Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Luis Felipe Sarmiento | -- | 2013 | 2023-04-14 18:34:25 | | | |
| 2 | Lindsay Dong | + 4 word(s) | 2017 | 2023-04-17 02:37:10 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Sarmiento, L.F.; Ríos-Flórez, J.A.; Lima De Sousa, P.S.; Olivera-La Rosa, A.; Oliveira Da Silva, A.M.H.; Gouveia, A. Pharmacological Modulation of Temporal Discounting. Encyclopedia. Available online: https://encyclopedia.pub/entry/43075 (accessed on 09 March 2026).
Sarmiento LF, Ríos-Flórez JA, Lima De Sousa PS, Olivera-La Rosa A, Oliveira Da Silva AMH, Gouveia A. Pharmacological Modulation of Temporal Discounting. Encyclopedia. Available at: https://encyclopedia.pub/entry/43075. Accessed March 09, 2026.
Sarmiento, Luis Felipe, Jorge Alexander Ríos-Flórez, Pêssi Socorro Lima De Sousa, Antonio Olivera-La Rosa, Anderson Manoel Herculano Oliveira Da Silva, Amauri Gouveia. "Pharmacological Modulation of Temporal Discounting" Encyclopedia, https://encyclopedia.pub/entry/43075 (accessed March 09, 2026).
Sarmiento, L.F., Ríos-Flórez, J.A., Lima De Sousa, P.S., Olivera-La Rosa, A., Oliveira Da Silva, A.M.H., & Gouveia, A. (2023, April 14). Pharmacological Modulation of Temporal Discounting. In Encyclopedia. https://encyclopedia.pub/entry/43075
Sarmiento, Luis Felipe, et al. "Pharmacological Modulation of Temporal Discounting." Encyclopedia. Web. 14 April, 2023.
Copy Citation
Temporal discounting is a phenomenon where a reward loses its value as a function of time (e.g., a reward is more valuable immediately than when it delays in time). This is a type of intertemporal decision-making that has an association with impulsivity and self-control. Many pathologies exhibit higher discounting rates, meaning they discount more the values of rewards, such as addictive behaviors, bipolar disorder, attention-deficit/hyperactivity disorders, social anxiety disorders, and major depressive disorder, among others.
temporal discounting
decision-making
intertemporal decisions
impulsivity
self-control
1. Introduction
Intertemporal decision-making is choosing between different outcomes at different times [1]. A reward or outcome will lose value while time passes; this phenomenon is known as ‘temporal discounting’ or ‘delayed discounting’ [2]. As the value of the delayed in-time outcome is discounted, there is a higher bias for the sooner choice [3]. This decision is prevalent in daily life, specifically in the areas of health, education, investment, and even clinical conditions [4].
Temporal discounting is measured with tasks in which participants have to choose between a smaller amount of money delivered immediately or after a short amount of time and a large amount of cash given in the future. e.g., ‘Would you prefer 40 dollars today or 100 dollars in three months?’. A person has a higher discount rate when his or her preference goes towards the smaller and more immediate reward. On the other hand, the person has a lower discount rate when he has a stronger preference for the delayed and bigger reward [5].
Thus, the preference for the small, immediate reward over the higher, delayed one is considered an impulsive decision. The preference for the higher, delayed reward over the smaller, immediate reward is a self-controlled decision [6]. Non-human animals prefer impulsive decisions, such as monkeys, rats, mice, and pigeons [1]. The discounting is best described by a hyperbolic curve that shows how the reward loses value with the passage of time (Figure 1). Furthermore, the tasks that measure temporal discounting have a variable known as the k-value. It is a free parameter that measures the sensitivity to the delay. When the k value is low, it means the individual discount is lower with the delay in time, and when the k-value is high, the individual is susceptible to the delay [7].
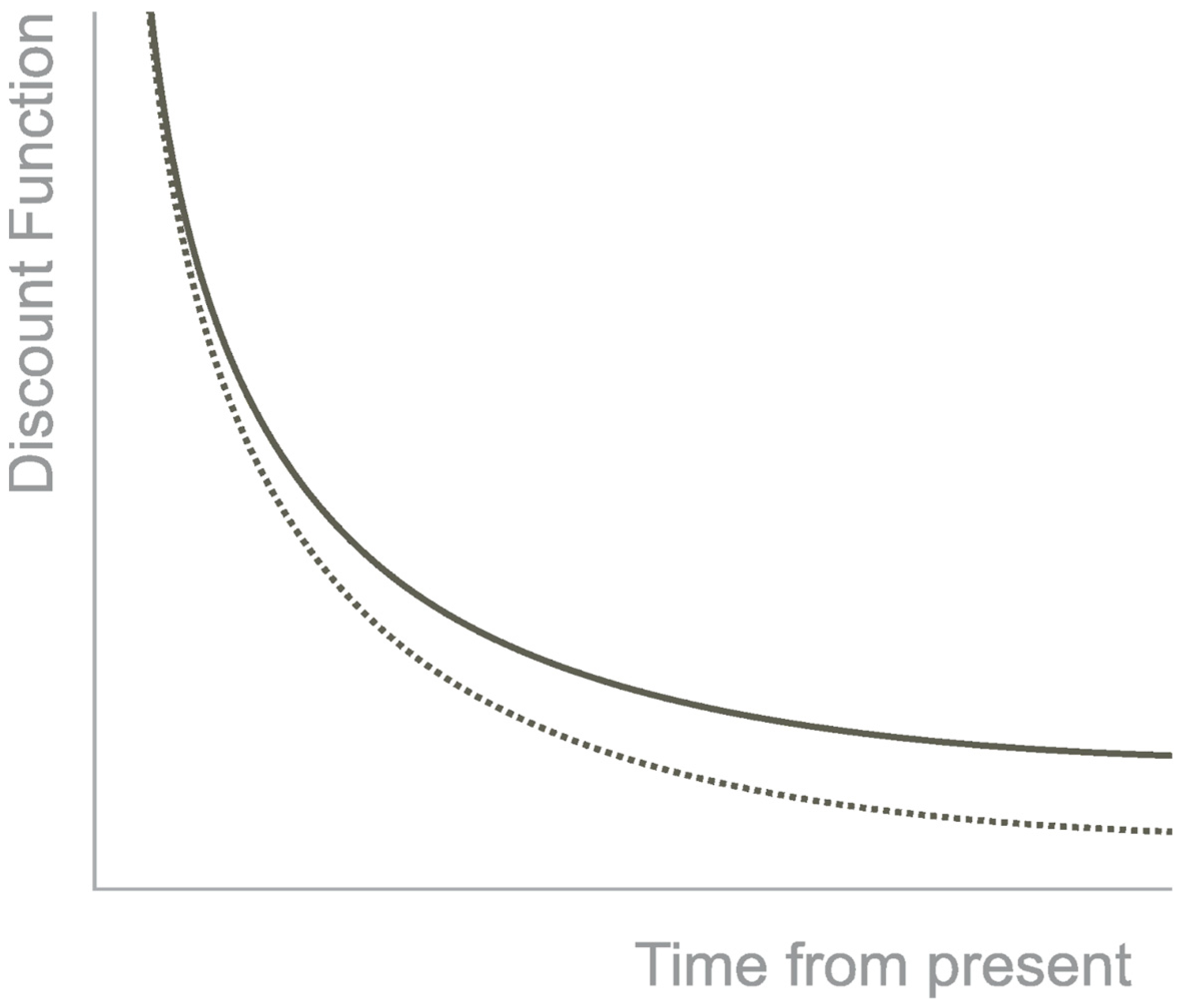
Figure 1. Hyperbolic Discounting. Note. The discounting of the outcome has a hyperbolic shape in function of time. The continuous line represents the hyperbolic function, and the dotted line an exponential function.
This type of decision has higher discount rates in many pathologies such as addictive behavior [8], heroin addiction [9], cocaine addiction [10], alcohol addiction [11], opioid-dependents [12], smokers [13], bipolar disorder [14], attention-deficit/hyperactivity disorder [15], antisocial personality disorder, social anxiety disorder [16], borderline personality disorder [17], major depressive disorder [18], schizophrenia and schizoaffective disorders [19], pathological gambling, and orbitofrontal cortex lesions [20].
2. Pharmacological Modulation of Temporal Discounting
2.1. Dopaminergic System
Dopamine is a crucial neurotransmitter for animals that have been involved in a variety of functions and behaviors. This neuromodulator has been strongly associated with impulsivity and clinical disorders involving impulsive behaviors. Preclinical and human studies suggest a crucial role for dopaminergic function in temporal discounting. Parkinson’s disease patients, a disease known for dopamine deficiency, have shown altered temporal discounting compared with healthy volunteers [21]. Some of the drugs that act in the dopaminergic system, such as Tolcapone and L-dopa from the included studies are employed in the treatment of Parkinson’s disease.
There is a consistent finding within the studies. The dopamine receptor D2 modulation is involved in choosing the later reward over the sooner reward [22][23][24]. Three drugs act on the D2 receptor: amisulpride [24], haloperidol [23], and metoclopramide [22]. This D2 receptor has been associated with impulsive behavior.
In mice, the absence of the D2 receptor increased impulsive behavior, while restoration of the expression of the D2 receptor decreased impulsivity [25]. Another study knocked down these receptors in rats and found that these knockdown rats had a higher preference for the smaller and immediate reward than the control rats in a delay-discounting task [26]. Furthermore, lower ventral striatal D2 density is associated with impulsivity and greater temporal discounting, i.e., preference for the sooner option [27].
Following this reasoning, a study with methamphetamine-dependent subjects found they had lower striatal dopamine D2/D3 receptor availability than healthy controls, and higher impulsiveness was related to it [28]. Furthermore, another study found that methamphetamine-dependent subjects with lower striatal D2/D3 receptors have steeper temporal discounting [29].
2.2. D-Amphetamine
D-Amphetamine is a drug known to have a high potential for abuse but is also used in the treatment of adults with Attention-Deficit Hyperactivity Disorder attention deficit hyperactivity disorder (ADHD) [30]. It can produce a feeling of well-being and euphoria and has helpful behavioral effects on ADHD patients. It increases dopamine in the synapses by binding to the dopamine transporter DAT, reversing DAT function in the medial prefrontal cortex, and inhibiting dopamine uptake [31]. Bupropion is commonly used to decrease impulsivity [32]. Bupropion is a drug that improves impulse control and attention in some patient populations, including those with ADHD [33]. Bupropion is a norepinephrine/dopamine-reuptake inhibitor. The administration of Bupropion had no effect on temporal discounting [34], the same as the administration of d-Amphetamine [34] and a low dose (10 mg) of d-Amphetamine [35]. However, administration of a higher dose (20 mg) in the same sample produced a preference for the later reward [35]. The higher dose by de Wit et al. [35] and the dose used by Acheson et al. [34] were the same (20 mg). However, the results differ, making the relationship between the acute pharmacological relationship and the task unclear. This is highlighted even more with the decrease in impulsivity (e.g., Go/No-Go task, Stop Task) found as other impulsivity measures were applied [35]. This could be explained by the dopamine U-shape action, as has been observed in mice and rats after d-amphetamine administration, where the shifted their response toward the sooner choice [36][37].
Another possible hypothesis Maguire et al. [38] found was that the effects of amphetamine differ depending on the manner in which the delayed reward is presented. This suggests that the changes in the results of the studies and the diverse performance were in part due to the sensitivity to the reward delay.
2.3. Levodopa (L-Dopa)
L-dopa is a dopamine precursor that passes the blood-brain barrier and converts it into dopamine. Dopamine is not able to pass the blood-brain barrier. This drug is often used in the treatment of Parkinson´s disease.
A secondary result from Petzold et al. [39] analyzed that low-impulsive individuals exhibit a preference for the sooner reward, converging with Pine et al. [40] results, but more impulsive individuals showed the opposite effect. These could be explained by the dopamine baseline levels, because, as mentioned, before dopamine behaves in a U-shape. Another possible hypothesis to explain this different result could be the difference in sample size between the two studies; Petzold et al. [39] had a sample size of 87 participants, while Pine et al. [40] had 14 participants. Animal studies have found that L-dopa leads to impulsive-like behaviors [41] and increases impulsivity in Parkinson´s disease even when improving some cognitive tasks [42].
2.4. Tolcapone
Tolcapone is a drug that inhibits the enzyme catechol-O-methyl transferase (COMT). This drug is used in the treatment of Parkinson´s disease. By inhibiting COMT, the degradation of L-dopa is prevented, permitting higher concentrations to cross the blood-brain barrier, and become dopamine [43]. After the administration of Tolcapone, Kayser et al. [44] found an increased preference for the later reward.
Congruently with this result, the genotype for the enzyme COTM predicts impulsive choice behavior. Specifically, subjects carrying enzymatically fewer active alleles encoding the COMT gene show a decrease in choosing the immediate reward [45][46]. Tolcapone increases dopamine tone prefernetially in the frontal cortex, and COMT is mainly in charge of degrading dopamine in the frontal cortex. Thus, low dopamine levels in the frontal cortex may predispose to higher impulsivity.
2.5. Hypothalamic-Pituitary-Adrenal Axis (HPA-Axis)
The HPA axis is a major neuroendocrine system that aims to maintain physiological homeostasis and modulates many important processes such as the stress response, metabolism, fertility, and immunity. The hypothalamus secretes the corticotropin-releasing hormone (CRH), which releases the Adrenocorticotropic hormone (ACTH) from the pituitary gland. This stimulates the production of corticosteroids from the adrenal gland. Cortisol is the main corticosteroid and a stress biomarker. Cornelisse et al. [47] and Riis-Vestergaard et al. [2] administered hydrocortisone and found that after 15 min there was a change in the preference for the sooner reward but not the later. These results suggest a link between HPA axis activation and the mentioned acute stress-induced studies [3][48], where similar results were found. Administration of exogenous cortisol and increasing levels of endogenous cortisol produce the same response in intertemporal decisions. It should be noted that this response is time-dependent, as measures taken 185 min later did not reveal the results.
2.6. Sympathetic-Adreno-Medullar System (SAM-System)
Herman et al. [49] and Lempert et al. [48] administered drugs that modulate the SAM system. The former used yohimbine to stimulate the system, and the latter used propranol to suppress it. Both found no effect. The Sympathetic-Adreno-Medullar System is part of the sympathetic nervous system. It gives a quick physiological response to face the challenge of outside stimuli. This reaction is mediated by catecholamines, especially epinephrine and norepinephrine, and leads to an increase in the heart rate and blood pressure. It is also one of the major systems involved in the stress response, along with the Hyphotalamic-Pituitary-Adrenal axis. Some studies administering protocols that produce acute stress have found that stressed individuals prefer the earlier option over the delayed option [3][50]. These pharmacological results could suggest that the SAM-system is not the main reason for the choice selected, but the HPA-axis should be further investigated.
2.7. Testosterone
Testosterone is a major sex hormone in men and women [51]. The two studies that looked for the effects of testosterone in temporal discounting found different results. While Wu et al. [52] found a preference for the smaller reward, Ortner et al. [53] found no effect. These differences could be dose-dependent; Wu et al. [52] administered 150 mg of testosterone, whereas Ortner et al. [53] used 50 mg. A meta-analysis reported a positive association between circulating testosterone and impulsivity [54]. This relationship could be possible due to the effects of testosterone in the dopaminergic system, as testosterone receptors are in dopaminergic neurons that project to the ventral striatum [55].
2.8. Opioids and Endocannabinoids
Opioidergic and cannabinoid systems involved receptors located throughout the brain and body. The opioid system is involved in affective processing, pain, pleasure, and reward [56]. The endocannabinoid system is involved in regulating physiological and cognitive processes, appetite, pain sensation, mood, and the pharmacological effects of cannabis [57]. Two studies administered drugs that act in the opioid system [24][58], and one in the endocannabinoid system [59]. All these studies were looking for the effects of these systems that have been implicated in drug addiction. None of the studies found any effect of the drugs used in participants.
These findings in the opioid system differ from preclinical studies in rats where the opioid receptor agonist, morphine, increased impulsive behavior in a temporal discounting task [60][61]. This difference could be explained by considering the differences between the tasks measured in the clinical and preclinical models. In the rats’ studies, the rats performed the task when the animal was in a drugged state, whereas in humans, the measure could be after the drug’s effects have dissipated. Furthermore, it is possible the temporal discounting is unaffected by drug effects, even when they affect other types of impulsivity. Some other acute administrations of drugs have failed to affect temporal discounting, for example, alcohol [62] and benzodiazepines [13].
2.9. Diazepam
Diazepam is a benzodiazepine that increases the inhibitory effects of gamma-aminobutyric acid (GABA). Some of the GABA functions are sleep induction, memory, anxiety, and epilepsy. Diazepam, as well as other benzodiazepines, are used for the treatment of anxiety, as a muscle relaxant, as an anticonvulsant, and sometimes are abused [63]. Acheson et al. [64] and Reynolds et al. [13] found no effect of diazepam in temporal discounting.
These results are divergent from the literature that suggests Diazepam affects impulsive behavior in human and non-human models [13][65]. Deakin et al. [66] found in healthy volunteers that after administration of 29 mg of diazepam there were disinhibitory cognitive effects. There are reports that benzodiazepines produce disinhibition and increase aggression [67], and even benzodiazepines such as flunitrazepam, increase impulsivity and aggression [68]. Interestingly, it appears that Diazepam just increases some specific types of impulsive behavior but not others.
References
- Kalenscher, T.; Pennartz, C.M. Is a bird in the hand worth two in the future? The neuroeconomics of intertemporal decision-making. Prog. Neurobiol. 2008, 84, 284–315.
- Riis-Vestergaard, M.I.; van Ast, V.; Cornelisse, S.; Joëls, M.; Haushofer, J. The effect of hydrocortisone administration on intertemporal choice. Psychoneuroendocrinology 2018, 88, 173–182.
- Haushofer, J.; Jain, P.; Musau, A.; Ndetei, D. Stress May Increase Choice of Sooner Outcomes, But Not Temporal Discounting. J. Econ. Behav. Organ. 2021, 183, 377–396.
- Madden, G.J.; Petry, N.M.; Johnson, P.S. Pathological gamblers discount probabilistic rewards less steeply than matched controls. Exp. Clin. Psychopharmacol. 2009, 17, 283–290.
- Scheres, A.; Tontsch, C.; Thoeny, A.L.; Kaczkurkin, A. Temporal reward discounting in attention-deficit/hyperactivity disorder: The contribution of symptom domains, reward magnitude, and session length. Biol. Psychiatry 2010, 67, 641–648.
- Kalenscher, T.; Ohmann, T.; Güntürkün, O. The neuroscience of impulsive and self-controlled decisions. Int. J. Psychophysiol. 2006, 62, 203–211.
- Basile, A.G.; Toplak, M.E. Four converging measures of temporal discounting and their relationships with intelligence, executive functions, thinking dispositions, and behavioral outcomes. Front. Psychol. 2015, 6, 728.
- Amlung, M.; Vedelago, L.; Acker, J.; Balodis, I.; MacKillop, J. Steep delay discounting and addictive behavior: A meta-analysis of continuous associations. Addiction 2017, 112, 51–62.
- Kirby, K.N.; Petry, N.M.; Bickel, W.K. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J. Exp. Psychol. Gen. 1999, 128, 78–87.
- Coffey, S.F.; Gudleski, G.D.; Saladin, M.E.; Brady, K.T. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Exp. Clin. Psychopharmacol. 2003, 11, 18–25.
- Petry, N.M. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology 2001, 154, 243–250.
- Karakula, S.L.; Weiss, R.D.; Griffin, M.L.; Borges, A.M.; Bailey, A.J.; McHugh, R.K. Delay discounting in opioid use disorder: Differences between heroin and prescription opioid users. Drug Alcohol Depend. 2016, 169, 68–72.
- Reynolds, B.; Richards, J.B.; Horn, K.; Karraker, K. Delay discounting and probability discounting as related to cigarette smoking status in adults. Behav. Process. 2004, 65, 35–42.
- Mason, L.; O’Sullivan, N.; Blackburn, M.; Bentall, R.; El-Deredy, W. I want it now! Neural correlates of hypersensitivity to immediate reward in hypomania. Biol. Psychiatry 2012, 71, 530–537.
- Jackson, J.N.; MacKillop, J. Attention-deficit/hyperactivity disorder and monetary delay discounting: A meta-analysis of case-control studies. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2016, 1, 316–325.
- Hurlemann, R.; Scheele, D.; Kinfe, T.M.; Berger, R.; Philipsen, A.; Voncken, M.J.; Kuypers, K.P.; Schruers, K. Increased temporal discounting in social anxiety disorder normalizes after oxytocin treatment. Psychother. Psychosom. 2019, 88, 55–57.
- Berlin, H.A.; Rolls, E.T. Time perception, impulsivity, emotionality, and personality in self-harming borderline personality disorder patients. J. Pers. Disord. 2004, 18, 358–378.
- Pulcu, E.; Trotter, P.D.; Thomas, E.J.; McFarquhar, M.; Juhász, G.; Sahakian, B.J.; Deakin, J.F.; Zahn, R.; Anderson, I.M.; Elliott, R. Temporal discounting in major depressive disorder. Psychol. Med. 2014, 44, 1825–1834.
- Brown, H.E.; Hart, K.L.; Snapper, L.A.; Roffman, J.L.; Perlis, R.H. Impairment in delay discounting in schizophrenia and schizoaffective disorder but not primary mood disorders. NPJ Schizophr. 2018, 4, 9.
- Sellitto, M.; Ciaramelli, E.; di Pellegrino, G. Myopic discounting of future rewards after medial orbitofrontal damage in humans. J. Neurosci. 2010, 30, 16429–16436.
- Milenkova, M.; Mohammadi, B.; Kollewe, K.; Schrader, C.; Fellbrich, A.; Wittfoth, M.; Dengler, R.; Münte, T.F. Intertemporal choice in Parkinson’s disease. Mov. Disord. 2011, 26, 2004–2010.
- Arrondo, G.; Aznárez-Sanado, M.; Fernández-Seara, M.A.; Goñi, J.; Loayza, F.R.; Salamon-Klobu, T.E.; Heukamp, F.H.; Pastor, M.A. Dopaminergic modulation of the trade-off between probability and time in economic decision-making. Eur. Neuropsychopharmacol. 2015, 25, 817–827.
- Wagner, B.; Clos, M.; Sommer, T.; Peters, J. Dopaminergic Modulation of Human Intertemporal Choice: A Diffusion Model Analysis Using the D2-Receptor Antagonist Haloperidol. J. Neurosci. 2020, 40, 7936–7948.
- Weber, S.C.; Beck-Schimmer, B.; Kajdi, M.E.; Müller, D.; Tobler, P.N.; Quednow, B.B. Dopamine D2/3-and μ-opioid receptor antagonists reduce cue-induced responding and reward impulsivity in humans. Transl. Psychiatry 2016, 6, e850.
- Kim, B.; Yoon, S.; Nakajima, R.; Lee, H.J.; Lim, H.J.; Lee, Y.K.; Choi, J.S.; Yoon, B.J.; Augustine, G.J.; Baik, J.H. Dopamine D2 receptor-mediated circuit from the central amygdala to the bed nucleus of the stria terminalis regulates impulsive behavior. Proc. Natl. Acad. Sci. USA 2018, 115, E10730–E10739.
- Bernosky-Smith, K.A.; Qiu, Y.Y.; Feja, M.; Lee, Y.B.; Loughlin, B.; Li, J.X.; Bass, C.E. Ventral tegmental area D2 receptor knockdown enhances choice impulsivity in a delay-discounting task in rats. Behav. Brain Res. 2018, 341, 129–134.
- Dalley, J.W.; Fryer, T.D.; Brichard, L.; Robinson, E.S.; Theobald, D.E.; Lääne, K.; Peña, Y.; Murphy, E.R.; Shah, Y.; Probst, K.; et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science 2007, 315, 1267–1270.
- Lee, B.; London, E.D.; Poldrack, R.A.; Farahi, J.; Nacca, A.; Monterosso, J.R.; Mumford, J.A.; Bokarius, A.V.; Dahlbom, M.; Mukherjee, J.; et al. Striatal dopamine D2/D3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J. Neurosci. 2009, 29, 14734–14740.
- Ballard, M.E.; Mandelkern, M.A.; Monterosso, J.R.; Hsu, E.; Robertson, C.L.; Ishibashi, K.; Dean, A.C.; London, E.D. Low dopamine D2/D3 receptor availability is associated with steep discounting of delayed rewards in methamphetamine dependence. Int. J. Neuropsychopharmacol. 2015, 18, pyu119.
- Castells, X.; Blanco-Silvente, L.; Cunill, R. Amphetamines for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database Syst. Rev. 2018, 8, CD007813.
- Perry, J.L.; Stairs, D.J.; Bardo, M.T. Impulsive choice and environmental enrichment: Effects of d-amphetamine and methylphenidate. Behav. Brain Res. 2008, 193, 48–54.
- Song, J.; Park, J.H.; Han, D.H.; Roh, S.; Son, J.H.; Choi, T.Y.; Lee, H.; Kim, T.H.; Lee, Y.S. Comparative study of the effects of bupropion and escitalopram on Internet gaming disorder. Psychiatry Clin. Neurosci. 2016, 70, 527–535.
- Verbeeck, W.; Bekkering, G.E.; van den Noortgate, W.; Kramers, C. Bupropion for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database Syst. Rev. 2017, 10, CD0095.
- Acheson, A.; de Wit, H. Bupropion improves attention but does not affect impulsive behavior in healthy young adults. Exp. Clin. Psychopharmacol. 2008, 16, 113–123.
- de Wit, H.; Enggasser, J.L.; Richards, J.B. Acute administration of d-amphetamine decreases impulsivity in healthy volunteers. Neuropsychopharmacology 2002, 27, 813–825.
- Cardinal, R.N.; Robbins, T.W.; Everitt, B.J. The effects of d-amphetamine, chlordiazepoxide, α-flupenthixol and behavioural manipulations on choice of signalled and unsignalled delayed reinforcement in rats. Psychopharmacology 2000, 152, 362–375.
- Helms, C.M.; Reeves, J.M.; Mitchell, S.H. Impact of strain and D-amphetamine on impulsivity (delay discounting) in inbred mice. Psychopharmacology 2006, 188, 144–151.
- Maguire, D.R.; Henson, C.; France, C.P. Effects of amphetamine on delay discounting in rats depend upon the manner in which delay is varied. Neuropharmacology 2014, 87, 173–179.
- Petzold, J.; Lee, Y.; Pooseh, S.; Oehme, L.; Beuthien-Baumann, B.; London, E.D.; Goschke, T.; Smolka, M.N. Presynaptic dopamine function measured with fluorodopa and L-DOPA effects on impulsive choice. Sci. Rep. 2019, 9, 17927.
- Pine, A.; Shiner, T.; Seymour, B.; Dolan, R.J. Dopamine, time, and impulsivity in humans. J. Neurosci. 2010, 30, 8888–8896.
- Carvalho, M.M.; Campos, F.L.; Marques, M.; Soares-Cunha, C.; Kokras, N.; Dalla, C.; Leite-Almeida, H.; Sousa, N.; Salgado, A.J. Effect of levodopa on reward and impulsivity in a rat model of Parkinson’s disease. Front. Behav. Neurosci. 2017, 11, 145.
- Cools, R.; D’Esposito, M. Inverted-U–shaped dopamine actions on human working memory and cognitive control. Biol. Psychiatry 2011, 69, e113–e125.
- Rivest, J.; Barclay, C.L.; Suchowersky, O. COMT Inhibitors in Parkinson’s Disease. Can. J. Neurol. Sci. 1999, 2, S34–S38.
- Kayser, A.S.; Allen, D.C.; Navarro-Cebrian, A.; Mitchell, J.M.; Fields, H.L. Dopamine, corticostriatal connectivity, and intertemporal choice. J. Cogn. Neurosci. 2012, 32, 9402–9409.
- Boettiger, C.A.; Mitchell, J.M.; Tavares, V.C.; Robertson, M.; Joslyn, G.; D’Esposito, M.; Fields, H.L. Immediate reward bias in humans: Front-parietal networks and a role for the catechol-O-methyltransferase 158Val/Val genotype. J. Neurosci. 2007, 27, 14383–14391.
- Paloyelis, Y.; Asherson, P.; Mehta, M.A.; Faraone, S.V.; Kuntsi, J. DAT1 and COMT effects on delay discounting and trait impulsivity in male adolescents with attention deficit/hyperactivity disorder and healthy controls. Neuropsychopharmacology 2010, 35, 2414–2426.
- Cornelisse, S.; van Ast, V.; Haushofer, J.; Seinstra, M.; Joels, M. Time-dependent effect of hydrocortisone administration on intertemporal choice. SSRN 2013, 1–22.
- Lempert, K.M.; Lackovic, S.F.; Tobe, R.H.; Glimcher, P.W.; Phelps, E.A. Propranolol reduces reference-dependence in intertemporal choice. Soc. Cogn. Affect. Neurosci. 2017, 12, 1394–1401.
- Herman, A.M.; Critchley, H.D.; Duka, T. The impact of Yohimbine-induced arousal on facets of behavioral impulsivity. Psychopharmacology 2019, 236, 1783–1795.
- Lempert, K.M.; Porcelli, A.J.; Delgado, M.R.; Tricomi, E. Individual differences in delay discounting under acute stress: The role of trait perceived stress. Front. Psychol. 2012, 3, 251.
- Eisenegger, C.; Haushofer, J.; Fehr, E. The role of testosterone in social interaction. Trends Cogn. Sci. 2011, 15, 263–271.
- Wu, Y.; Shen, B.; Liao, J.; Li, Y.; Zilioli, S.; Li, H. Single dose testosterone administration increases impulsivity in the intertemporal choice task among healthy males. Horm. Behav. 2020, 118, 104634.
- Ortner, G.R.; Wibral, M.; Becker, A.; Dohmen, T.; Klingmüller, D.; Falk, A.; Weber, B. No evidence for an effect of testosterone administration on delay discounting in male university students. Psychoneuroendocrinology 2013, 38, 1814–1818.
- Kurath, J.; Mata, R. Individual differences in Risk-taking and endogenous levels of testosterone, estradiol, and cortisol: A systematic literature search and three independent meta-analyses. Neurosci. Biobehav. Rev. 2018, 90, 428–446.
- Creutz, L.M.; Kritzer, M.F. Mesostriatal and mesolimbic projections of midbrain neurons immunoreactive for estrogen receptor beta or androgen receptors in rats. J. Comp. Neurol. 2004, 476, 348–362.
- van Steenbergen, H.; Eikemo, M.; Leknes, S. The role of the opioid system in decision making and cognitive control: A review. Cogn. Affect. Behav. Neurosci. 2019, 19, 435–458.
- Aizpurua-Olaizola, O.; Elezgarai, I.; Rico-Barrio, I.; Zarandona, I.; Etxebarria, N.; Usobiaga, A. Targeting the endocannabinoid system: Future therapeutic strategies. Drug Discov. Today 2017, 22, 105–110.
- Zacny, J.P.; de Wit, H. The prescription opioid, oxycodone, does not alter behavioral measures of impulsivity in healthy volunteers. Pharmacol. Biochem. Behav. 2009, 94, 108–113.
- McDonald, J.; Schleifer, L.; Richards, J.B.; de Wit, H. Effects of THC on behavioral measures of impulsivity in humans. Neuropsychopharmacology 2003, 28, 1356–1365.
- Kieres, A.K.; Hausknecht, K.A.; Farrar, A.M.; Acheson, A.; de Wit, H.; Richards, J.B. Effects of morphine and naltrexone on impulsive decision making in rats. Psychopharmacology 2004, 173, 167–174.
- Pattij, T.; Schetters, D.; Janssen, M.C.; Wiskerke, J.; Schoffelmeer, A.N. Acute effects of morphine on distinct forms of impulsive behavior in rats. Psychopharmacology 2009, 205, 489–502.
- de Wit, H.; Crean, J.; Richards, J.B. Effects of d-amphetamine and ethanol on a measure of behavioral inhibition in humans. Behav. Neurosci. 2000, 114, 830–837.
- Gelkopf, M.; Bleich, A.; Hayward, R.; Bodner, G.; Adelson, M. Characteristics of benzodiazepine abuse in methadone maintenance treatment patients: A 1-year prospective study in an Israeli clinic. Drug Alcohol Depend. 1999, 55, 63–68.
- Acheson, A.; Reynolds, B.; Richards, J.B.; de Wit, H. Diazepam impairs behavioral inhibition but not delay discounting or risk-taking in healthy adults. Exp. Clin. Psychopharmacol. 2006, 14, 190–198.
- Evenden, J.; Ko, T. The psychopharmacology of impulsive behavior in rats VIII: Effects of amphetamine, methylphenidate, and other drugs on responding maintained by a fixed consecutive number avoidance schedule. Psychopharmacology 2005, 180, 294–305.
- Deakin, J.B.; Aitken, M.R.; Dowson, J.H.; Robbins, T.W.; Sahakian, B.J. Diazepam produces disinhibitory cognitive effects in male volunteers. Psychopharmacology 2004, 173, 88–97.
- Fava, M. Psychopharmacologic treatment of pathologic aggression. Psychiatr. Clin. N. Am. 1997, 20, 427–451.
- Dåderman, A.M.; Fredriksson, B.; Kristiansson, M.; Nilsson, L.H.; Lidberg, L. Violent behavior, impulsive decision-making, and anterograde amnesia while intoxicated with flunitrazepam and alcohol or other drugs: A case study in forensic psychiatric patients. J. Am. Acad. Psychiatry Law 2002, 30, 238–251. Available online: https://pubmed.ncbi.nlm.nih.gov/12108561/ (accessed on 13 December 2022).
More
Information
Subjects:
Psychology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
17 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





