Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Roberto Santambrogio | -- | 2131 | 2023-04-11 10:08:45 | | | |
| 2 | Conner Chen | Meta information modification | 2131 | 2023-04-13 07:20:22 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Santambrogio, R.; Vertemati, M.; Barabino, M.; Zappa, M.A. Use of 3D Reconstruction in Laparoscopic Microwave Ablation. Encyclopedia. Available online: https://encyclopedia.pub/entry/42924 (accessed on 07 February 2026).
Santambrogio R, Vertemati M, Barabino M, Zappa MA. Use of 3D Reconstruction in Laparoscopic Microwave Ablation. Encyclopedia. Available at: https://encyclopedia.pub/entry/42924. Accessed February 07, 2026.
Santambrogio, Roberto, Maurizio Vertemati, Matteo Barabino, Marco Antonio Zappa. "Use of 3D Reconstruction in Laparoscopic Microwave Ablation" Encyclopedia, https://encyclopedia.pub/entry/42924 (accessed February 07, 2026).
Santambrogio, R., Vertemati, M., Barabino, M., & Zappa, M.A. (2023, April 11). Use of 3D Reconstruction in Laparoscopic Microwave Ablation. In Encyclopedia. https://encyclopedia.pub/entry/42924
Santambrogio, Roberto, et al. "Use of 3D Reconstruction in Laparoscopic Microwave Ablation." Encyclopedia. Web. 11 April, 2023.
Copy Citation
Laparoscopic ablation of hepatic tumors is a demanding procedure. In the preoperative period, 3D reconstruction of radiological imaging associated with virtual reality permits to evaluate of exactly the position of the lesions. During the operation, augmented reality and intraoperative ultrasound examination are useful tools to perform a safe ablation of the lesion. ICG fluorescence imaging can be used to identify new subglissonian nodules, to guide with more precision microwave antenna e to verify the effect of associated procedures as well as the intra-hepatic vascular occlusion.
hepatic surgery
thermoablation
laparoscopy
1. Introduction
Hepatic resections and thermoablation procedures represent important options in the therapeutic strategies for hepatocellular carcinoma (HCC). Very often, they are performed by a laparoscopic approach [1][2] that can be a complicated procedure for the surgeon.
In recent years, several technologies have been developed to facilitate the surgical maneuvers and to improve the results in terms of radicality and complications.
These technologies have evolved simultaneously with the rapid development of laparoscopic surgery in which preoperative and intraoperative planning was more necessary due to accuracy than open surgery [3][4].
2. Use of 3D Reconstruction
The complexity of liver vascular anatomy and the anatomic variations and the relationship with focal liver lesions emphasize the importance of surgical planning in liver cancer surgery. In this context, 3D reconstruction has on the one hand increased our level of knowledge regarding the anatomical variants (i.e., 3D reconstruction is tailored on patient-specific anatomy), on the other hand, it allows the possibility of developing new laparoscopic procedures and approaches [5]. Jiang et al. carried out a systematic meta-analysis to compare the difference between 3D reconstruction and 2D CT scans before surgery for primary hepatic carcinoma. They found that preoperative 3D reconstruction has a positive impact on liver surgery reducing damage to liver blood vessels, avoiding intraoperative bleeding during the operation, and achieving the accuracy of the tumor resection [6]. Moreover, 3D reconstruction in conjunction with 2D imaging could be a useful model for improving trainees’ understanding of liver anatomy and surgical resection [7].
During the laparoscopy, to help with surgical maneuvers, the ultrasound view of the spatial relationship of intra-hepatic structures is settled on two-dimensional (2D) images using a high-frequency transducer with a limited depth (about 6 cm) that does not provide a panoramic visualization of the intra-hepatic anatomy [8]. The identification of the exact location of a nodule within the hepatic parenchyma is established by the relationships with the vascular-biliary structures that define the segmental anatomy of the liver and which is very often different in many patients [9][10]. Based on the two-dimensional information obtained from preoperative imaging (CT and MRI), expert surgeons can have a mental picture of a 3D representation scan to perform the operation successfully, but it can be a serious challenge for surgeons to identify the presence of anatomical variants using only the LUS evaluation [11][12]. In this setting, 3D reconstruction from 2D images and virtual reality technologies can clearly show the exact spatial anatomy of a nodule and can help LUS in planning the thermoablation procedures [13][14][15]. Unlike traditional imaging, virtual reality (as well as augmented reality) allows a three-dimensional view of the patient in the form of a copy of the original. This 3D representation increases the visibility of the organs to be examined making them more perceptible in their real form, thus allowing the surgeon to immerse themselves in the image that was created, interact with it, and navigate within its space.
This technology is based on three fundamental principles: immersion, navigation and interaction. The immersion corresponds to the feeling of being “submerged” inside the 3D image. Navigation corresponds to movement within the image. The interaction consists of the manipulation of the structures of the image created in real-time (Figure 1).
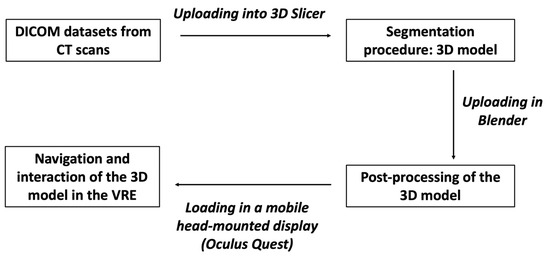
Figure 1. The figure shows the different steps to obtain a 3D model from CT scans to be visualized in the HDMI.
The 3D reconstruction procedure is not yet an easy technique and requires different steps before reaching the final product. Of course, a thin-layer, three-phase CT is required. MRI is definitely a more precise imaging technique in the diagnostic definition of the tumor, but 3D reconstruction is more efficient using CT images. In addition, CT should be performed in the three different stages of hepatic perfusion: arterial, portal and late. These phases, indispensable for the diagnostic definition of the nodule, represent the basis for the correct reconstruction of the vascular architecture of the organ and its relationship with the nodule. The result will be improved for the finer (1 mm) layers obtained from the CT images resulting in a more precise and defined 3D reconstruction.
CT images are loaded and examined with a free, open-source medical image viewer (Horos software Version 3.3.6 or Osirix software version 4.1; Pixmeo, Geneva, Switzerland), designed for Digital Imaging and Communications in Medicine (DICOM) images [16][17]. This software allows the surgeon to perform multiplanar reconstructions of the liver in a very simple way (Figure 2). Such reconstructions are useful for visualizing the lesion, and by analyzing its relationships with contiguous vascular structures, it is possible to identify the exact location of the nodule. In this way, the surgeon is able to program in advance how to place the patient on the operating table in order to have the easiest access to the nodule to be treated.
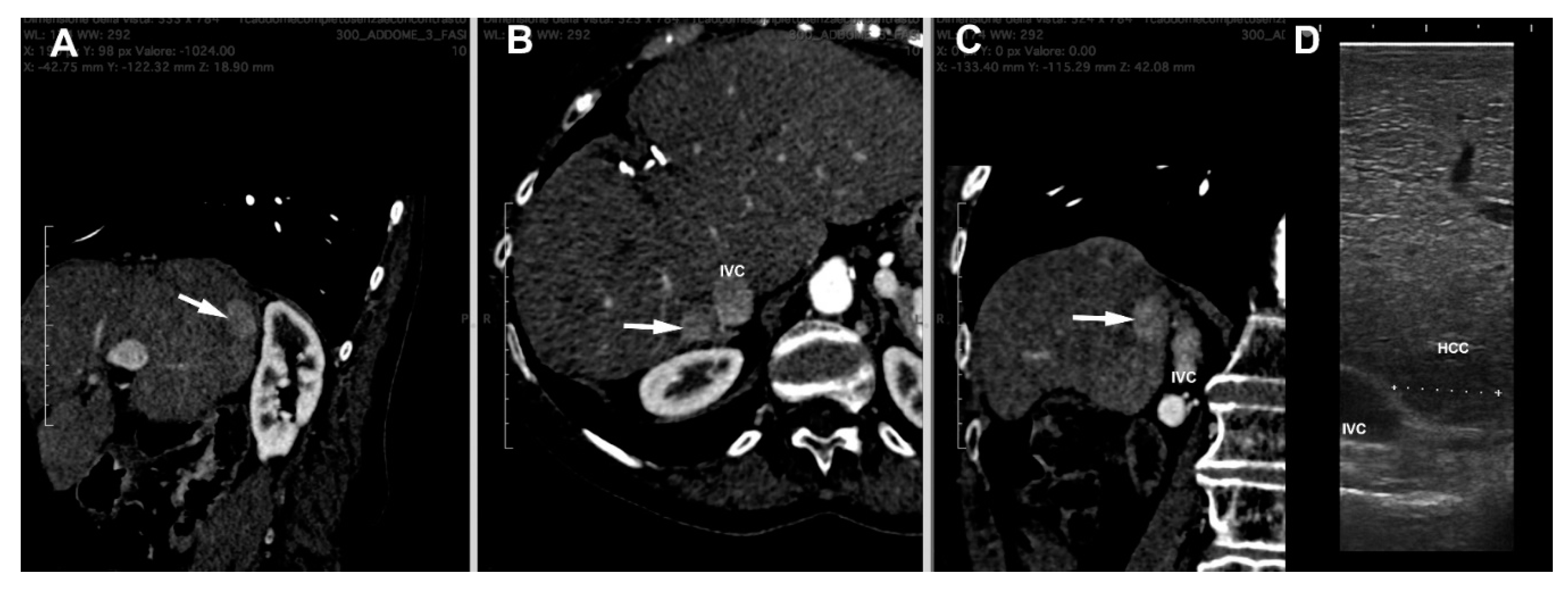
Figure 2. Multiplanar reconstruction of CT scan ((A) = sagittal, (B) = axial and (C) = coronal planes) of a HCC nodule (arrow) in the seventh segment contiguous to inferior vena cava (IVC). (D) = laparoscopic ultrasound image of the nodule.
At this point, the procedure becomes more complex and requires the help of experienced staff. Starting from the DICOM CT datasets, a 3D reconstruction of liver structures (parenchyma, arterial and venous vessels) is obtained using a segmentation procedure. Different methodologies may be used [18][19], but for 3D liver reconstructions, manual segmentation is definitely the preferable one, although it is tedious and requires much more time. In the first step, 3D Slicer is used, a free open-source software for the advanced analysis and processing of medical imaging [20]. With this program, it is possible to obtain a 3D reconstruction of the liver parenchyma and its vascular structures contained within it as well as tumor nodules using semiautomatic algorithms based on in-built region-growing and threshold algorithms in Hounsfield units, with manual adjustment of the boundary to refine little branches of vessels. This human–machine interface allows for the realistic observation of reconstructed structures, which, depending on the needs, can become transparent, ensuring a better visualization of the vascular relationships with the nodule, highlighting only those of interest for the next surgical procedure. The organ can thus be manipulated in real-time.
The 3D model thus obtained is then re-evaluated by a radiologist expert to confirm the correct reproduction of the CT images. Then, it can be exported in STL file format and then adjusted and converted by using Blender v.2.80 (Blender Foundation, Amsterdam, Netherlands), a 3D computer graphics open-source software. Lastly, the converted file is uploaded in a dedicated Virtual Reality Environment (VRE) developed by one team and based on the game engine Unity (Unity Technologies, San Francisco, CA) [21]. The VRE is visualized in a mobile head-mounted display (i.e., Oculus Quest 2) to provide the surgical team with an immersive visualization of the 3D model. Once immersed in the virtual reality environment, the operator can navigate and interact with the 3D reconstruction in an immersive way, can isolate the structures that interest them making the others transparent, and rotate the organ so as to display the nodule from different angles (Figure 3).
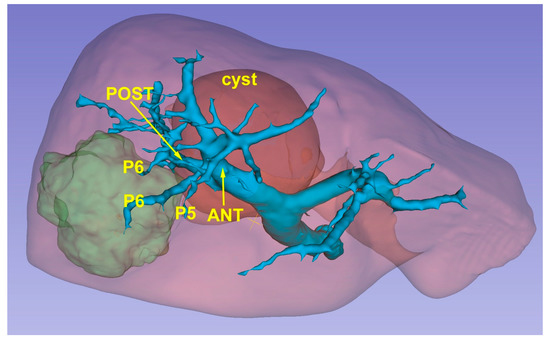
Figure 3. A 3D reconstruction which permits the identification of the lesion’s position (green) in relationship with glissonean pedicles.
In recent years, technological development has allowed access to the world of augmented reality (i.e., Microsoft HoloLens), which can be used to project three-dimensional holograms on the surrounding physical environment, also allowing interaction with holographic objects and spatial tracking.
This technology can be used in the operating room in association with the images of the laparoscopy column monitor and the surgeon can then interact with the 3D model. All of this can be transmitted on an additional monitor allowing other operators to view the same images, thus also playing an educational role [22]. HoloLens is worn by the surgeon without determining any impediments in its action. They can perform the intraoperative ultrasound by comparing the ultrasound images with the 3D model in front of them. The surgeon can interact with HoloLens by moving or zooming in without running the risk of contaminating the operating field. Surgical maneuvers can be stopped at any time to recheck the information that the 3D model can provide. In case the ultrasound or intraoperative images raises doubts about the vascular distribution, the surgeon can compare in real time such images with those of the 3D model and better address the positioning of the antenna or the direction of the dissection slice (Figure 4).
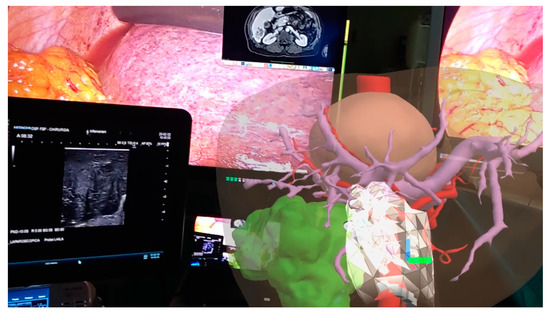
Figure 4. Augmented reality in operating room: surgeon can manipulate the model comparing its features with laparoscopic ultrasound images.
Although 3D reconstructions in the virtual environment based on VR and AR technologies allow liver surgical approaches to be planned by evaluating intrahepatic liver segmental branches and their spatial relationships with the lesion(s), some limitations are still present. Development of VR and AR, for example, is still expensive and time-consuming [23]. Moreover, the soft and deformable nature of the liver parenchyma and the liver movement during operation because of respiratory cycles may determine a change in relationships between the lesion and intrahepatic vascular and biliary structures and an incorrect match between the 3D reconstruction image and the real intrahepatic structures [24]. In this respect, advancements in hardware and tracking technologies (i.e., sensor-based, marker-based, hybrid tracking technologies) with AR systems can increase the matching and mixing of virtual objects (the 3D liver) with the real environment.
Therefore, the use of 3D reconstructions and the development of models for virtual and augmented reality are key aids to:
-
Choosing the patient’s position on the operating table according to the correct location of the nodule;
-
Once the patient is positioned, the choice of the trocar entry point to be used for the laparoscopic probe can be chosen based on the location of the nodule as visualized by virtual reality [25];
-
The technique of lesion centering using the laparoscopic ultrasound probe is totally free-hand. Comparison of images obtained with intraoperative ultrasound and augmented reality allow the surgeon greater accuracy in locating the antenna insertion point in the liver, so that the nodule can be reached with greater precision [14];
-
Finally, in case the intra-hepatic vascular occlusion (IHVO) technique is to be used, 3D reconstruction together with virtual and augmented reality techniques allow the exact individualization of the vessel feeding the nodule, by obtaining a coagulative ablation of the vessel [26].
As indicated at the beginning of this section, all virtual and augmented reality techniques allow the manipulation of the model: in this way they are of valuable help in a situation such as the laparoscopic approach, which does not allow for the easy manipulation of the liver, in addition because of the use of rigid instrumentation. Certainly, the use of robotic surgery with an immersive vision technique and the use of articulated instruments will be of further help in surgical procedures on the liver. Even more so, laparoscopic ultrasound, using linear, high-frequency transducers and therefore with a limited field, may have difficulty in detecting nodules located in deep sites: the 3D reconstruction can tell the surgeon which areas of the liver to examine most carefully for their location [14][25].
However, as already mentioned at the beginning, 3D reconstruction and the creation of virtual models require time and dedicated personnel who are specialized in handling this software. The process of making a 3D reconstruction from DICOM data and transforming it into a virtual and augmented reality model can take more than 2 h, and in centers with a high volume of patients, it can be a problem to accomplish this.
In most cases that come to the attention of centers of liver surgery, the use of CT images with multiplanar reconstructions and possibly the use of 3D reconstruction only on the computer are sufficient to identify the exact location of the lesion and its main vascular relationships. Only in the presence of complex cases, with important vascular abnormalities that make it difficult to understand their relationship with the nodule, might 3D reconstruction with virtual and augmented reality be necessary, as well as the use of 3D printing that in addition to its real use during surgical procedures has an important educational and training role. In fact, the use of models in 3D printing allows a precise liver segmentation to be obtained, thus improving the spatial understanding of the lesions and their relationship with the vascular structures and favoring the professional growth of all the staff [27]. However, the costs and time needed to build these models currently limit their use.
References
- Goh, B.K.P.; Prieto, M.; Syn, N.; Koh, Y.-X.; Teo, J.-Y.; Lee, S.-Y.; Chung, A.Y.; Chan, C.Y. Validation and comparison of the Iwate, IMM, Southampton and Hasegawa difficulty scoring systems for primary laparoscopic hepatectomies. HPB 2021, 23, 770–776.
- Santambrogio, R.; Barabino, M.; De Nicola, E.; Galfrascoli, E.; Giovenzana, M.; Zappa, M.A. Laparoscopic ablation therapies for hepatocellular carcinoma: Could specific indications for the laparoscopic approach influence the effectiveness? Updates Surg. 2020, 72, 435–443.
- Schneider, C.; Allam, M.; Stoyanov, D.; Hawkes, D.J.; Gurusamy, K.; Davidson, B.R. Performance of image guided navigation in laparoscopic liver surgery—A systematic review. Surg. Oncol. 2021, 38, 101637.
- Berardi, G.; Colasanti, R.L.; Meniconi, R.L.; Ferretti, S.; Guglielmo, N.; Mariano, G.; Burocchi, M.; Campanelli, A.; Scotti, A.; Pecoraro, A.; et al. The applications of 3D imaging and indocyanine green dye fluorescence in laparoscopic liver surgery. Diagnostics 2021, 11, 2169.
- Dupré, A.; De Crignis, L. Liver anatomy is king, three-dimensional reconstruction is queen, liver resections are princesses and princes. Ann. Transl. Med. 2022, 10, 1296.
- Jiang, J.; Pei, L.; Jiang, R. Clinical efficacy and safety of 3D vascular reconstruction combined with 3D navigation in laparoscopic hepatectomy: Systematic review and meta-analysis. J. Gastrointest. Oncol. 2022, 13, 1215–1223.
- Yeo, C.T.; MacDonald, A.; Ungi, T.; Lasso, A.; Jalink, D.; Zevin, B.; Fichtinger, G.; Nanji, S. Utility of 3D Reconstruction of 2D Liver Computed Tomography/Magnetic Resonance Images as a Surgical Planning Tool for Residents in Liver Resection Surgery. J. Surg. Educ. 2017, 75, 792–797.
- Gavriilidis, P.; Edwin, B.; Pelanis, E.; Hidalgo, E.; De’Angelis, N.; Memeo, R.; Aldrighetti, L.; Sutcliffe, R.P. Navigated liver surgery: State of the art and future perspectives. Hepatobiliary Pancreat Dis. Int. 2021, 21, 226–233.
- Nagino, M.; DeMatteo, R.; Lang, H.; Cherqui, D.; Malago, M.; Kawakatsu, S.; DeOliveira, M.L.; Adam, R.; Aldrighetti, L.; Boudjema, K.; et al. Proposal of a new comprehensive notation for hepatectomy. The “New World” terminology. Ann. Surg. 2021, 274, 1–3.
- Ichida, H.; Imamura, H.; Yoshioka, R.; Mizuno, T.; Mise, Y.; Kuwatsuru, R.; Kawasaki, S.; Saiura, A. Re-evaluation of the Couinaud classification for segmental anatomy of the right liver, with particular attention to the relevance of cranio-caudal boundaries. Surgery 2021, 169, 333–340.
- Hagopian, E.J. Liver ultrasound: A key procedure in the surgeon’s toolbox. J. Surg. Oncol. 2020, 122, 61–69.
- Torzilli, G.; McCormack, L.; Pawlik, T. Parenchyma-sparing liver resections. Int. J. Surg. 2020, 82, 192–197.
- Boedecker, C.; Huettl, F.; Saalfeld, P.; Paschold, M.; Kneist, W.; Baumgart, J.; Preim, B.; Hansen, C.; Lang, H.; Huber, T. Using virtual 3D-models in surgical planning: Workflow of an immersive virtual reality application in liver surgery. Langenbeck’s Arch. Surg. 2021, 406, 911–915.
- An, C.; Zhang, M.; Yang, J.; Cheng, Z.; Yu, X.; Han, Z.; Liu, F.; Dong, L.; Yu, J.; Liang, P. 3D visualization ablation planning system assisted microwave ablation for hepatocellular carcinoma (diameter >3): A precise clinical application. BMC Cancer 2020, 20, 44–58.
- Shin, J.; Lee, S.; Yoon, J.K.; Chung, Y.E.; Choi, J.Y.; Park, M.S. LI-RADS major features on MRI for diagnosing hepatocellular carcinoma: A systematic review and meta-analysis. J. Magn. Reson. Imaging 2021, 54, 518–525.
- Soon, D.S.C.; Chae, M.P.; Pilgrim, C.H.C.; Rozen, W.M.; Spychal, R.T.; Hunter-Smith, D.J. 3D haptic modelling for preoperative planning of hepatic resection: A systematic review. Ann. Med. Surg. 2016, 10, 1–7.
- Lodewick, T.M.; Arnoldussen, C.W.; Lahaye, M.J.; van Mierlo, K.M.; Neumann, U.P.; Beets-Tan, R.G.; Dejong, C.H.; van Dam, R.M. Fast and accurate liver volumetry prior to hepatectomy. HPB 2016, 18, 764–772.
- Heimann, T.; van Ginneken, B.; Styner, M.A.; Arzhaeva, Y.; Aurich, V.; Bauer, C.; Beck, A.; Becker, C.; Beichel, R.; Bekes, G.; et al. Comparison and evaluation of methods for liver segmentation from CT datasets. IEEE Trans. Med. Imaging 2009, 28, 1251–1265.
- Willatt, J.; Ruma, J.A.; Azar, S.F.; Dasika, N.L.; Syed, F. Imaging of hepatocellular carcinoma and image guided therapies—How we do it. Cancer Imaging 2017, 17, 9.
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.-C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an Image Computing Platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341.
- Vertemati, M.; Cassin, S.; Rizzetto, F.; Vanzulli, A.; Elli, M.; Sampogna, G.; Gallieni, M. Virtual Reality Environment to Visualize Three-Dimensional Patient-Specific Models by a Mobile Head-Mounted Display. Surg. Innov. 2019, 26, 359–370.
- Al Janabi, H.F.; Aydin, A.; Palaneer, S.; Macchione, N.; Al-Jabir, A.; Shamim Khan, M.; Dasgupta, P.; Ahmed, A. Effectiveness of the HoloLens mixed-reality headset in minimally invasive surgery: A simulation-based feasibility study. Surg. Endosc. 2020, 34, 1143–1149.
- Van Nguyen, S.; Thanh Le, S.; Tran, M.K.; Tran, H.M. Reconstruction of 3D digital heritage objects for VR and AR applications. J. Inf. Telecommun. 2022, 6, 254–269.
- Giannone, F.; Felli, E.; Cherkaoui, Z.; Mascagni, P.; Pessaux, P. Augmented Reality and Image-Guided Robotic Liver Surgery. Cancers 2021, 13, 6268.
- Santambrogio, R.; Barabino, M.; Opocher, E. Non-resection: Radiofrequency Ablation, Cryo, Microwave. In Surgical Principles of Minimally Invasive Procedures: Manual of the European Association of Endoscopic Surgery (EAES); Bonjer, H.J., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 131–137.
- Santambrogio, R.; Costa, M.; Barabino, M.; Opocher, E. Laparoscopic radiofrequency of hepatocellular carcinoma using ultrasound-guided selective intra-hepatic vascular occlusion. Surg. Endosc. 2008, 22, 2051–2055.
- Yang, T.; Lin, S.; Xie, Q.; Ouyang, W.; Tan, T.; Li, J.; Chen, Z.; Yang, J.; Wu, H.; Pan, J.; et al. Impact of 3D printing technology on the comprehension of surgical liver anatomy. Surg. Endosc. 2019, 33, 411–417.
More
Information
Subjects:
Health Care Sciences & Services
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
557
Revisions:
2 times
(View History)
Update Date:
13 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




