoyasaponin I [10][12]. The degradation process is presented in Figure 2. The effect of soyasaponin βb on the bitterness and astringency of pea protein isolate has been reported by calculating dose–over-threshold factors (the ratio of compound concentration over bitter/astringency threshold for each compound). The factor for astringency is 1.8 and 0.7 for bitterness, suggesting that soyasaponin I is more involved in astringency than in bitter taste [14]. However, DDMP soyasaponin has not been detected and has probably been degraded due to high-temperature extraction, which can reduce the bitter intensity. In another study, the areas of the main phytochemical compounds identified through ultrahigh-performance liquid chromatography–diode array detector–mass spectrometry (UHPLC-DAD-MS) of pea-based samples were correlated with sensory attributes to model bitterness and astringency (prediction). According to the modelling, saponin B, saponin derivates, and soyasapogenol B are not involved in bitterness, whereas they contribute to astringency [15]. However, the DDMP soyasaponin has a priori not been detected in these samples (absence of a peak with a mass of approximatively 1067 g/mol [3][4]), probably due to the high-temperature extraction used (40 °C) that degrades it into soyasaponin βb and/or soyasaponin E [12]. In soybeans, different saponin fractions have been extracted and sensorially characterised. Soyasaponins A, B, and E have bitter characteristics and exhibit 10−5, 5.10−4 and 10−3 mM taste threshold values, respectively. Aglycones A and E are slightly bitter and present a lower threshold value. An astringent perception has been identified in soyasaponin A and soyasapogenol B [16]. Soybean extracts with different soyasaponin βb concentrations exhibit the same off-flavour intensities. The DDMP saponin (and malonyl-β-glucoside isoflavones) should contribute more to bitterness and astringency than the other saponins and isoflavones identified in these extracts [17]. Although the sensory contribution of saponins has only been studied in soybeans and peas, it is important to note that DDMP soyasaponin and soyasaponin βb have been identified in other pulses including adzuki beans, common beans, lentils, chickpeas, lupins and broad beans [3][4][18]. However, the level of saponins in lupin seeds is very low, suggesting no significant contribution to bitterness and astringency [19].
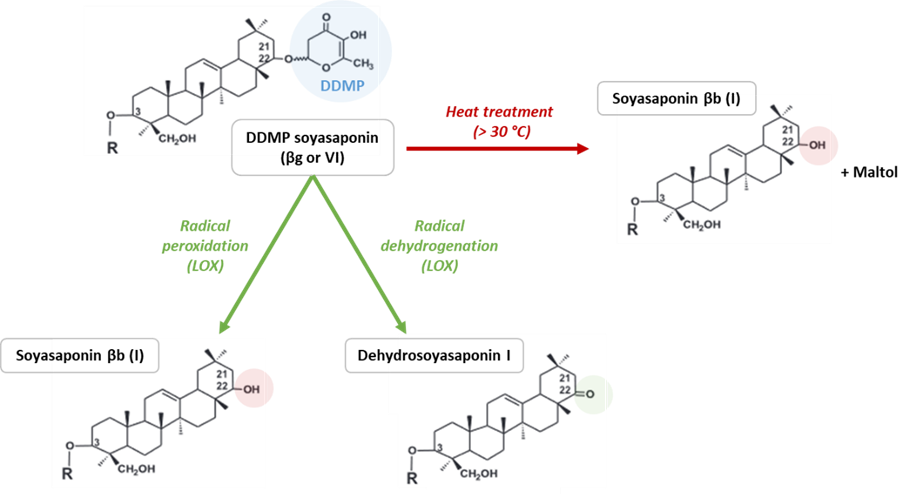
Figure 2. Degradation of DDMP soyasaponin into soyasaponin βb and dehydrosoyasaponin I through heat treatment and enzymatic reactions (adapted from [12]). LOX: lipoxygenase; R: glucuronic acid-galactose-rhamnose.
Researchers have produced saponin-free pea varieties (the study was focused on DDMP and βb soyasaponins) [11]; however, it would have been interesting to compare the bitter and astringent intensities between wild and mutant cultivars to verify the effect of saponin content on sensory properties. A possible drawback of such an approach is that both saponins and volatile compounds interact with proteins; consequently, the decrease in saponin content may increase protein-volatile compound interactions and impact the odour/aroma of pulses [20]. The 10-day germination of broad beans increases the saponin content [4] and may intensify bitter and astringent perceptions. Finally, the heating of pulses appears to be a simple and efficient strategy to reduce these off-flavours in pulses due to the degradation of the DDMP soyasaponin [12][21][22].
2. Phenolic Compounds
Phenolic compounds are a large class of plant secondary metabolites exhibiting a diversity of structures. They have one or more hydroxyl groups attached directly to the aromatic ring and vary from simple molecules to highly complex polymers [23]. In pulses, many groups have been identified, including phenolic acids (and their derivates), stilbenes, flavonoids, and condensed tannins [24]. Their content in legumes depends on the cultivar, location (including abiotic and biotic stress conditions), and transformation [24][25][26][27][28]. They protect plant tissues against UV (ultraviolet) irradiation and participate in plant defence against herbivores, fungi, and viruses [24]. White-flowered faba beans are associated with low tannin due to breeding. These low-tannin genotypes have been reported to be more susceptible to soil-borne diseases [26]. Moreover, phenolic compounds are distributed differently in the parts of the seed (Table 1). The hulls of chickpeas, faba beans, and lentils contain higher amounts of tannins, whereas the cotyledons are richer in other phenolic compounds, including flavonoids [27][28]. During food processing and storage, plant phenolic compounds are converted to a variety of reaction products that modify the product flavour [24].
Table 1. Concentration (mg/g DM) of phenolic compounds, flavonoids and tannins in different seed parts of pulses (chickpeas, faba beans and lentils).
| Pulses | Concentration (mg/g DM) | Reference | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Whole Seed | Cotyledon | Hull | Embryonic Axe | ||||||||||
| PC | F | T | PC | F | T | PC | F | T | PC | F | T | ||
| Chickpeas | - | - | - | 15.2 | 7.5 | 5.2 | 75.9 | 12.6 | 32.4 | 46.1 | 9.3 | 11.4 | [27] |
| Faba beans | 39.69 | - | 6.85 | 39.17 | - | 7.22 | 22.30 | - | 16.23 | - | - | - | [28] |
| Lentils | 6.30 | - | 1.27 | 4.27 | - | 0.40 | 57.19 | - | 46.27 | - | - | - | [28] |
DM: dry matter; PC: phenolic compounds; F: flavonoids; T: tannins.
2.1. Phenolic Acids and Derivates
Many phenolic acids and their derivates have been identified in pulses. Phenolic acids contribute to bitterness but more significantly to astringency in wine or corn germ protein flour [35][36][37]. Beans, lentils, and peas exhibit a low concentration of p-hydroxybenzoic acid (0.32–1.0 µg/g), whereas peas are richer in protocatechuic acid (2.06–221 µg/g) and lentils in p-coumaric acid (3.22–3.42 µg/g) [38]. m-Coumaric acid has been detected in faba beans and peas [4][39]. Chickpeas and lentils contain a high amount of gallic acid, and chickpeas are richer in caffeic acid than other pulses [27][40][41]. These six phenolic acids identified in pulses are well-known to be responsible for astringency in wine. Protocatechuic, p-coumaric, m-coumaric, gallic, and caffeic acids exhibit moderate bitterness at a concentration of 2 g/L in water, whereas p-hydroxybenzoic acid is described as being slightly more bitter [36]. These phenolic acids could contribute to pulse bitterness. According to the correlation model, caffeic acid contributes to both bitterness and astringency in peas, with an estimated concentration of 90.7 ng/g [15].
2.2. Stilbenes
Two stilbenes have been identified in faba beans, resveratrol and polydatin [42]. Resveratrol is often produced by plants as a defence against microbial infections, upon exposure to UV and other stresses [43][44]. Bitterness and astringency have only been studied for resveratrol, which is known to contribute to the bitterness and astringency of wine [45]. This molecule is described as bitter at a concentration of 47 mg/L in water [46]. Moreover, cellular tests have shown that resveratrol activates the bitter receptors TAS2R14 and TAS2R39. TAS2R14 is more sensitive to this compound than TAS2R39 [31]. It would have been interesting to quantify resveratrol in faba beans [42] and to compare this concentration with the cellular test results [31] to determine its involvement in bitterness.
2.3. Flavonoids
Flavonoids are the largest group of phenolic compounds. Some flavonols, flavanols, flavones, flavanones, and isoflavones are identified as astringent and/or bitter (sensory and in vitro tests), which may imply their potential impact on pulse off-flavours. The structures of these flavonoids are presented in Figure 3.
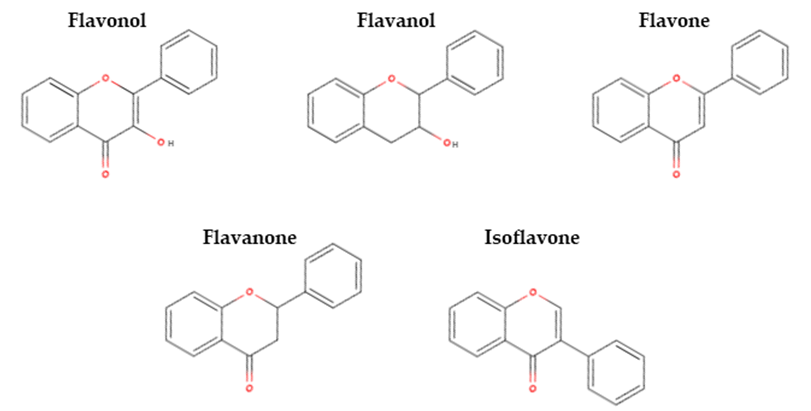
Figure 3. Chemical structures of the flavonoids (adapted from [24]).
-
Flavonols
Flavonols have a 3-hydroxyflavone backbone according to the place and number of OH groups. Kaempferol, quercetin and myricetin activate TAS2R14 and TAS2R39. Kaempferol exhibits lower AT (8 µM for TAS2R14 and 0.5 µM for TAS2R39) than myricetin (250 µM for TAS2R14 and 0.5 µM for TAS2R39), while quercetin leads to ambiguous activation at 500 µM [31]. Chickpeas contain a high content of these three flavonols (5.5–97.5 µg of kaempferol/g dry matter (DM)), but the variation in concentration depends on genotype, location, and seed sample (whole, dehulled, seed coat, and embryonic axe) [27][40]. Adzuki beans are also rich in quercetin (36.2 µg/g) [47]. In addition, many derivates from these three flavonols have also been identified in legumes [15][23][48][49][50]. In particular, quercetin-3-O-glucoside, quantified in pea flour at a concentration of 14.8 ng/g, was found to contribute to pea flour astringency according to the correlation model [15].
-
Flavanols
Flavanols are isomers of (+)-catechin and/or (+)-gallocatechin, and participate in the formation of condensed tannins. Several flavanols have been detected in pulses, some of which have been characterised as bitter and astringent, such as (+)-catechin, (-)-epicatechin, (-)-epigallocatechin, (-)-epicatechin gallate, (-)-epigallocatechin gallate and theaflavin [34][36][51][52]. Beans and chickpeas exhibit higher concentrations of (+)-catechin, whereas faba beans have high concentrations of (-)-epicatechin gallate and (-)-epigallocatechin gallate [40][41][53][54][55].These six flavanols identified in pulses are described as astringent but exhibit different DTs between 16 and 930 µmol/L. Theaflavin, only detected in faba beans, has a very low DT (16 µmol/L) compared to (+)-catechin (410 µmol/L) and (-)-epicatechin (930 µmol/L) [52]. Their sensory description suggests their involvement in the astringent characteristics of pulses.
The activation of bitter receptors by these flavanols has been studied. The receptor TAS2R39 is activated by all the flavanols identified in pulses. According to different studies, the AT and EC50 are different for (-)-epicatechin, (-)-epicatechin gallate, and (-)-epigallocatechin gallate [31][33][34][56][57]. Theaflavin exhibits a very low EC50 (2.79 µM) compared to (-)-epicatechin gallate (21.3 µM) and (-)-epigallocatechin gallate (112 µM) [57]. The same ranking was observed in another study but with a higher EC50, 88.2 µM for (-)-epicatechin gallate and 181.6 µM for (-)-epigallocatechin gallate [56]. Moreover, (-)-epigallocatechin and (-)-epicatechin exhibit higher EC50 values for TAS2R39 [33][56]. Concerning TAS2R14, (-)-epicatechin and theaflavin have not activated this receptor, in contrast to TAS2R39 [57]. Flavonoids are made of two to three OH groups, which should be involved in hydrogen bonds with TAS2R39. Moreover, the receptor binding pocket of TAS2R39 exhibits an additional acceptor site compared to TAS2R14, which could explain its high affinity [31]. (-)-Epigallocatechin gallate exhibits a higher TAS2R4 and TAS2R5 AT than (-)-epicatechin [33][34]. Finally, (-)-epigallocatechin gallate activates both TAS2R30 and TAS2R43 [34]. This flavanol should be more involved in pulse off-flavours due to the activation of many receptors and low AT compared to the other compounds. Further studies on a wider range of flavanols should allow a better overview of bitter taste receptor activation by these compounds.
-
Flavones
Only three flavones have been detected in pulses, for which the activation of TAS2Rs has been studied. Chrysin has been identified in adzuki beans (0.00–0.09 g/kg) and faba beans [42][54][58], and activates TAS2R14 and TAS2R39 at concentrations of 63 µM and 16 µM, respectively [31]. 7,4′-Dihydroxyflavone, detected in faba beans, activates TAS2R14 at a lower threshold (16 µM) than chrysin, whereas it is higher for TAS2R39 (125 µM) [31][58]. Finally, luteolin exhibits very low AT for these two receptors in comparison with the previous flavones: 2 µM for TAS2R14 and 0.5 µM for TAS2R39 [31]. Pea flour contains 81.7 ng/g luteolin, but this compound does not contribute to bitterness and astringency according to the correlation model [15]. The concentrations of luteolin and caffeic acid are similar in pea flour, although only caffeic acid contributes to its bitterness and astringency [15]. One explanation is that the bitter receptor threshold activation of caffeic acid was lower than that of luteolin. However, this remains to be demonstrated.
-
Flavanones
Roland et al. (2013) [31] have shown the activation of TAS2R14 and TAS2R39 by two flavanones, pinocembrin and naringenin. The TA and EC50 are similar for both molecules and bitter receptors [31]. Pinocembrin has been identified in common beans and faba beans, whereas lentils contain naringenin [42][53][59].
-
Isoflavones
Three isoflavones, daidzein, formononetin, and genistein, have been identified in beans, chickpeas, faba beans, lentils, and peas. The extracts of daidzein and genistein from soybeans are described as slightly bitter and astringent by a trained panel [16]. These isoflavones are involved in the bitter taste and astringency of soybeans due to their very high content compared to other legumes [60][61]. Genistein (extracted from soybeans) has AT for TAS2R14 and TAS2R39 at concentrations of 4 and 8 µM, respectively [32], and these ATs are similar to naringenin (flavanone) [31]. These low threshold values could explain the important role of genistein in the perception of soybean bitterness. However, the relationship between the AT (receptor level) and DT (sensory level) has never been demonstrated for this compound. Moreover, daidzein and formononetin (also extracted from soybeans) also activate these two receptors at a higher concentration (500 µM) [32]. The number and positions of hydroxyl groups should be an important parameter for TAS2R activation [31][34]. Indeed, genistein exhibits three OH groups whereas formononetin and daidzein have one and two hydroxyl groups. However, chickpeas, soybeans, and peas are more concentrated in daidzein than in genistein. These two isoflavones could be equally involved in the bitter sensation of these pulses (balance between concentration and AT). In addition, malonyl-β-glucosides such as malonyl daidzein, malonyl glycitin and malonyl genistein have been identified in soybean flakes [17]. These are derived from the malonylation of β-glucosides [62]. These malonyl-β-glucosides should contribute as much to soybean bitterness and astringency as DDMP saponin and more than the other saponins and isoflavones [17]. Currently, none of these malonyl-β-glucosides have been identified in beans, chickpeas, faba beans, lentils and peas. Finally, heat treatment reduces the isoflavone content, whereas germination increases it [63][64].
2.4. Condensed Tannins
Condensed tannins are oligomers or polymers composed of derivates from (+)-catechin and its isomers. Unlike hydrolysable tannins, they are resistant to hydrolysis and are degraded using chemical treatments [65]. Prodelphinidins and procyanidins have been identified in pulses. Hulls contain higher concentrations of these condensed tannins than cotyledons [27][28][66]. These compounds may be responsible for bitterness and astringency in grapes and wines [67]. In lupin, condensed tannins may be more involved in bitterness than flavanols and alkaloids [19]. Moreover, the evaluation of the bitter and astringent intensities of low- and high-tannin faba beans would have made it possible to verify their involvement [26].
Procyanidins B4 and C2 are described as astringent and bitter at a concentration of 0.9 g/L in aqueous ethanol [51]. Dimers and trimers of procyanidins are more astringent than monomers ((+)-catechin and (-)-epicatechin) [51]. The astringent DT of (-)-epicatechin is five times higher than that of procyanidin B2 [35]. Hufnagel and Hofmann (2008) suggested that the more polymerised the molecules are, the more bitter they are, as shown by the ranking obtained according to the intensity of bitterness perceived in wines: procyanidin B1 and C1 > procyanidin B2 > procyanidin B3 > (-)-epicatechin > (+)-catechin [35]. Indeed, a similar ranking of TAS2R5 receptor DTs has been established [33]. Conversely, Peleg et al. (1999) demonstrated using sensory analysis that the more polymerised the molecules are, the less bitter they are. In wine, (-)-epicatechin is more bitter than (+)-catechin, which is more bitter than procyanidin trimers [51]. These contradictory results can be explained by the presence of ethanol in the wines, which increases the intensity of the bitter perception in the mouth. Indeed, Fischer and Noble (1994) have shown that an increase of 3% (v/v) ethanol in wine is equivalent to an increase in bitterness (+50%) caused by the addition of 1400 mg/L catechin [68]. However, it is not possible to verify these results with bitter receptors in vitro in the presence of ethanol, as this molecule is 1% toxic to cells. Some sensorial results are therefore consistent with those obtained via cell tests: the degree of polymerisation of these phenolic compounds should increase the intensity of bitterness. (+)-Epicatechin activates TAS2R4, TAS2R5 and TAS2R39 from a concentration above 1000 µM, while procyanidin C2 (trimer) activates TAS2R5 from 30 µM [33]. Roland et al. (2011) suggest that a molecule with many hydroxyl groups could have a better affinity for TAS2R5 [32]. Indeed, dimers (procyanidins B) and trimers (procyanidins C) have more OH groups than monomers. The ability of seven procyanidins (five dimers and two trimers also identified in pulses) to activate the 25 TAS2Rs has been tested [33][34]. Only TAS2R5, TAS2R7 and TAS2R39 are activated by at least one procyanidin. Procyanidins B2, B3, and C1 did not activate the 25 TAS2Rs at the tested concentrations. TAS2R7 is only activated by procyanidin B1, and TAS2R39 is activated by procyanidin B2g. In addition, TAS2R5 is activated by procyanidins B1, B2g, B4 and C2. Procyanidin B2g exhibits the lowest EC50 followed by procyanidins C2 and then B1. However, the EC50 of dimer B4 has not been determined due to the observation of unspecific responses in the control condition (mock) [33][34]. These results suggest a role for condensed tannins, especially procyanidins, in the bitterness and astringency of pulses. However, it would be interesting to investigate the potential bitter taste and astringency of prodelphinidins.
3. Alkaloids
Some alkaloids contribute to the bitterness of food products such as caffeine [69]. Approximatively sixteen alkaloids have been detected in different lupin varieties and could be partially responsible for their bitterness. They are distributed in the quinolizidine, indole, and piperidine classes [19][70][71]. For example, lupanine is the most abundant alkaloid in white and narrow-leafed lupins and sparteine in yellow lupins [71]. However, quinolizidine alkaloids are considered human antinutritional factors due to neurological, cardiovascular, and gastrointestinal disturbances [71][72]. Lupins are classified into two varieties: the “bitter” and the “sweet” which differ in their alkaloid content [19][73]. DuPont et al. established the relationship between the bitter intensity of milled lupins and their alkaloid content [19]. The bitter mean scores of the “bitter” varieties are higher than those of the “sweet” ones (7.8 and 2.0 over 10, respectively). The “sweet” varieties exhibited 0.1 mg/g DM of mean alkaloids compared to 15.0 mg/g DM for the “bitter” varieties. Concerning the “bitter” varieties, the lupin evaluated as the least bitter contains 4.8 mg/g (dry matter) of alkaloids, including lupinine and gramine, whereas the one with the highest bitter intensity contains 26.9 mg/g (dry matter) composed of sparteine, lupanine and 13-hydroxylupanine. This research has highlighted the role of alkaloids in lupin bitterness; however, the authors suggest that intense bitterness in “bitter” varieties could also be attributed to the presence of tannins [19]. Moreover, treatments to eliminate alkaloids in lupin are called “debittering treatments” [70].
Faba bean is another pulse containing two alkaloids, vicine and convicine [74][75]. These molecules are pyrimidine glucosides and cause favism in people who express a genetically inherited glucose-6-phosphate dehydrogenase (G6PD) deficiency; they are considered antinutritional factors [26][76][77]. Such as lupins, there are new cultivars of faba bean breeding lines with a significantly lower amount of alkaloids; the reduced level of vicine and convicine can considerably vary among cultivars [26][76]. However, the sensorial aspect of these molecules has never been studied. It would be interesting to compare the bitter intensity of the high- and low-vicine/convicine cultivars. A study correlated the flavour-related components and the sensorial attributes of faba bean flour, concentrate and isolate using partial least squares (PLS) regression. Bitterness is related to vicine and convicine, although other compounds, including free phenolic compounds and amino acids (phenylalanine, tryptophan, and histidine), also contribute [78].
Thus, the main disadvantages of alkaloids are their antinutritional effect and their potential contribution to bitterness of lupins and faba beans. However, the main advantage of these secondary metabolites is their involvement in the plant mechanism, which limits herbivory attacks and ensures harvest yields [79][80]. For example, the low vicine and convicine faba bean genotypes are more sensitive to bruchid attack [26]. These compounds are beneficial for the plant in the field and can be eliminated after harvesting by many strategies including cooking, soaking, germination, and fermentation [70][77][81][82].
References
- Oleszek, M.; Oleszek, W. Saponins in Food. In Handbook of Dietary Phytochemicals; Xiao, J., Sarker, S.D., Asakawa, Y., Eds.; Springer: Singapore, 2020; pp. 1–40. ISBN 9789811317453.
- Sparg, S.G.; Light, M.E.; van Staden, J. Biological Activities and Distribution of Plant Saponins. J. Ethnopharmacol. 2004, 94, 219–243.
- Ha, T.J.; Lee, B.W.; Park, K.H.; Jeong, S.H.; Kim, H.-T.; Ko, J.-M.; Baek, I.-Y.; Lee, J.H. Rapid Characterisation and Comparison of Saponin Profiles in the Seeds of Korean Leguminous Species Using Ultra Performance Liquid Chromatography with Photodiode Array Detector and Electrospray Ionisation/Mass Spectrometry (UPLC–PDA–ESI/MS) Analysis. Food Chem. 2014, 146, 270–277.
- Mekky, R.H.; Thabet, M.M.; Rodríguez-Pérez, C.; Elnaggar, D.M.Y.; Mahrous, E.A.; Segura-Carretero, A.; Abdel-Sattar, E. Comparative Metabolite Profiling and Antioxidant Potentials of Seeds and Sprouts of Three Egyptian Cultivars of Vicia Faba L. Food Res. Int. 2020, 136, 109537.
- Oomah, B.D.; Patras, A.; Rawson, A.; Singh, N.; Compos-Vega, R. 2-Chemistry of Pulses. In Pulse Foods; Tiwari, B.K., Gowen, A., McKenna, B., Eds.; Academic Press: San Diego, CA, USA, 2011; pp. 9–55. ISBN 978-0-12-382018-1.
- Krishnamurthy, P.; Tsukamoto, C.; Singh, R.J.; Lee, J.-D.; Kim, H.-S.; Yang, S.-H.; Chung, G. The Sg-6 Saponins, New Components in Wild Soybean (Glycine Soja Sieb. and Zucc.): Polymorphism, Geographical Distribution and Inheritance. Euphytica 2014, 198, 413–424.
- Price, K.R.; Griffiths, N.M.; Curl, C.L.; Fenwick, G.R. Undesirable Sensory Properties of the Dried Pea (Pisum Sativum). The Rôle of Saponins. Food Chem. 1985, 17, 105–115.
- Ikedo, S.; Shimoyamada, M.; Watanabe, K. Interaction between Bovine Serum Albumin and Saponin As Studied by Heat Stability and Protease Digestion. J. Agric. Food Chem. 1996, 44, 792–795.
- Fenwick, D.E.; Oakenfull, D. Saponin Content of Food Plants and Some Prepared Foods. J. Sci. Food Agric. 1983, 34, 186–191.
- Heng, L.; Vincken, J.-P.; van Koningsveld, G.; Legger, A.; Gruppen, H.; van Boekel, T.; Roozen, J.; Voragen, F. Bitterness of Saponins and Their Content in Dry Peas. J. Sci. Food Agric. 2006, 86, 1225–1231.
- Vernoud, V.; Lebeigle, L.; Munier, J.; Marais, J.; Sanchez, M.; Pertuit, D.; Rossin, N.; Darchy, B.; Aubert, G.; Le Signor, C.; et al. β-Amyrin Synthase1 Controls the Accumulation of the Major Saponins Present in Pea (Pisum Sativum). Plant Cell Physiol. 2021, 62, 784–797.
- Chitisankul, W.T.; Shimada, K.; Omizu, Y.; Uemoto, Y.; Varanyanond, W.; Tsukamoto, C. Mechanism of DDMP-Saponin Degradation and Maltol Production in Soymilk Preparation. LWT 2015, 64, 197–204.
- Daveby, Y.D.; Åman, P.; Betz, J.M.; Musser, S.M. Effect of Storage and Extraction on Ratio of Soyasaponin I to 2,3-Dihydro-2,5-Dihydroxy-6-Methyl-4-Pyrone-Conjugated Soyasaponin I in Dehulled Peas (Pisum Sativum L). J. Sci. Food Agric. 1998, 78, 141–146.
- Gläser, P.; Dawid, C.; Meister, S.; Bader-Mittermaier, S.; Schott, M.; Eisner, P.; Hofmann, T. Molecularization of Bitter Off-Taste Compounds in Pea-Protein Isolates (Pisum Sativum L.). J. Agric. Food Chem. 2020, 68, 10374–10387.
- Cosson, A.; Meudec, E.; Ginies, C.; Danel, A.; Lieben, P.; Descamps, N.; Cheynier, V.; Saint-Eve, A.; Souchon, I. Identification and Quantification of Key Phytochemicals in Peas—Linking Compounds with Sensory Attributes. Food Chem. 2022, 385, 132615.
- Okubo, K.; Iijima, M.; Kobayashi, Y.; Yoshikoshi, M.; Uchida, T.; Kudou, S. Components Responsible for the Undesirable Taste of Soybean Seeds. Biosci. Biotechnol. Biochem. 1992, 56, 99–103.
- Aldin, E.; Reitmeier, H.A.; Murphy, P. Bitterness of Soy Extracts Containing Isoflavones and Saponins. J. Food Sci. 2006, 71, S211–S215.
- Donat, P.V.; Caprioli, G.; Conti, P.; Maggi, F.; Ricciutelli, M.; Torregiani, E.; Vittori, S.; Sagratini, G. Rapid Quantification of Soyasaponins I and Βg in Italian Lentils by High-Performance Liquid Chromatography (HPLC)–Tandem Mass Spectrometry (MS/MS). Food Anal. Methods 2014, 7, 1024–1031.
- Dupont, M.S.; Muzquiz, M.; Estrella, I.; Fenwick, G.R.; Price, K.R. Relationship between the Sensory Properties of Lupin Seed with Alkaloid and Tannin Content. J. Sci. Food Agric. 1994, 65, 95–100.
- Heng, L. Flavour Aspects of Pea and Its Protein Preparations in Relation to Novel Protein Foods. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2005.
- Heng, L.; Vincken, J.-P.; Hoppe, K.; van Koningsveld, G.A.; Decroos, K.; Gruppen, H.; van Boekel, M.A.J.S.; Voragen, A.G.J. Stability of Pea DDMP Saponin and the Mechanism of Its Decomposition. Food Chem. 2006, 99, 326–334.
- Martínez Noguera, P.; Lantoine, J.; Roux, E.L.; Yang, S.; Jakobi, R.; Krause, S.; Saint-Eve, A.; Bonazzi, C.; Rega, B. Saponins from Pea Ingredients to Innovative Sponge Cakes and Their Association with Perceived Bitterness. Foods 2022, 11, 2919.
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic Composition and Antioxidant Potential of Grain Legume Seeds: A Review. Food Res. Int. 2017, 101, 1–16.
- Cheynier, V. Phenolic Compounds: From Plants to Foods. Phytochem. Rev. 2012, 11, 153–177.
- Johnson, J.B.; Skylas, D.J.; Mani, J.S.; Xiang, J.; Walsh, K.B.; Naiker, M. Phenolic Profiles of Ten Australian Faba Bean Varieties. Molecules 2021, 26, 4642.
- Duc, G. Faba Bean (Vicia Faba L.). Field Crops Res. 1997, 53, 99–109.
- Sreerama, Y.N.; Sashikala, V.B.; Pratape, V.M. Variability in the Distribution of Phenolic Compounds in Milled Fractions of Chickpea and Horse Gram: Evaluation of Their Antioxidant Properties. J. Agric. Food Chem. 2010, 58, 8322–8330.
- Boudjou, S.; Oomah, B.D.; Zaidi, F.; Hosseinian, F. Phenolics Content and Antioxidant and Anti-Inflammatory Activities of Legume Fractions. Food Chem. 2013, 138, 1543–1550.
- Roland, W.S.U.; Pouvreau, L.; Curran, J.; van de Velde, F.; de Kok, P.M.T. Flavor Aspects of Pulse Ingredients. Cereal Chem. 2017, 94, 58–65.
- Amarowicz, R.; Troszyńska, A.; Baryłko-Pikielna, N.; Shahidi, F. Polyphenolics Extracts from Legume Seeds: Correlations Between Total Antioxidant Activity, Total Phenolics Content, Tannins Content and Astringency. J. Food Lipids 2004, 11, 278–286.
- Roland, W.S.U.; van Buren, L.; Gruppen, H.; Driesse, M.; Gouka, R.J.; Smit, G.; Vincken, J.-P. Bitter Taste Receptor Activation by Flavonoids and Isoflavonoids: Modeled Structural Requirements for Activation of HTAS2R14 and HTAS2R39. J. Agric. Food Chem. 2013, 61, 10454–10466.
- Roland, W.S.U.; Vincken, J.-P.; Gouka, R.J.; van Buren, L.; Gruppen, H.; Smit, G. Soy Isoflavones and Other Isoflavonoids Activate the Human Bitter Taste Receptors HTAS2R14 and HTAS2R39. J. Agric. Food Chem. 2011, 59, 11764–11771.
- Soares, S.; Kohl, S.; Thalmann, S.; Mateus, N.; Meyerhof, W.; De Freitas, V. Different Phenolic Compounds Activate Distinct Human Bitter Taste Receptors. J. Agric. Food Chem. 2013, 61, 1525–1533.
- Soares, S.; Silva, M.S.; García-Estevez, I.; Groβmann, P.; Brás, N.; Brandão, E.; Mateus, N.; de Freitas, V.; Behrens, M.; Meyerhof, W. Human Bitter Taste Receptors Are Activated by Different Classes of Polyphenols. J. Agric. Food Chem. 2018, 66, 8814–8823.
- Hufnagel, J.C.; Hofmann, T. Quantitative Reconstruction of the Nonvolatile Sensometabolome of a Red Wine. J. Agric. Food Chem. 2008, 56, 9190–9199.
- Ferrer-Gallego, R.; Hernández-Hierro, J.M.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Sensory Evaluation of Bitterness and Astringency Sub-Qualities of Wine Phenolic Compounds: Synergistic Effect and Modulation by Aromas. Food Res. Int. 2014, 62, 1100–1107.
- Huang, C.J.; Zayas, J.F. Phenolic Acid Contributions to Taste Characteristics of Corn Germ Protein Flour Products. J. Food Sci. 1991, 56, 1308–1310.
- López-Amorós, M.L.; Hernández, T.; Estrella, I. Effect of Germination on Legume Phenolic Compounds and Their Antioxidant Activity. J. Food Compos. Anal. 2006, 19, 277–283.
- Troszyńska, A.; Amarowicz, R.; Lamparski, G.; Wołejszo, A.; Baryłko-Pikielna, N. Investigation of Astringency of Extracts Obtained from Selected Tannins-Rich Legume Seeds. Food Qual. Prefer. 2006, 17, 31–35.
- Quintero-Soto, M.F.; Saracho-Peña, A.G.; Chavez-Ontiveros, J.; Garzon-Tiznado, J.A.; Pineda-Hidalgo, K.V.; Delgado-Vargas, F.; Lopez-Valenzuela, J.A. Phenolic Profiles and Their Contribution to the Antioxidant Activity of Selected Chickpea Genotypes from Mexico and ICRISAT Collections. Plant Foods Hum. Nutr. 2018, 73, 122–129.
- Giusti, F.; Capuano, E.; Sagratini, G.; Pellegrini, N. A Comprehensive Investigation of the Behaviour of Phenolic Compounds in Legumes during Domestic Cooking and in Vitro Digestion. Food Chem. 2019, 285, 458–467.
- Abu-Reidah, I.; Contreras, M.d.M.; Arráez-Román, D.; Fernández-Gutiérrez, A.; Segura Carretero, A. UHPLC-ESI-QTOF-MS Based Metabolic Profiling of Vicia Faba L. Fabaceae) Seeds as a Key Strategy for Characterization in Foodomics. Electrophoresis 2014, 35, 1571–1581.
- Pandey, R.P.; Parajuli, P.; Shin, J.Y.; Lee, J.; Lee, S.; Hong, Y.-S.; Park, Y.I.; Kim, J.S.; Sohng, J.K. Enzymatic Biosynthesis of Novel Resveratrol Glucoside and Glycoside Derivatives. Appl. Environ. Microbiol. 2014, 80, 7235–7243.
- Poklar Ulrih, N.; Opara, R.; Skrt, M.; Košmerl, T.; Wondra, M.; Abram, V. Part I. Polyphenols Composition and Antioxidant Potential during ‘Blaufränkisch’ Grape Maceration and Red Wine Maturation, and the Effects of Trans-Resveratrol Addition. Food Chem. Toxicol. 2020, 137, 111122.
- Sahar, A.; ur Rahman, U.; Ishaq, A.; Munir, M.S.; Aadil, R.M. 12-Health-Promoting Perspectives of Fruit-Based Functional Energy Beverages. In Sports and Energy Drinks; Grumezescu, A.M., Holban, A.M., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 399–439. ISBN 978-0-12-815851-7.
- Koga, C.C.; Becraft, A.R.; Lee, Y.; Lee, S.-Y. Taste Detection Thresholds of Resveratrol. J. Food Sci. 2015, 80, S2064–S2070.
- Amarowicz, R.; Estrella, I.; Hernández, T.; Troszyńska, A. Antioxidant Activity of Extract of Adzuki Bean and Its Fractions. J. Food Lipids 2008, 15, 119–136.
- Dueñas, M.; Sarmento, T.; Aguilera, Y.; Benitez, V.; Mollá, E.; Esteban, R.M.; Martín-Cabrejas, M.A. Impact of Cooking and Germination on Phenolic Composition and Dietary Fibre Fractions in Dark Beans (Phaseolus Vulgaris L.) and Lentils (Lens Culinaris L.). LWT 2016, 66, 72–78.
- Dueñas, M.; Martínez-Villaluenga, C.; Limón, R.I.; Peñas, E.; Frias, J. Effect of Germination and Elicitation on Phenolic Composition and Bioactivity of Kidney Beans. Food Res. Int. 2015, 70, 55–63.
- Liu, R.; Cai, Z.; Xu, B. Characterization and Quantification of Flavonoids and Saponins in Adzuki Bean (Vigna Angularis L.) by HPLC–DAD–ESI–MSn Analysis. Chem. Cent. J. 2017, 11, 93.
- Peleg, H.; Gacon, K.; Schlich, P.; Noble, A.C. Bitterness and Astringency of Flavan-3-ol Monomers, Dimers and Trimers. J. Sci. Food Agric. 1999, 79, 1123–1128.
- Scharbert, S.; Holzmann, N.; Hofmann, T. Identification of the Astringent Taste Compounds in Black Tea Infusions by Combining Instrumental Analysis and Human Bioresponse. J. Agric. Food Chem. 2004, 52, 3498–3508.
- Aguilera, Y.; Estrella, I.; Benitez, V.; Esteban, R.M.; Martín-Cabrejas, M.A. Bioactive Phenolic Compounds and Functional Properties of Dehydrated Bean Flours. Food Res. Int. 2011, 44, 774–780.
- Sangsukiam, T.; Duangmal, K. Changes in Bioactive Compounds and Health-Promoting Activities in Adzuki Bean: Effect of Cooking Conditions and in Vitro Simulated Gastrointestinal Digestion. Food Res. Int. 2022, 157, 111371.
- García-Lafuente, A.; Moro, C.; Manchón, N.; Gonzalo-Ruiz, A.; Villares, A.; Guillamón, E.; Rostagno, M.; Mateo-Vivaracho, L. In Vitro Anti-Inflammatory Activity of Phenolic Rich Extracts from White and Red Common Beans. Food Chem. 2014, 161, 216–223.
- Narukawa, M.; Noga, C.; Ueno, Y.; Sato, T.; Misaka, T.; Watanabe, T. Evaluation of the Bitterness of Green Tea Catechins by a Cell-Based Assay with the Human Bitter Taste Receptor HTAS2R39. Biochem. Biophys. Res. Commun. 2011, 405, 620–625.
- Yamazaki, T.; Sagisaka, M.; Ikeda, R.; Nakamura, T.; Matsuda, N.; Ishii, T.; Nakayama, T.; Watanabe, T. The Human Bitter Taste Receptor HTAS2R39 Is the Primary Receptor for the Bitterness of Theaflavins. Biosci. Biotechnol. Biochem. 2014, 78, 1753–1756.
- Valente, I.M.; Cabrita, A.R.J.; Malushi, N.; Oliveira, H.M.; Papa, L.; Rodrigues, J.A.; Fonseca, A.J.M.; Maia, M.R.G. Unravelling the Phytonutrients and Antioxidant Properties of European Vicia Faba L. Seeds. Food Res. Int. 2019, 116, 888–896.
- Mirali, M.; Ambrose, S.J.; Wood, S.A.; Vandenberg, A.; Purves, R.W. Development of a Fast Extraction Method and Optimization of Liquid Chromatography–Mass Spectrometry for the Analysis of Phenolic Compounds in Lentil Seed Coats. J. Chromatogr. B 2014, 969, 149–161.
- Belitz, H.; Wieser, H. Bitter Compounds: Occurrence and Structure-activity Relationships. Food Rev. Int. 1985, 1, 271–354.
- Huang, A.-S.; Hsieh, O.A.-L.; Chang, S.S. Characterization of the Nonvolatile Minor Constituents Responsible for the Objectionable Taste of Defatted Soybean Flour. J. Food Sci. 1982, 47, 19–23.
- Yang, S.-E.; Lien, J.-C.; Tsai, C.-W.; Wu, C.-R. Therapeutic Potential and Mechanisms of Novel Simple O-Substituted Isoflavones against Cerebral Ischemia Reperfusion. Int. J. Mol. Sci. 2022, 23, 10394.
- Liggins, J.; Bluck, L.J.C.; Runswick, S.; Atkinson, C.; Coward, W.A.; Bingham, S.A. Daidzein and Genistein Contents of Vegetables. Br. J. Nutr. 2000, 84, 717–725.
- Guajardo-Flores, D.; García-Patiño, M.; Serna-Guerrero, D.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Characterization and Quantification of Saponins and Flavonoids in Sprouts, Seed Coats and Cotyledons of Germinated Black Beans. Food Chem. 2012, 134, 1312–1319.
- Sarni-Manchado, P.; Cheynier, V. Les Polyphénols en Agroalimentaire; Éditions Tec & Doc: Paris, France, 2006; ISBN 978-2-7430-0805-5.
- Mattila, P.H.; Pihlava, J.-M.; Hellström, J.; Nurmi, M.; Eurola, M.; Mäkinen, S.; Jalava, T.; Pihlanto, A. Contents of Phytochemicals and Antinutritional Factors in Commercial Protein-Rich Plant Products. Food Qual. Prefer 2018, 2, 213–219.
- Brossaud, F.; Cheynier, V.; Noble, A.C. Bitterness and Astringency of Grape and Wine Polyphenols. Aust. J. Grape Wine Res. 2001, 7, 33–39.
- Fischer, U.; Noble, A. The Effect of Ethanol, Catechin Concentration, and PH on Sourness and Bitterness of Wine. Am. J. Enol. Vitic. 1994, 45, 6–10.
- Briand, L.; Salles, C. Taste Perception and Integration. In Flavor from Food to Behaviors, Wellbeing and Health; Elsevier Ltd.: Duxford, UK, 2016; pp. 101–119. ISBN 978-0-08-100295-7.
- Cristian, J.-M.; Rosalva, M.-E.; Anaberta, C.M.; Mercedes, M.; Mercedes, M.P.; Gloria, D.-O. Effect of Aqueous, Acid, and Alkaline Thermal Treatments on Antinutritional Factors Content and Protein Quality in Lupinus Campestris Seed Flour. J. Agric. Food Chem. 2010, 58, 1741–1745.
- Magalhães, S.C.Q.; Fernandes, F.; Cabrita, A.R.J.; Fonseca, A.J.M.; Valentão, P.; Andrade, P.B. Alkaloids in the Valorization of European Lupinus Spp. Seeds Crop. Ind. Crops Prod. 2017, 95, 286–295.
- Frick, K.M.; Foley, R.C.; Kamphuis, L.G.; Siddique, K.H.M.; Garg, G.; Singh, K.B. Characterization of the Genetic Factors Affecting Quinolizidine Alkaloid Biosynthesis and Its Response to Abiotic Stress in Narrow-Leafed Lupin (Lupinus Angustifolius L.). Plant Cell Environ. 2018, 41, 2155–2168.
- Sbihi, H.M.; Nehdi, I.A.; Tan, C.P.; Al-Resayes, S.I. Bitter and Sweet Lupin (Lupinus Albus L.) Seeds and Seed Oils: A Comparison Study of Their Compositions and Physicochemical Properties. Ind. Crops Prod. 2013, 49, 573–579.
- Pulkkinen, M.; Gautam, M.; Lampi, A.-M.; Ollilainen, V.; Stoddard, F.; Sontag-Strohm, T.; Salovaara, H.; Piironen, V. Determination of Vicine and Convicine from Faba Bean with an Optimized High-Performance Liquid Chromatographic Method. Food Res. Int. 2015, 76, 168–177.
- Duc, G.; Marget, P.; Esnault, R.; Guen, J.L.; Bastianelli, D. Genetic Variability for Feeding Value of Faba Bean Seeds (Vicia Faba): Comparative Chemical Composition of Isogenics Involving Zero-Tannin and Zero-Vicine Genes. J. Agric. Sci. 1999, 133, 185–196.
- Purves, R.W.; Khazaei, H.; Vandenberg, A. Quantification of Vicine and Convicine in Faba Bean Seeds Using Hydrophilic Interaction Liquid Chromatography. Food Chem. 2018, 240, 1137–1145.
- Multari, S.; Stewart, D.; Russell, W.R. Potential of Fava Bean as Future Protein Supply to Partially Replace Meat Intake in the Human Diet. Compr. Rev. Food Sci. Food Saf. 2015, 14, 511–522.
- Tuccillo, F.; Kantanen, K.; Wang, Y.; Martin Ramos Diaz, J.; Pulkkinen, M.; Edelmann, M.; Knaapila, A.; Jouppila, K.; Piironen, V.; Lampi, A.-M.; et al. The Flavor of Faba Bean Ingredients and Extrudates: Chemical and Sensory Properties. Food Res. Int. 2022, 162, 112036.
- Ulyanych, O.; Poltoretskyi, S.; Liubych, V.; Yatsenko, A.; Yatsenko, V.; Lazariev, O.; Kravchenko, V. Effect of Surface Drip Irrigation and Cultivars on Physiological State and Productivity of Faba Bean Crop. J. Agric. Sci. 2021, 32, 139–149.
- Wink, M. Chemical Defense of Lupins. Mollusc-Repellent Properties of Quinolizidine Alkaloids. Z. Naturforsch. C J. Biosci. 1984, 39, 553–558.
- Jiménez-Martínez, C.; Hernández-Sánchez, H.; Dávila-Ortiz, G. Diminution of Quinolizidine Alkaloids, Oligosaccharides and Phenolic Compounds from Two Species of Lupinus and Soybean Seeds by the Effect of Rhizopus Oligosporus. J. Sci. Food Agric. 2007, 87, 1315–1322.
- Abd Allah, M.A.; Foda, Y.H.; Abu Salem, F.M.; Abd Allah, Z.S. Treatments for Reducing Total Vicine in Egyptian Faba Bean (Giza 2 Variety). Plant Food Hum. Nutr. 1988, 38, 201–210.
More
Information
Subjects:
Food Science & Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.2K
Revisions:
4 times
(View History)
Update Date:
20 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




