Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Wei Boon Yap | -- | 3546 | 2023-04-19 13:01:22 | | | |
| 2 | Camila Xu | Meta information modification | 3546 | 2023-04-20 03:05:17 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
A. Rahman, N.A.; Balasubramaniam, V.R.M.T.; Yap, W.B. Activities of Interleukin-12 Family during Virus Infections. Encyclopedia. Available online: https://encyclopedia.pub/entry/43238 (accessed on 03 March 2026).
A. Rahman NA, Balasubramaniam VRMT, Yap WB. Activities of Interleukin-12 Family during Virus Infections. Encyclopedia. Available at: https://encyclopedia.pub/entry/43238. Accessed March 03, 2026.
A. Rahman, Nur Azizah, Vinod R. M. T. Balasubramaniam, Wei Boon Yap. "Activities of Interleukin-12 Family during Virus Infections" Encyclopedia, https://encyclopedia.pub/entry/43238 (accessed March 03, 2026).
A. Rahman, N.A., Balasubramaniam, V.R.M.T., & Yap, W.B. (2023, April 19). Activities of Interleukin-12 Family during Virus Infections. In Encyclopedia. https://encyclopedia.pub/entry/43238
A. Rahman, Nur Azizah, et al. "Activities of Interleukin-12 Family during Virus Infections." Encyclopedia. Web. 19 April, 2023.
Copy Citation
The interleukin (IL)-12 family consists of pro- and anti-inflammatory cytokines that are able to signal the activation of host antiviral immunity while preventing over-reactive immune reactions due to active virus replication and viral clearance.
cytokines
antiviral
IL-12
innate immunity
adaptive immunity
1. Introduction
Disease-causing viruses can be transmitted from persons to persons or from animals to humans, leading to various disease manifestations [1][2]. Generally, there are several mechanisms that support virus entry. Enveloped viruses can enter host cells by receptor-mediated endocytosis and subsequently release their genomes into the cells to initiate genome replication [3]. Endocytosis can occur via clathrin proteins, macropinocytosis and caveolae [4]. Upon viral invasion into vulnerable host cells, the host immune response is activated, particularly the synthesis and release of pro-inflammatory cytokines such as interleukin (IL)-8 and IL-6 at significant levels [5][6]. Given the significant roles of cytokines in modulating the antiviral immunity in hosts, they are usually associated with the manifestation of viral disease symptoms and disease severity.
Unlike antimicrobial treatments for bacterial, fungal and parasitic infections, antivirals available for virus infections are very limited and their usage is also restricted to particular types of virus infections due to unique viral genetic make-ups and the architectures of antigenic viral epitopes. An antiviral treatment becomes more complex when the contagious viral agent develops resistance and abilities to inhibit cellular antiviral responses such as downregulation of type-1 interferon (IFN) signalling by degrading the signal transducer and activator of transcription (STAT)-2 [7]. As a result, efforts to seek effective antiviral drugs and therapeutic agents are required in order to better prepare the public health sector to face the occurrence of seasonal viral outbreaks and the occasional viral pandemics [8].
2. Activities of IL-12, IL-23, IL-27 and IL-35 during Virus Infections
The members of the IL-12 family encompass IL-12, IL-23, IL-27 and IL-35. The vast majority of the IL-12 family members are secreted by myeloid-origin cells expressing compatible receptors [9]. Despite similarities in their molecular structures, individually, these cytokines have unique actions and mechanisms in combating virus infections [10]. In this light, understanding their respective activities and functions in response to virus infections can be a great step in developing them into effective treatment agents for virus infections.
2.1. Pro-Inflammatory IL-12 and IL-23
Being a pro-inflammatory cytokine, IL-12 has been proven to be effective in inhibiting virus infections and ameliorating the infection symptoms when administered prophylactically and post-infection [11]. Relative to other cytokines (such as IL-18, TNF-α and IFN-α, -β, and -γ), IL-12 is synthesized more significantly in hosts to initiate the antiviral immune response as early as day-1 post-infection [12]. IL-12 is synthesized and released profoundly by innate immune cells, for instance macrophages at the early stage of infections following the binding of viral antigens to PRR (PAMP recognition receptors) such as Toll-like receptor (TLR)-3, TLR-7, and TLR-8 [13][14][15]. Upon release, IL-12 binds to the compatible receptors on T cells and regulates the expression of T-bet, the transcription factor that is responsible for CD4+ T-cell proliferation into helper T cell (Th)-1 (Figure 1). The formation of Th-1 subsequently activates the synthesis of immune effectors such as IFN-γ, tumor necrosis factor (TNF)-α, CD-107a, IL-2 and granzyme [16]. Those effectors help remove infectious virus particles and infected cells, thus preventing the systemic spread of the contagious entity in hosts. For instance, synthesis of IFN-γ by Th-1 through Janus kinase (Jak)/STAT signalling promotes the proliferation and activation of macrophages and natural killer (NK) T cells that are responsible for engulfing virus particles and clearance of virally infected cells, respectively (Figure 1) [17]. Bhardwaj et al. (1996) showed that IL-12 enhanced the proliferation of T cells at a relatively low dose in the course of a virus infection [18]. In view of its promising antiviral effects even at a relatively low dose, utilization of IL-12 in antiviral therapies can reduce the chances of patients experiencing adverse side effects that are usually seen in antiviral chemotherapy.
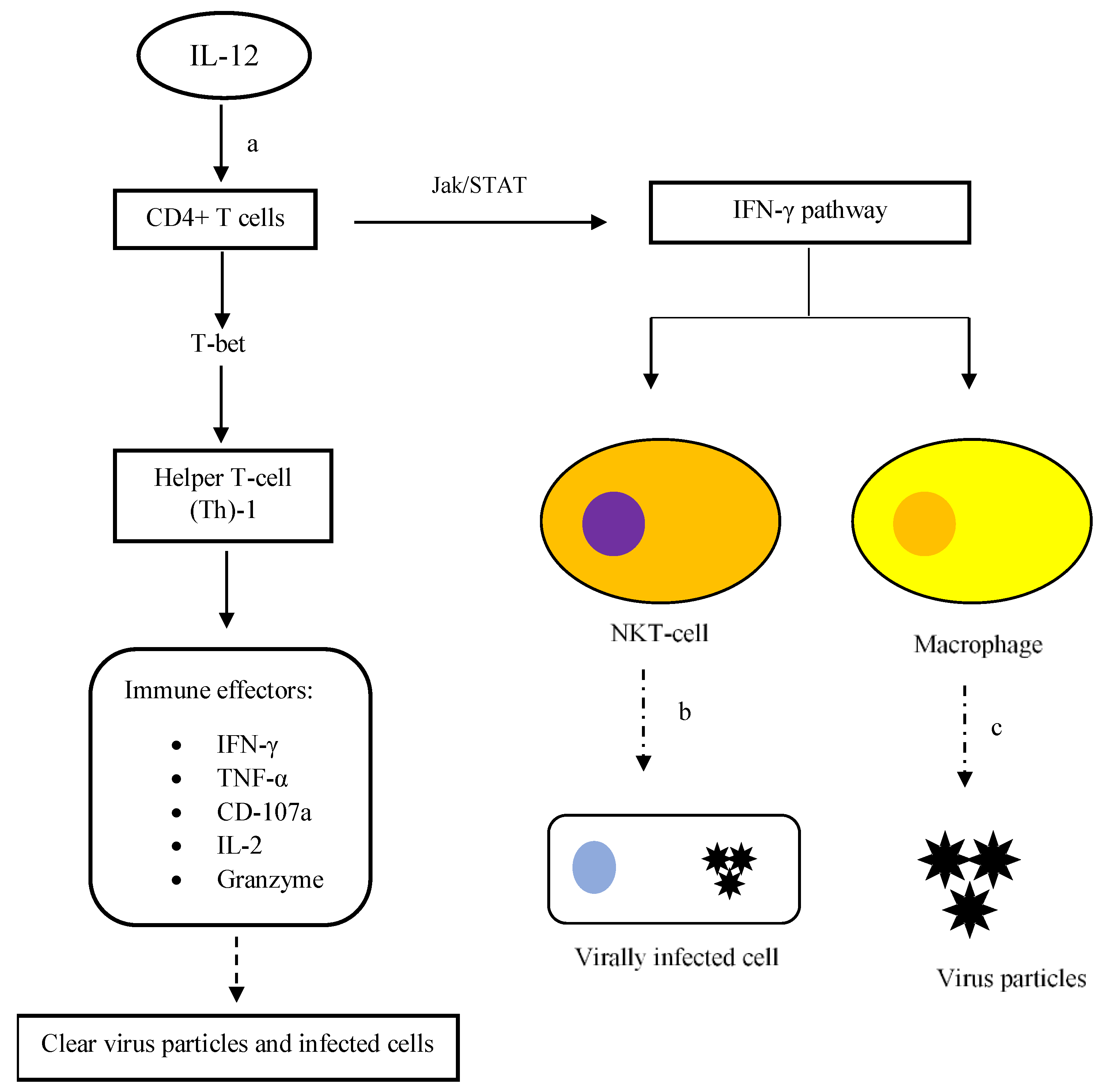
Figure 1. Activation of CD4+ T cells and IFN-γ pathway via IL-12 signalling. (a) Upon binding to cell receptors, IL-12 initiates the proliferation of CD4+ T cells into Th-1 cells that subsequently activates immune effectors to further enhance host defence against virus infections. Eventually, clearance of virus particles and infected cells can be carried out by the host immune system. Secondly, the activated Th-1 cells also synthesize IFN-γ via Jak/STAT signalling. The release of IFN-γ into the surroundings encourages the proliferation and activation of macrophages and NK T cells. (b) NK T cells are important cellular effectors in eliminating infected cells. (c) Once armed, macrophages actively engulf virus particles to prevent virus spread. The dashed-line arrows represent the antiviral activities.
In terms of the cellular immune response, the activation of CD4+ T cells through the actions of IL-12 helps sustain the number of CD8+ T cells in chronic viral infections. The presence of activated CD8+ T cells is crucial in ensuring successful clearance of viral infections via cytolytic and cytotoxic mechanisms [19]. The identified cytolytic and cytotoxic mechanisms include granule protein-mediated and receptor-mediated cell death. Binding of degradative granule proteins to the cell membrane and engagement of death receptors with their ligands causes pore formation and activation of the cell death mechanism, respectively. Upon activation of cell death, membrane blebbing, nuclear DNA fragmentation and cytoplasm vacuolization take place in infected cells [20]. Altogether, it emphasizes the importance of IL-12 in antiviral immunity as it links innate and cellular immunity and promotes the production of effector antiviral biomolecules in order to expedite the elimination of viruses.
IL-23, another member of the IL-12 family, demonstrates similar regulatory properties in promoting the secretion of effector cytokines. Innate immune cells such as NK cells are actively involved in IL-23 antiviral activity. NK cells express IL-23 receptors (IL-23 Rs) on their cell surface, which initiate the expression of effector IFN-γ by NK cells upon binding to IL-23. IFN-γ is released to promote CD8+ T-cell responses such as perforin-dependent cytotoxicity in virally infected cells (Figure 2). As a result, it prevents the spread of virus infections to neighbouring host cells [21][22][23].
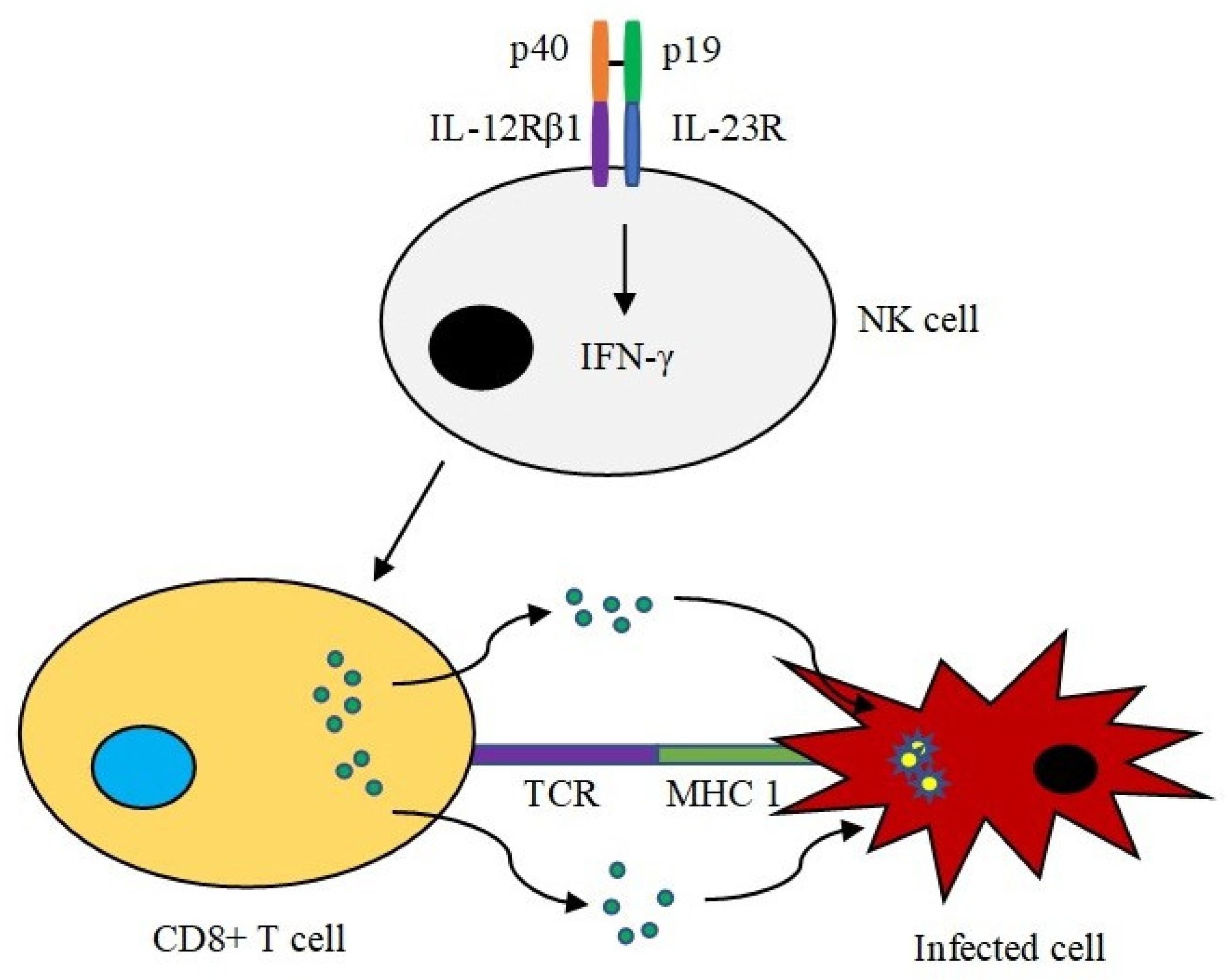
Figure 2. IL-23 activity in NK cells. IL-23 (p40/p19) binds to its receptor on the surface of NK cells. The binding leads to the production of effector IFN-γ. IFN-γ is responsible for promoting CD8+ T-cell responses such as eliminating infected cells in a perforin-dependent manner. The targeted cell undergoes programmed cell death, preventing spread of the virus.
The roles of IL-23 in host antiviral immunity were verified using a recombinant vaccinia virus model expressing IL-23 (vv-IL-23) [24]. The recombinant virus was less virulent than the wild-type vaccinia virus in the challenged mice. The lower pathogenicity was likely attributable to the enhanced cytotoxic T cell activity mediated by IL-23. The IL-23-mediated anti-VV mechanism was also reportedly independent of IFN-γ, which nonetheless is essential to promote IL-12-mediated immunity. Surprisingly, the study also reported the involvement of IL-17 in the IL-23-regulated anti-VV response, albeit to a lesser extent. Inhibition of IL-17 using a monoclonal antibody resulted in a significant increase in vaccinia viral load. In addition, IL-17-deficient mice were more susceptible to the wild-type VV infection. In this light, the IL-23/IL-17-mediated antiviral immunity plays a subdominant anti-VV response.
IL-23 also helps upregulate the expression of several other immune effectors such as IL-21, IL-22, IFN-γ and the antiviral protein myxovirus resistance protein A (MxA) in peripheral blood mononuclear cells (PBMCs) in the course of virus infections (Figure 3). IL-21 was shown to be responsible for enhancing the expansion of CD8+ T memory stem cells (TSCMS) in inhibiting human immunodeficiency virus (HIV)-1 replication [25]. In chronic viral infections, IL-21 helps maintain the functions of CD8 T cells by inducing the expression of basic leucine zipper ATF-like transcription factor (BATF) through the STAT3-dependent pathway [26]. BATF, together with interferon regulatory factor-4 (IRF-4), up-regulates the expression of Blimp-1, which is crucial for the maintenance of virus-specific CD8 T cells. In order to further enhance antiviral activities in hosts, IL-23 limits the expression of transcriptional factors such as FoxP3 and GATA3, which are responsible for the differentiation of regulatory T cells (Treg). Eventually, the magnitude of the anti-inflammatory response that antagonizes IL-23-mediated pro-inflammatory reactions during virus removal is tightly regulated [27]. Collectively, IL-12 and IL-23 are crucial effector cytokines that promote a pro-inflammatory response for virus elimination in hosts.
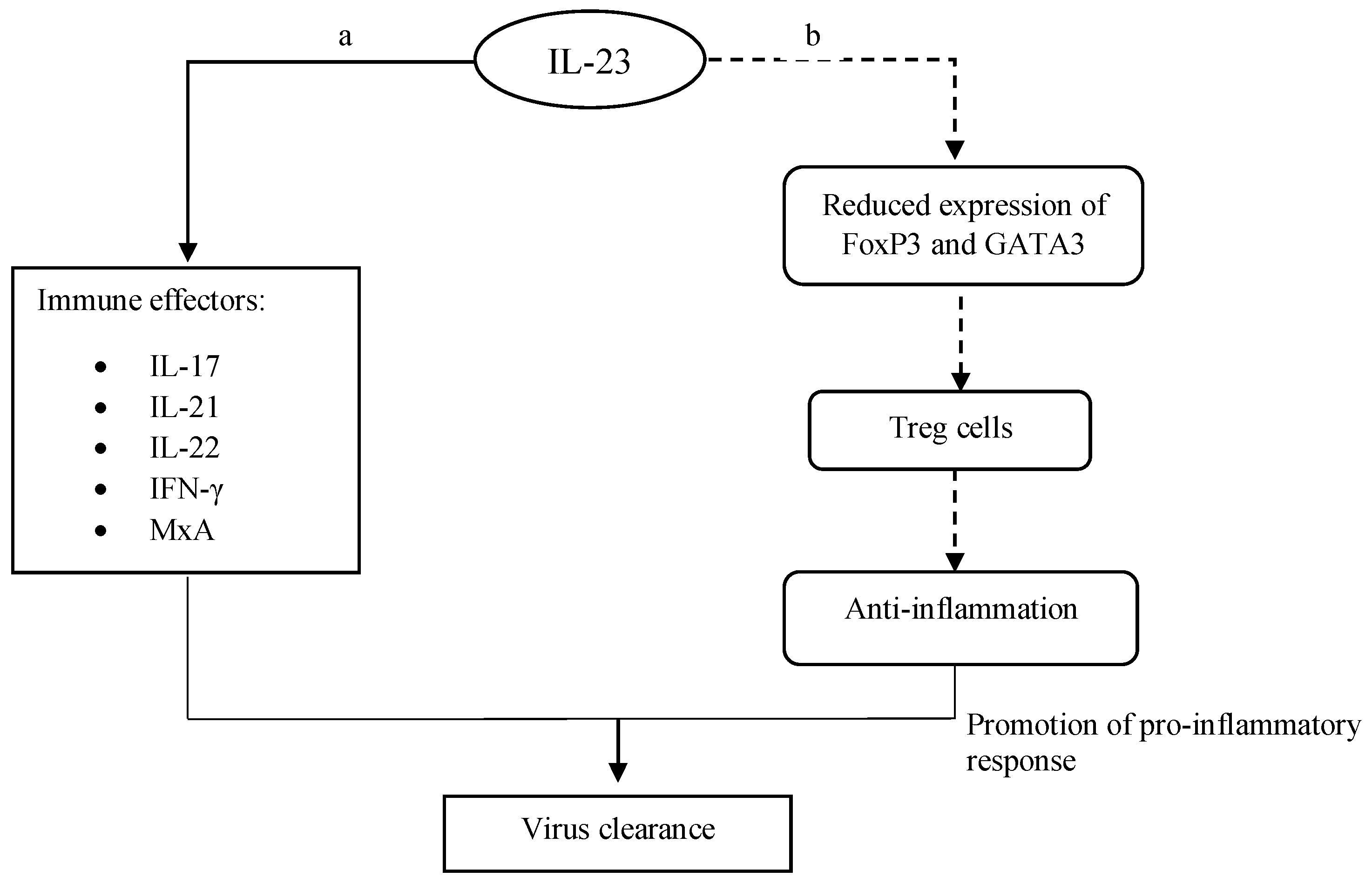
Figure 3. Pro-inflammatory reactions by IL-23. (a) IL-23 induces the expression of immune effectors. Upon their expressions, those effectors enhance the host immune response, which subsequently elevates virus clearance. (b) IL-23 limits anti-inflammatory activities (dashed-line arrows) via the downregulation of Treg. It later increases the pro-inflammatory response that aids in the removal of virus particles.
Despite their remarkable antiviral properties, developing IL-12 and IL-23 into effective antiviral molecules requires greater understanding and more rigorous empirical evidence. This is especially important because both IL-12 and IL-23 mostly regulate antiviral activities via pro-inflammatory reactions, and it is proven that an immoderate inflammatory response is greatly associated with an increased risk of developing severe diseases or immune-directed complications [28]. Furthermore, owing to the superior adaptations to the host’s physiology, viruses have developed multiple antagonizing strategies to inhibit the synthesis or actions of effector cytokines in infected hosts. For instance, the binding of HIV-derived T20 peptide to the formyl peptide receptors on monocytes has been shown to inhibit the expression of IL-12 and thereby renders this anti-HIV defence ineffective [29].
Considering the importance of immunomodulation and the ever-evolving immune escape strategies of viruses, it is noteworthy to further explore the precise roles of IL-12 and IL-23 as part of antiviral therapies. For example, upregulating the upstream cytokine synthesis, which in turn enhances the expression of IL-12, could be an alternative to overcome the challenge. IFN-γ is responsible for initiating the transcription and translation of IL-12 in response to human herpes virus (HHV)-6 infections and the synthesis of IFN-γ can be tightly regulated via the inhibition of endogenous IL-10 [30]. Thus, manoeuvring cytokine synthesis involved in the positive or negative feedback loops might be able to facilitate antiviral activities in hosts. In addition, it has also been suggested that pro- and anti-inflammatory cytokines may be administered in pair, for instance, IL-12 and IL-35, in order to combat virus infections and control the magnitude of host immunity simultaneously [31]. While IL-12 functions to promote the host’s immunity against virus infections, IL-35 is co-administered to control the pro-inflammatory actions in order to prevent immune-directed complications in the host. Given the roles of IL-35 in regulating the host’s inflammatory response, its involvement in host antiviral immunity will be detailed in the next section. Of note, a precisely concerted immune response through pro- and anti-inflammatory cytokine actions can be one of the key antiviral approaches.
2.2. Anti-Inflammatory and Antiviral Actions of Interleukin (IL)-35
Considering the increasing number of emerging and re-emerging viral diseases, it is indispensable to embark on the effort to seek effective antiviral agents to control the spread of viral diseases [32][33][34]. As one of the newest members of the IL-12 family, the antiviral properties of IL-35 are actively being studied; nonetheless, there is very limited empirical evidence elucidating the practical use of IL-35 in treating viral diseases and their related complications compared to the other cytokine members.
IL-35 is composed of two subunits, namely Epstein–Barr virus-induced gene 3 (EBI3) and IL-12A (alpha subunit of IL-12) or p35 [35]. IL-35 shares the same alpha subunit with IL-12; therefore, it is able to exert similar inhibitory actions as IL-12 in combating virus infections. CD4+ T cells isolated from hepatitis B virus (HBV)-infected patients were shown to express higher levels of EBI3 and p35 mRNAs than the cells of healthy counterparts [36]. As aforementioned, IL-35 is able to exert anti-inflammatory effects to counterbalance pro-inflammation in hosts during virus infections. This prevents exacerbation of the viral diseases due to the vigorous immune response in the host. Under the influence of IL-35, anti-inflammatory Treg cells are formed to control and regulate overly reactive immune responses (Figure 4a). In hepatitis C, following the proliferation and activation of Treg, the synthesis of proinflammatory cytokines such as IFN-γ, IL-6, IL-8, and TNF-α was tightly regulated upon IL-35 treatment [37]. As a result, hepatitis C-associated liver inflammation was greatly improved, which manifested as a significant reduction in the serum ALT level and necrosis of hepatocytes [38].
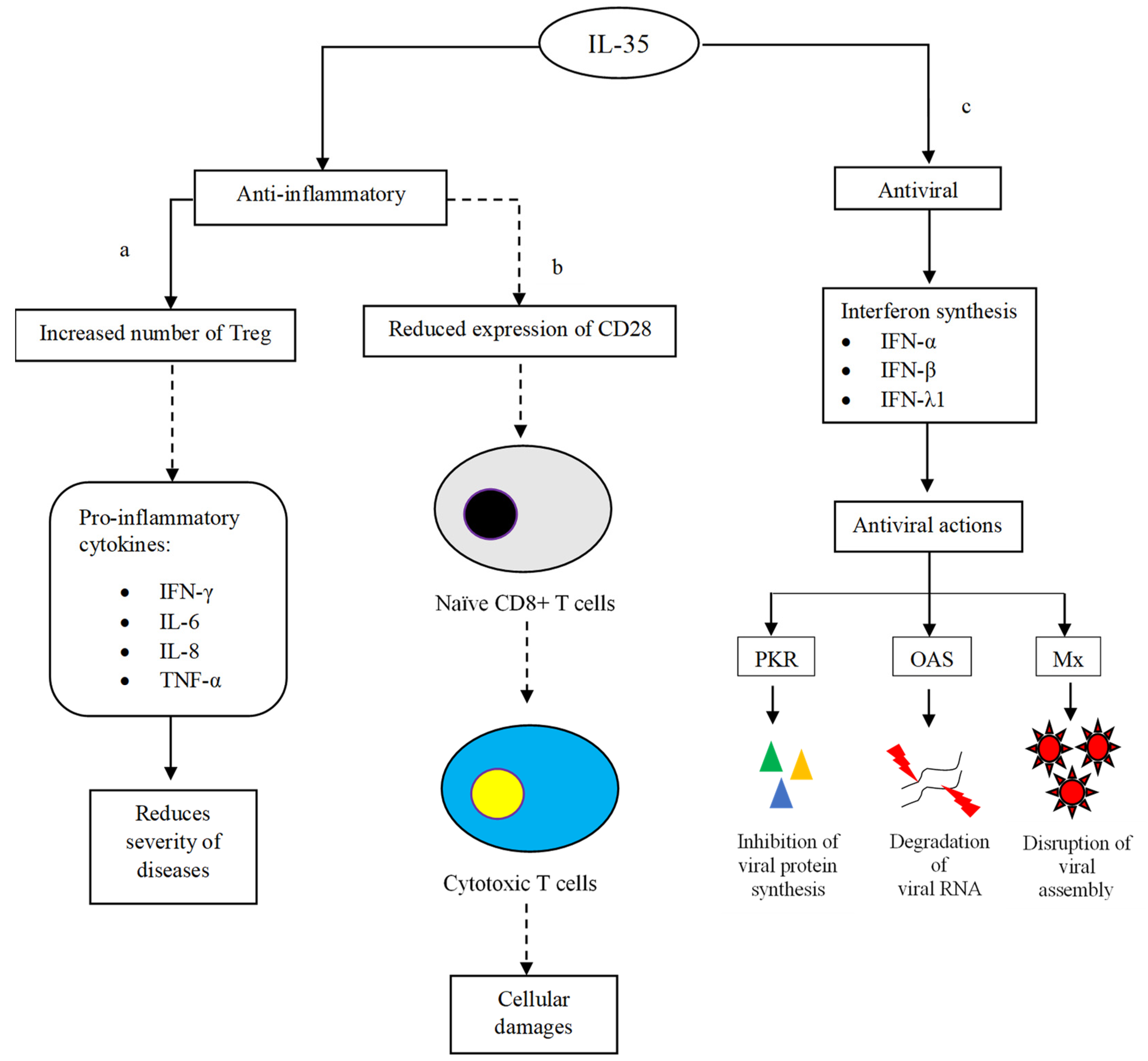
Figure 4. Anti-inflammatory and antiviral actions of IL-35. (a) IL-35 induces the proliferation of Treg in order to suppress the synthesis of pro-inflammatory cytokines. The inhibition subsequently improves inflammation and tissue damage that are usually manifested as the severity of virus infections. (b) IL-35 controls the maturation of naïve CD8+ T cells into cytotoxic T cells. The limiting number of mature cytotoxic T cells prevents the occurrence of cellular damage. (c) IL-35 induces antiviral effects via interferon production. Interferons are known to inhibit viral protein synthesis, genome replication and assembly. The inhibitory activities prevent virus replication and limit virus spreading. The dashed-line arrows represent inhibitory effects.
Besides reducing immune-directed tissue damages and maintaining the physiological functions of vital organs, IL-35 also suppresses the differentiation of naïve CD8+ T cells into cytotoxic T cells (CTLs) via the downregulation of the costimulatory molecule CD28 (Figure 4b) [39]. In this process, the JAK1/TYK2/STAT1/STAT4 pathway is believed to concert the reaction cascade, while the reaction is governed by iTR35 cells [40]. It is thus said that the suppressive iTR35 is part of the regulatory elements that contribute to the downregulation of CTLs. Owing to the reduced number of mature CTLs, the intensity of cytotoxic and cytolytic events decreases, leading to less severe cellular damage [41]. In addition, iTR35 also suppresses the differentiation of Th2, particularly by regulating the production of Th2 cytokines [42].
Despite the exceptional immune-modulating properties of IL-35, deducing the pro- and anti-inflammatory mechanisms involved can be quite tricky. This is because any imbalance between pro- and anti-inflammatory immune responses can fail to eliminate the infecting virus; in the worst-case scenario, it possibly leads to undesired complications and disease manifestations [43]. Eventually, the infected person may undergo prolonged inflammation due to perpetual uncontrollable virus replication. This condition further results in chronic viral diseases and system failure [44]. Of note, in addition to its anti-inflammatory actions, IL-35 also induces antiviral activities via the synthesis of interferons which in turn undermines virus infections through several virucidal pathways. For instance, in influenza A, administration of commercially available recombinant human IL-35 (rhIL-35) was found to induce the production of antiviral cytokines such as IFN-α, β and λ1 (Figure 4c) [45]. Following the upregulation of antiviral interferons, the expression of antiviral proteins, including protein kinase (PKR), 2′,5′-oligoadenylate synthetase (OAS) and myxovirus resistance protein (Mx), is initiated. The activated PKR then phosphorylates eukaryotic initiation factor 2-alpha, (eIF2α) which is responsible for blocking viral protein synthesis [46]. OAS, on the other hand, is responsible for activating RNaseL endonuclease, which degrades viral RNA genomes [47]. To further enhance the antiviral activities in infected cells, MxA prevents the assembly of viral nucleocapsid protein (N), which is essential for viral genome transcription [48]. As a result, it inhibits the formation of new virus particles and stops the virus from spreading to neighbouring cells.
Indeed, IL-35 plays substantial roles in modulating the host immune response and eliminating infecting virus particles through various effector antiviral molecules. However, data pertaining to its exact mode of action are somewhat limited. In this light, more rigorous evidence-based research is required in order to better understand the mechanism of action of IL-35 in targeting virus infections and their disease complications so that IL-35 can be precisely applied as a prospective antiviral drug. Having the same EBI3 subunit as IL-35, a newly discovered but not fully studied member, IL-39, is worth noting for its antiviral potential. IL-39 is composed of p19/Ebi3 subunits and binds to the IL-23R/gp130 cellular receptor in order to orchestrate signalling pathways in affected cells. Activated B cells have been found to express IL-39 [49]. Additional empirical investigations are warranted to further disclose the role of IL-39 in the host immune response against virus infections.
2.3. Antiviral Properties of IL-27
IL-27 is a member of the IL-12 family and it consists of two subunits, p28 and EBI3 [50][51]. The compatible IL-27 receptor is also made up of two subunits, namely WSX-1 and gp130 (a shared receptor of IL-35). The receptor is found abundantly on the majority of cell types [52]. Immune cells such as B cells and macrophages are known to produce IL-27 [53][54]. IL-27 has shown promising antiviral potential due to its pro-inflammatory and anti-inflammatory properties. The duality of IL-27 can be explained as follows: IL-27-induced pro-inflammatory actions include upregulation of CXC motif ligand-10 (CXCL-10), which is an IFN-γ-responsive gene, as well as increased expression of transporter associated with antigen processing 1 (TAP1) and low molecular weight polypeptide 7 (LMP7) genes that are actively involved in the antigen presenting process (Figure 4a) [55]. The interaction between CXCL-10 and its receptor CXCR-3 (also known as CXCL-10/CXCR-3 axis) promotes pro-inflammatory responses such as greater levels of pro-inflammatory cytokines and infiltration of inflammatory cells into the affected areas [56]. Meanwhile, the activation of the TAP1/LMP2/LMP7 pathway aids in processing and transporting intracellular peptides derived from viral antigens, which are subsequently associated with MHC class-I molecules to form complexes which are then displayed on the surface of antigen-presenting cells (Figure 5a) [57]. In addition, IL-27R-deficient NK cells were unable to restore their abilities to produce IFN-γ upon IL-27 stimulation [58]. It is highly possible that this phenomenon is due to the loss of intact receptors on the NK cell surface. Likewise, the level of IFN-γ was also reduced in mice whose EBI3 gene was knocked out. These observations highlight two important key messages: (i) the structural intactness of IL-27 is mandatory to ensure cytokine signalling, and (ii) IL-27 is an important mediator for promoting the synthesis of pro-inflammatory cytokines such as IFN-γ and their downstream pro-inflammatory reactions.
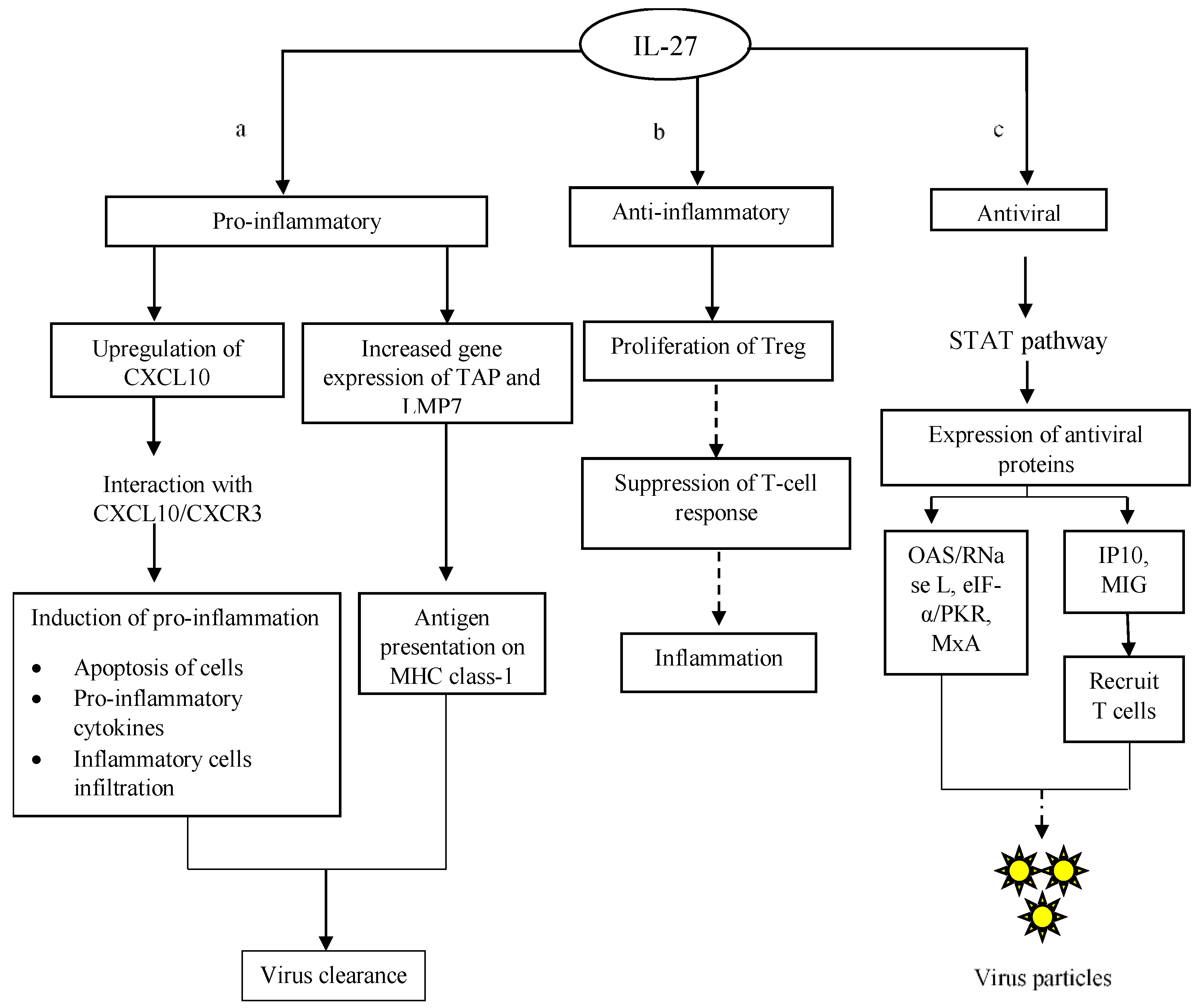
Figure 5. IL-27-mediated antiviral response in infected cells. (a) IL-27 triggers the production of effector mediators and chemokines to promote inflammation. These mediators enhance the host immune response in its targeting of virus infections, for example antigen presentation, cell apoptosis, synthesis of pro-inflammatory cytokines and infiltration of immune cells. Those pro-inflammatory reactions are responsible for virus clearance. (b) In order to control the occurrence of pro-inflammations in infected cells, IL-27 promotes the formation of Treg cells that execute anti-inflammatory responses in the host. Treg controls T-cell-mediated inflammation in order to prevent undesired immune-mediated complications. (c) Upon binding to receptors, IL-27 activates STAT signalling pathways. The activation of STAT signalling results in the transcription and translation of antiviral products that help target virus replication. The dashed-line arrows indicate inhibitory actions on cellular inflammation and the synthesis of new virus progeny.
In contrast, the anti-inflammatory properties of IL-27 are greatly associated with its ability to induce the proliferation of Treg cells and restore their suppressive capabilities [59]. As discussed earlier, Treg cells display anti-inflammatory effects via direct or indirect suppression on T cells in order to tightly regulate T-cell-mediated inflammatory responses (Figure 5b) [60]. IL-27 indeed has a broad spectrum of functions during a virus infection; whilst regulating the host immune response to prevent prolonged, damaging inflammation, it also functions to inhibit virus replication. In order to initiate antiviral activities in host cells, IL-27 activates the STAT-1/3 signalling pathway (Figure 5c). Upon the binding of IL-27 to the compatible cellular receptors, STAT-1 and -3 are phosphorylated and recruited in the cytosol of infected cells. The activated STAT-1 and -3 are then translocated into the cell nucleus to initiate the expression of antiviral genes, leading to inhibition of virus replication in infected cells [61]. This is well demonstrated through the inhibition of HIV replication by IL-27 via the STAT-1 signalling pathway, which promotes the synthesis of OAS-2 [62]. As mentioned earlier, OAS is responsible for activating RNase L, which digests viral RNA. Subsequently, the RNA genome of HIV is degraded, preventing the formation of new virus progeny. Likewise, the involvement of STAT-1/2/3 signalling has also been reported in IL-27-induced immunity against influenza A virus infection [63]. In the study, STAT-1, -2 and -3 were first activated through phosphorylation. The activation of STAT-1/2/3 then promoted the expression of PKR, which in turn inhibited the viral protein translation via eIF-2α. Besides OAS and PKR, the expression of MxA is also upregulated following the activation of STAT-1/3 signalling by IL-27 in hepatitis C [64]. Ultimately, the inhibitory actions circumvent the production of new virions and the spread of the virus infection in the host.
In addition, IL-27 also promotes the expression of chemokines such as IFN-γ-inducible protein-10 (IP-10) and monokine-induced-by-interferon-γ (MIG) through the activation of the STAT 1/3 signalling pathway (Figure 4c) [65]. IP-10 and MIG attract T lymphocytes, especially cytotoxic T lymphocytes, to the affected area in order to remove infected cells and prevent the spreading of the virus infection [66]. Interestingly, the antiviral activities of IL-27 are not only restricted to human viruses; it was also shown to inhibit fowl plague virus infection in birds. When targeting the fowl plaque virus infection, STAT-1 signalling was initiated by IL-27 in order to elevate the expression of antiviral products to reduce the viral load in infected birds [67]. More recently, a study on Zika virus infection showed that IL-27 induced the expression of antiviral genes in vitro and in vivo in a STAT-1-dependent manner [68]. These studies strongly suggest that IL-27 is able to execute immune defence against a broad range of virus infections, especially in STAT-dependent manners, in order to combat virus infections in vitro and in vivo.
References
- Modes of Transmission of Virus Causing COVID-19: Implications for IPC Precaution Recommendations. Available online: https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations (accessed on 16 May 2022).
- Zoonoses. Available online: https://www.who.int/news-room/fact-sheets/detail/zoonoses (accessed on 16 May 2022).
- Smit, J.M.; Moesker, B.; Rodenhuis-Zybert, I.; Wilschut, J. Flavivirus Cell Entry and Membrane Fusion. Viruses 2011, 3, 160–171.
- Sieczkarski, S.B.; Whittaker, G.R. Dissecting virus entry via endocytosis. J. Gen. Virol. 2002, 83, 1535–1545.
- Alhetheel, A.; Albarrag, A.; Shakoor, Z.; Alswat, K.; Abdo, A.; Al-Hamoudi, W. Assessment of pro-inflammatory cytokines in sera of patients with hepatitis C virus infection before and after anti-viral therapy. J. Infect. Dev. Ctries. 2016, 10, 1093–1098.
- He, L.; Ding, Y.; Zhang, Q.; Che, X.; He, Y.; Shen, H.; Wang, H.; Li, Z.; Zhao, L.; Geng, J.; et al. Expression of elevated levels of pro-inflammatory cytokines in SARS-CoV-infected ACE2+ cells in SARS patients: Relation to the acute lung injury and pathogenesis of SARS. J. Pathol. 2006, 210, 288–297.
- Grant, A.; Ponia, S.S.; Tripathi, S.; Balasubramaniam, V.; Miorin, L.; Sourisseau, M.; Schwarz, M.C.; Sánchez-Seco, M.P.; Evans, M.J.; Best, S.M.; et al. Zika Virus Targets Human STAT2 to Inhibit Type i Interferon Signaling. Cell Host Microbe 2016, 19, 882–890.
- Cohen, F.S. How Viruses Invade Cells. Biophys. J. 2016, 110, 1028–1032.
- Thompson, A.; Orr, S.J. Emerging Il-12 Family Cytokines in the Fight against Fungal Infections. Cytokine 2018, 111, 398–407.
- Guo, Y.; Cao, W.; Zhu, Y. Immunoregulatory Functions of the Il-12 Family of Cytokines in Antiviral Systems. Viruses 2019, 11, 772.
- Carr, J.; Rogerson, J.; Mulqueen, M.; Roberts, N.; Booth, R. Interleukin-12 Exhibits Potent Antiviral Activity in Experimental Herpesvirus Infections. J. Virol. 1997, 71, 7799–7803.
- Hama, Y.; Kurokawa, M.; Imakita, M.; Yoshida, Y.; Shimizu, T.; Watanabe, W.; Shiraki, K. Interleukin 12 is a primary cytokine responding to influenza virus infection in the respiratory tract of mice. Acta Virol. 2009, 53, 233–240.
- Pirhonen, J.; Matikainen, S.; Julkunen, I. Regulation of Virus-Induced Il-12 and Il-23 Expression in Human Macrophages. J. Immunol. 2002, 169, 5673–5678.
- Park, A.; Iwasaki, A. Type I and type III interferons–induction, signaling, evasion, and application to combat COVID-19. Cell Host Microbe 2020, 27, 870–878.
- Ramasamy, S.; Subbian, S. Critical Determinants of Cytokine Storm and Type I Interferon Response in COVID-19 Pathogenesis. Clin. Microbiol. Rev. 2021, 34, e00299-20.
- Popescu, I.; Pipeling, M.R.; Mannem, H.; Shah, P.D.; Orens, J.B.; Connors, M.; Migueles, S.A.; Mcdyer, J.F. Il-12–Dependent Cytomegalovirus-Specific Cd4+ T Cell Proliferation, T-Bet Induction, and Effector Multifunction During Primary Infection Are Key Determinants for Early Immune Control. J. Immunol. 2016, 196, 877–890.
- Stubblefield Park, S.R.; Widness, M.; Levine, A.D.; Patterson, C.E. T Cell-, Interleukin-12-, and Gamma Interferon-Driven Viral Clearance in Measles Virus-Infected Brain Tissue. J. Virol. 2011, 85, 3664–3676.
- Bhardwaj, N.; Seder, R.A.; Reddy, A.; Feldman, M.V. Il-12 in Conjunction with Dendritic Cells Enhances Antiviral Cd8+ Ctl Responses in Vitro. J. Clin. Investig. 1996, 98, 715–722.
- Matloubian, M.; Concepcion, R.J.; Ahmed, R. Cd4+ T Cells Are Required to Sustain Cd8+ Cytotoxic T-Cell Responses During Chronic Viral Infection. J. Virol. 1994, 68, 8056–8063.
- Smyth, M.J.; Trapani, J.A. The Relative Role of Lymphocyte Granule Exocytosis Versus Death Receptor-Mediated Cytotoxicity in Viral Pathophysiology. J. Virol. 1998, 72, 1–9.
- Parham, C.; Chirica, M.; Timans, J.; Vaisberg, E.; Travis, M.; Cheung, J.; Pflanz, S.; Zhang, R.; Singh, K.P.; Vega, F.; et al. A Receptor for the Heterodimeric Cytokine Il-23 Is Composed of Il-12rβ1 and a Novel Cytokine Receptor Subunit, Il-23r. J. Immunol. 2002, 168, 5699–5708.
- Valentine, L.; Potts, R.; Premenko-Lanier, M. Cd8+ T Cell–Derived Ifn-Γ Prevents Infection by a Second Heterologous Virus. J. Immunol. 2012, 189, 5841–5848.
- Shrestha, B.; Samuel, M.A.; Diamond, M.S. Cd8+ T Cells Require Perforin to Clear West Nile Virus from Infected Neurons. J. Virol. 2006, 80, 119–129.
- Kohyama, S.; Ohno, S.; Isoda, A.; Moriya, O.; Belladonna, M.L.; Hayashi, H.; Iwakura, Y.; Yoshimoto, T.; Akatsuka, T.; Matsui, M. Il-23 Enhances Host Defense against Vaccinia Virus Infection Via a Mechanism Partly Involving Il-17. J. Immunol. 2007, 179, 3917–3925.
- Wu, K.; Zhang, S.; Zhang, X.; Li, X.; Hong, Z.; Yu, F.; Liu, B.; Pan, T.; Huang, Z.; Tang, X.P. Il-21 Expands Hiv-1-Specific Cd8+ T Memory Stem Cells to Suppress Hiv-1 Replication in Vitro. J. Immunol. Res. 2019, 2019, 1801560.
- Xin, G.; Schauder, D.M.; Lainez, B.; Weinstein, J.S.; Dai, Z.; Chen, Y.; Esplugues, E.; Wen, R.; Wang, D.; Parish, I.A. A Critical Role of Il-21-Induced Batf in Sustaining Cd8-T-Cell-Mediated Chronic Viral Control. Cell Rep. 2015, 13, 1118–1124.
- Meng, P.; Zhao, S.; Niu, X.; Fu, N.; Su, S.; Wang, R.; Zhang, Y.; Nan, Y.; Qiao, L. Involvement of the Interleukin-23/Interleukin-17 Axis in Chronic Hepatitis C Virus Infection and Its Treatment Responses. Int. J. Mol. Sci. 2016, 17, 1070.
- Zhao, L.; Huang, X.; Hong, W.; Qiu, S.; Wang, J.; Yu, L.; Zeng, Y.; Tan, X.; Zhang, F. Slow Resolution of Inflammation in Severe Adult Dengue Patients. BMC Infect. Dis. 2016, 16, 291.
- Braun, M.C.; Wang, J.M.; Lahey, E.; Rabin, R.L.; Kelsall, B.L. Activation of the Formyl Peptide Receptor by the Hiv-Derived Peptide T-20 Suppresses Interleukin-12 P70 Production by Human Monocytes. Blood J. Am. Soc. Hematol. 2001, 97, 3531–3536.
- Li, C.; Goodrich, J.; Yang, X. Interferon-Gamma (Ifn-Γ) Regulates Production of Il-10 and Il-12 in Human Herpesvirus-6 (Hhv-6)-Infected Monocyte/Macrophage Lineage. Clin. Exp. Immunol. 1997, 109, 421–425.
- Li, X.; Mai, J.; Virtue, A.; Yin, Y.; Gong, R.; Sha, X.; Gutchigian, S.; Frisch, A.; Hodge, I.; Jiang, X. Il-35 Is a Novel Responsive Anti-Inflammatory Cytokine—A New System of Categorizing Anti-Inflammatory Cytokines. PLoS ONE 2012, 7, e33628.
- Imperato, P.J. The Convergence of a Virus, Mosquitoes, and Human Travel in Globalizing the Zika Epidemic. J. Community Health 2016, 41, 674–679.
- Oladapo, O.T.; Souza, J.P.; De Mucio, B.; De León, R.G.P.; Perea, W.; Gülmezoglu, A.M. Who Interim Guidance on Pregnancy Management in the Context of Zika Virus Infection. Lancet Glob. Health 2016, 4, e510–e511.
- Petersen, E.; Abubakar, I.; Ihekweazu, C.; Heymann, D.; Ntoumi, F.; Blumberg, L.; Asogun, D.; Mukonka, V.; Lule, S.A.; Bates, M. Monkeypox—Enhancing Public Health Preparedness for an Emerging Lethal Human Zoonotic Epidemic Threat in the Wake of the Smallpox Post-Eradication Era. Int. J. Infect. Dis. 2019, 78, 78–84.
- Li, X.; Tian, L.; Dong, Y.; Zhu, Q.; Wang, Y.; Han, W.; Liu, X.; Ni, Q.; Chen, Y.; Li, L. Il-35 Inhibits Hbv Antigen-Specific Ifn-Γ-Producing Ctls in Vitro. Clin. Sci. 2015, 129, 395–404.
- Zhou, Y.; Zhang, H.; Li, Y. Il-35 Expression in Peripheral Blood Cd4+ T Cells from Chronic Hepatitis B Virus-Infected Patients Directly Correlates with Virus Load. Cytokine 2015, 73, 169–175.
- Liu, S.; Zhang, Q.; Shao, X.; Wang, W.; Zhang, C.; Jin, Z. An Immunosuppressive Function of Interleukin-35 in Chronic Hepatitis C Virus Infection. Int. Immunopharmacol. 2017, 50, 87–94.
- Teng, D.-K.; Liu, Y.; Lv, Y.-F.; Wang, L.; Zhang, W.; Wang, J.-P.; Li, Y. Elevated Interleukin-35 Suppresses Liver Inflammation by Regulation of T Helper 17 Cells in Acute Hepatitis B Virus Infection. Int. Immunopharmacol. 2019, 70, 252–259.
- Jiang, H.; Zhang, T.; Yan, M.-X.; Wu, W. Il-35 Inhibits Cd8+ T Cells Activity by Suppressing Expression of Costimulatory Molecule Cd28 and Th1 Cytokine Production. Transl. Cancer Res. 2019, 8, 1319.
- Dong, Y.; Li, X.; Yu, Y.; Lv, F.; Chen, Y. Jak/Stat Signaling Is Involved in Il-35-Induced Inhibition of Hepatitis B Virus Antigen-Specific Cytotoxic T Cell Exhaustion in Chronic Hepatitis B. Life Sci. 2020, 252, 117663.
- Fischbeck, A.J.; Ruehland, S.; Ettinger, A.; Paetzold, K.; Masouris, I.; Noessner, E.; Mendler, A.N. Tumor Lactic Acidosis: Protecting Tumor by Inhibiting Cytotoxic Activity through Motility Arrest and Bioenergetic Silencing. Front. Oncol. 2020, 10, 2528.
- Wang, W.; Wei, C.; Cheng, Z.; Yang, J. Aberrant Th2 Immune Responses Are Associated with a Reduced Frequency of Il-35-Induced Regulatory T Cells after Allergen Exposure in Patients with Allergic Asthma. Allergy Asthma Immunol. Res. 2020, 12, 1029.
- Shao, X.; Ma, J.; Jia, S.; Yang, L.; Wang, W.; Jin, Z. Interleukin-35 Suppresses Antiviral Immune Response in Chronic Hepatitis B Virus Infection. Front. Cell. Infect. Microbiol. 2017, 7, 472.
- Boquet, A.; Boulay, G.; Hautin, E.; Mottard, N. Septic Shock Complicated by Disseminated Herpes Simplex Virus-1 Infection: A Case Report. J. Med. Case Rep. 2021, 15, 394.
- Wang, L.; Zhu, S.; Xu, G.; Feng, J.; Han, T.; Zhao, F.; She, Y.-L.; Liu, S.; Ye, L.; Zhu, Y. Gene Expression and Antiviral Activity of Interleukin-35 in Response to Influenza a Virus Infection. J. Biol. Chem. 2016, 291, 16863–16876.
- Kang, J.-I.; Kwon, S.-N.; Park, S.-H.; Kim, Y.K.; Choi, S.-Y.; Kim, J.P.; Ahn, B.-Y. Pkr Protein Kinase Is Activated by Hepatitis C Virus and Inhibits Viral Replication through Translational Control. Virus Res. 2009, 142, 51–56.
- Chakrabarti, A.; Jha, B.K.; Silverman, R.H. New Insights into the Role of Rnase L in Innate Immunity. J. Interferon Cytokine Res. 2011, 31, 49–57.
- Haller, O.; Kochs, G. Interferon-Induced Mx Proteins: Dynamin-Like Gtpases with Antiviral Activity. Traffic 2002, 3, 710–717.
- Wang, X.; Wei, Y.; Xiao, H.; Liu, X.; Zhang, Y.; Han, G.; Chen, G.; Hou, C.; Ma, N.; Shen, B. A Novel Il-23p19/Ebi3 (Il-39) Cytokine Mediates Inflammation in Lupus-Like Mice. Eur. J. Immunol. 2016, 46, 1343–1350.
- Devergne, O.; Hummel, M.; Koeppen, H.; Le Beau, M.M.; Nathanson, E.C.; Kieff, E.; Birkenbach, M. A Novel Interleukin-12 P40-Related Protein Induced by Latent Epstein—Barr virus Infection in B Lymphocytes. J. Virol. 1996, 70, 1143–1153.
- Pflanz, S.; Timans, J.C.; Cheung, J.; Rosales, R.; Kanzler, H.; Gilbert, J.; Hibbert, L.; Churakova, T.; Travis, M.; Vaisberg, E. Il-27, a Heterodimeric Cytokine Composed of Ebi3 and P28 Protein, Induces Proliferation of Naive Cd4+ T Cells. Immunity 2002, 16, 779–790.
- Pflanz, S.; Hibbert, L.; Mattson, J.; Rosales, R.; Vaisberg, E.; Bazan, J.F.; Phillips, J.H.; Mcclanahan, T.K.; De Waal Malefyt, R.; Kastelein, R.A. Wsx-1 and Glycoprotein 130 Constitute a Signal-Transducing Receptor for Il-27. J. Immunol. 2004, 172, 2225–2231.
- Pratumchai, I.; Zak, J.; Huang, Z.; Min, B.; Oldstone, M.B.; Teijaro, J.R. B Cell–Derived Il-27 Promotes Control of Persistent Lcmv Infection. Proc. Natl. Acad. Sci. USA 2022, 119, e2116741119.
- Suwanpradid, J.; Lee, M.J.; Hoang, P.; Kwock, J.; Floyd, L.P.; Smith, J.S.; Yin, Z.; Atwater, A.R.; Rajagopal, S.; Kedl, R.M. Il-27 Derived from Macrophages Facilitates Il-15 Production and T Cell Maintenance Following Allergic Hypersensitivity Responses. Front. Immunol. 2021, 12, 3999.
- Ramamurthy, N.; Boninsegna, S.; Adams, R.; Sahgal, N.; Lockstone, H.; Baban, D.; Marchi, E.; Klenerman, P. Impact of Il-27 on Hepatocyte Antiviral Gene Expression and Function. Wellcome Open Res. 2016, 1, 17.
- Liang, R.; Chen, S.; Jin, Y.; Tao, L.; Ji, W.; Zhu, P.; Li, D.; Zhang, Y.; Zhang, W.; Duan, G. The Cxcl10/Cxcr3 Axis Promotes Disease Pathogenesis in Mice Upon Cva2 Infection. Microbiol. Spectr. 2022, 10, e02307–e02321.
- Jamaluddin, M.; Wang, S.; Garofalo, R.P.; Elliott, T.; Casola, A.; Baron, S.; Brasier, A.R. Ifn-Β Mediates Coordinate Expression of Antigen-Processing Genes in Rsv-Infected Pulmonary Epithelial Cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001, 280, L248–L257.
- Kumar, P.; Rajasekaran, K.; Nanbakhsh, A.; Gorski, J.; Thakar, M.S.; Malarkannan, S. Il-27 Promotes Nk Cell Effector Functions Via Maf-Nrf2 Pathway During Influenza Infection. Sci. Rep. 2019, 9, 4984.
- Pyle, C.J.; Uwadiae, F.I.; Swieboda, D.P.; Harker, J.A. Early Il-6 Signalling Promotes Il-27 Dependent Maturation of Regulatory T Cells in the Lungs and Resolution of Viral Immunopathology. PLoS Pathog. 2017, 13, e1006640.
- Schmidt, A.; Oberle, N.; Krammer, P.H. Molecular Mechanisms of Treg-Mediated T Cell Suppression. Front. Immunol. 2012, 3, 51.
- Chen, Q.; Swaminathan, S.; De Yang, L.D.; Sui, H.; Yang, J.; Hornung, R.L.; Wang, Y.; Da Wei Huang, X.H.; Lempicki, R.A.; Imamichi, T. Interleukin-27 Is a Potent Inhibitor of Cis Hiv-1 Replication in Monocyte-Derived Dendritic Cells Via a Type I Interferon-Independent Pathway. PLoS ONE 2013, 8, e59194.
- Imamichi, T.; Yang, J.; Huang, D.-W.; Brann, T.W.; Fullmer, B.A.; Adelsberger, J.W.; Lempicki, R.A.; Baseler, M.W.; Lane, H.C. Il-27, a Novel AntiHiv Cytokine, Activates Multiple Interferon-Inducible Genes in Macrophages. Aids 2008, 22, 39–45.
- Liu, L.; Cao, Z.; Chen, J.; Li, R.; Cao, Y.; Zhu, C.; Wu, K.; Wu, K.; Wu, J.; Liu, F.; et al. Influenza A virus induces interleukin-27 through cyclooxygenase-2 and protein kinase A signaling. J. Biol. Chem. 2012, 287, 11899–11910.
- Frank, A.C.; Zhang, X.; Katsounas, A.; Bharucha, J.P.; Kottilil, S.; Imamichi, T. Interleukin-27, an Anti-Hiv-1 Cytokine, Inhibits Replication of Hepatitis C Virus. J. Interferon Cytokine Res. 2010, 30, 427–431.
- Heikkilä, O.; Nygårdas, M.; Paavilainen, H.; Ryödi, E.; Hukkanen, V. Interleukin-27 Inhibits Herpes Simplex Virus Type 1 Infection by Activating Stat1 and 3, Interleukin-6, and Chemokines Ip-10 and Mig. J. Interferon Cytokine Res. 2016, 36, 617–629.
- Arai, K.; Liu, Z.-X.; Lane, T.; Dennert, G. Ip-10 and Mig Facilitate Accumulation of T Cells in the Virus-Infected Liver. Cell. Immunol. 2002, 219, 48–56.
- Bender, H.; Wiesinger, M.Y.; Nordhoff, C.; Schoenherr, C.; Haan, C.; Ludwig, S.; Weiskirchen, R.; Kato, N.; Heinrich, P.C.; Haan, S. Interleukin-27 Displays Interferon-γ–Like Functions in Human Hepatoma Cells and Hepatocytes. Hepatology 2009, 50, 585–591.
- Kwock, J.T.; Handfield, C.; Suwanpradid, J.; Hoang, P.; McFadden, M.J.; Labagnara, K.F.; Floyd, L.; Shannon, J.; Uppala, R.; Sarkar, M.K.; et al. IL-27 signaling activates skin cells to induce innate antiviral proteins and protects against Zika virus infection. Sci. Adv. 2020, 6, eaay3245.
More
Information
Subjects:
Virology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
519
Revisions:
2 times
(View History)
Update Date:
20 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





