
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Arantxa Montserrat Gonzalez Aguilar | -- | 3626 | 2023-04-07 06:20:08 | | | |
| 2 | Camila Xu | Meta information modification | 3626 | 2023-04-10 02:40:14 | | | | |
| 3 | Camila Xu | Meta information modification | 3626 | 2023-04-10 02:43:18 | | |
Video Upload Options
Global polystyrene (PS) production has been influenced by the lightness and heat resistance this material offers in different applications, such as construction and packaging. However, population growth and the lack of PS recycling lead to a large waste generation, affecting the environment. Pyrolysis has been recognized as an effective recycling method, converting PS waste into valuable products in the chemical industry. The conversion of PS into a liquid with high aromatic content (84.75% of styrene) can be achieved by pyrolysis. In addition, PS favors the production of liquid fuel when subjected to co-pyrolysis with biomass, improving its properties such as viscosity and energy content.
1. Introduction
2. Pyrolysis as an Alternative Plastic Waste Recycling Route
3. Polystyrene Characterization
4. Statistical Analysis of PS Pyrolysis
4.1 Thermal Degradation through TGA Analysis
Thermogravimetric analysis (TGA) determines the quantity and the frequency of the weight variation of the samples against temperature and time in a controlled atmosphere [66]. TGA is used to study the degradation behavior of polymeric materials, including homopolymers, copolymers, and others [67]. In addition, TGA helps determine degradation trends of operation parameters of the pyrolysis process, such as temperature, heating, oxygen absorption rates, and others [68]. In the literature, different studies on thermal or catalytic pyrolysis of PS have been developed; in these publications, the authors reported as a first step a thermogravimetric analysis of their samples to determine the optimum reaction conditions in the pyrolysis process and to develop their design of experiments.
Fuentes et al. [69] reported the initial, final, and maximum temperatures of two types of PS, purchased and waste. Their results showed slight differences in the degradation temperatures. It was observed that the purchased and the waste had a weight loss of 98.50 and 95.60% at a final temperature of 452 and 463 °C, respectively. Despite these differences, the maximum degradation temperature for both samples was about 420 °C.
Figure 1 visualizes box plots of data collected from the literature to visualize the distribution of virgin PS [69][70][71][72][73][74][75][76] and PS waste [77][78][79][69][71][80][81][82] degradation temperatures (initial, final, and maximum). The results contemplate data from TGA analysis of samples without catalyst in a nitrogen environment with a flow rate range of 10 to 100 mL min−1 and a heating rate of 3 to 100 °C min−1.
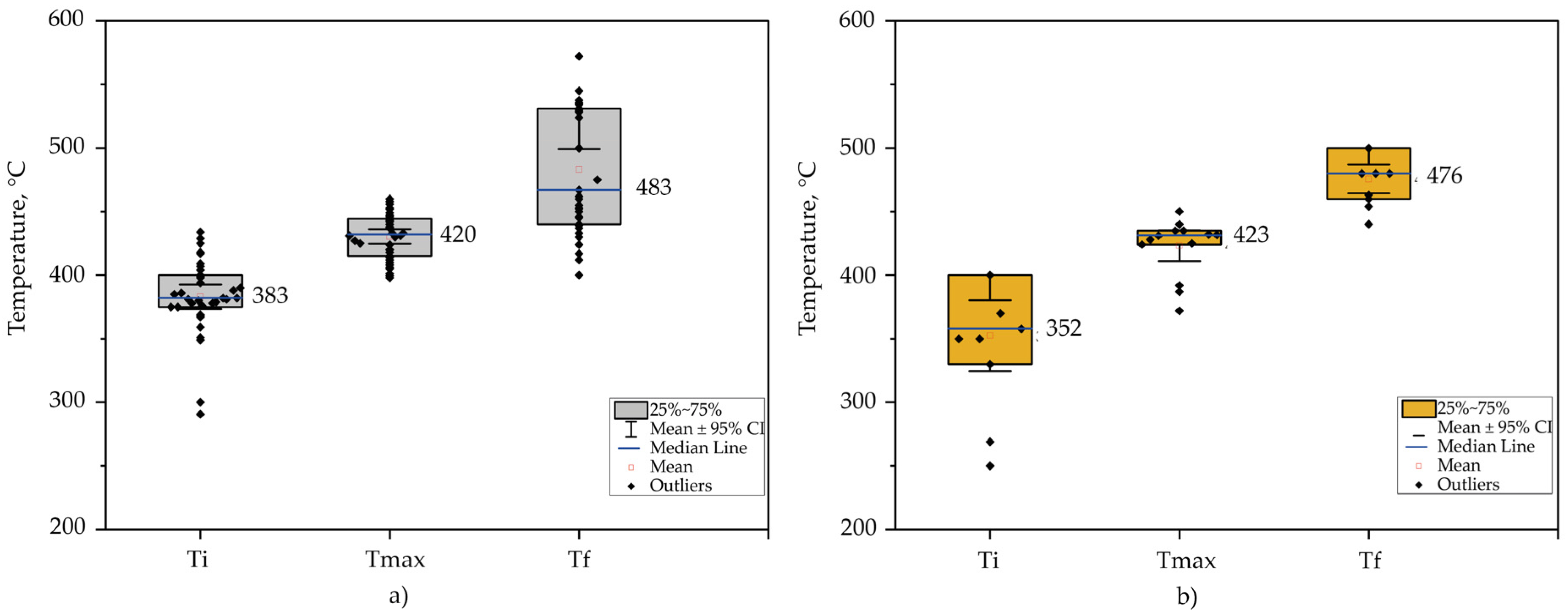
It can be observed in TGA results that the mean initial temperature of the PS waste is about 31 °C lower compared to virgin PS; an influential factor may be the impurities contained in the sample when the waste has not been pre-treated, such as in food. However, both maximum and final temperatures have similar behavior.
4.2. Thermal Pyrolysis of PS
Thermal pyrolysis is the simplest form of chemical recycling in which carbon-carbon bonds are broken by applying heat [76]. A simple process can recover valuable chemicals; an example is the study by Lu et al. [70]. In their experiments, pyrolysis was performed under an inert nitrogen atmosphere and heating of 5 °C min−1 until reaching a temperature of 420 °C for 2 hours. The investigation yielded 76.24, 13.01, and 10.75% of liquid, solid, and gas, respectively. The results stand out because they achieved a single styrene component in their liquid sample, reaching a 73% yield.
4.2.1. Temperature
Temperature controls the cracking reaction in the polymer chain, which is why it is considered one of the most influential parameters in the pyrolysis process [83][84]. Studies report that, in the pyrolysis process, the production of a liquid with long hydrocarbon chains is improved at low temperatures. In contrast, the liquid yield decreases at high temperatures, and gas production improves. Furthermore, high temperatures lead to secondary reactions within the reactor, which reduce the obtaining of solid products[85].
A study of the influence of temperature on the thermal pyrolysis of PS waste is that of Verma et al. [86]. They evaluated the process at temperatures from 400 to 700 °C with 50 °C intervals and a heating rate of 15 °C min−1. Their results showed that the pyrolytic liquid yield increases with increasing temperatures up to 650 °C. However, the liquid yield decreases at temperatures higher than 650 °C. Moreover, the same behavior was obtained even when a catalyst was used.
4.2.2. Reactor types
Reactor design is an essential parameter in the PS pyrolysis process since it affects how the reaction develops by influencing the method of heating, reactant mixing, residence time, and heat transfer.
Batch reactors are those where the system does not allow the flow of reactants or the exit of the products while the reaction is taking place; this allows the reactants to remain inside the reactor for a longer time, achieving a high conversion efficiency [87]. Moreover, these reactors are recognized for their simple design and ease of control of the operating parameters involved. Nevertheless, the main disadvantages of batch reactors are the requirements to refill feedstock and, in addition, that it is impossible to use them at a high production scale.
Unlike a batch reactor, in semi-batch reactors, it is possible to feed reactants and collect the resulting products simultaneously. However, coinciding with the disadvantage of the batch type, they are suitable for smaller-scale production. Finally, fixed-bed reactors are categorized as simple in design and are generally used as secondary reactors [88]. Yet, a two-stage process is not considered economically viable since the products obtained are very similar to single-stage processes [85].
Different types of reactors have been studied in the pyrolysis of PS to obtain products of interest in the chemical or petrochemical industry. The most used reactors are batch [89][78][76][90][91], semi-batch [92][68][76][80][82], fixed bed [93][94], and laboratory scale, which include basic systems such as a simple steel tube or using Pyrex material [79][70][81][95][96][97]. Lopez et al. [98] reported that reactor design is vital because an inadequate design leads to undesired reaction conditions, decreasing the quality of the products and promoting the formation of undesired by-products, such as solid residues or tar. Therefore, an optimal design must ensure a high heat transfer rate for rapid heating of the polymer and reliable temperature control to avoid operating problems due to the nature of the plastic melting process.
4.3. Catalytic Pyrolysis of PS
Thermo-catalytic pyrolysis has proven to be an alternative technology to reduce the impact of polymeric waste on the environment [99]. A catalyst is a substance that changes the performance of the chemical reaction without being altered in the process [100]. Using catalysts during a hydrocarbon degradation process can decrease the temperature and reaction time, increase the conversion rate, and promote a desired selectivity in the products [101]. These advantages make catalysts play an important role in waste pyrolysis processes [102].
The catalytic pyrolysis of PS has been less studied than other polyolefins; however, basic and acid catalysts have shown potential in the depolymerization of PS. Nevertheless, literature has shown differences using these catalyst types, such as the conversion efficiency of the products or their selectivity. An example is the study conducted by Inayat et al. [71], where they evaluated the catalytic pyrolysis of different PS wastes, experimenting with zeolite (HZSM-5) and MgO as acid and basic catalysts, respectively. Their results showed that implementing basic catalysts influenced the composition of the products slightly compared to those obtained by thermal pyrolysis. In conclusion, they indicated that the PS feedstock type affects the composition more than basic catalysts. Unlike acid catalysts, they significantly influenced the composition of the products.
Furthermore, in their experiments with zeolite, they added the operation mode as a factor (ex-situ and in situ). The results indicated that mixing the PS waste with the zeolite increases the production of waxes. In contrast, zeolite promotes mono aromatics' formation in the ex-situ mode, such as benzene, toluene, and xylenes (BTX). The authors concluded that acid catalysts have the potential to transform PS-based materials into higher-value compounds.
Figure 2 compares two types of catalysts used in the catalytic pyrolysis of PS. The analysis involves data collected from experiments with different types of bentonite [68][86][71][103][104] and zeolites [104][105] and evaluates the influence on liquid hydrocarbon yield and styrene formation. In this case, the effect of another operating parameter was not contemplated; the analysis includes temperatures from 300 to 600 °C. Despite the wide scatter of the data collected, the results show that zeolite achieves the highest performance for both liquid yield and styrene formation. The yields mean between 37.70 and 25.90% liquid and styrene, respectively, with the use of bentonite; on the other hand, with zeolite, it is possible to reach, on mean, up to 72.80% liquid and 63.40% styrene.
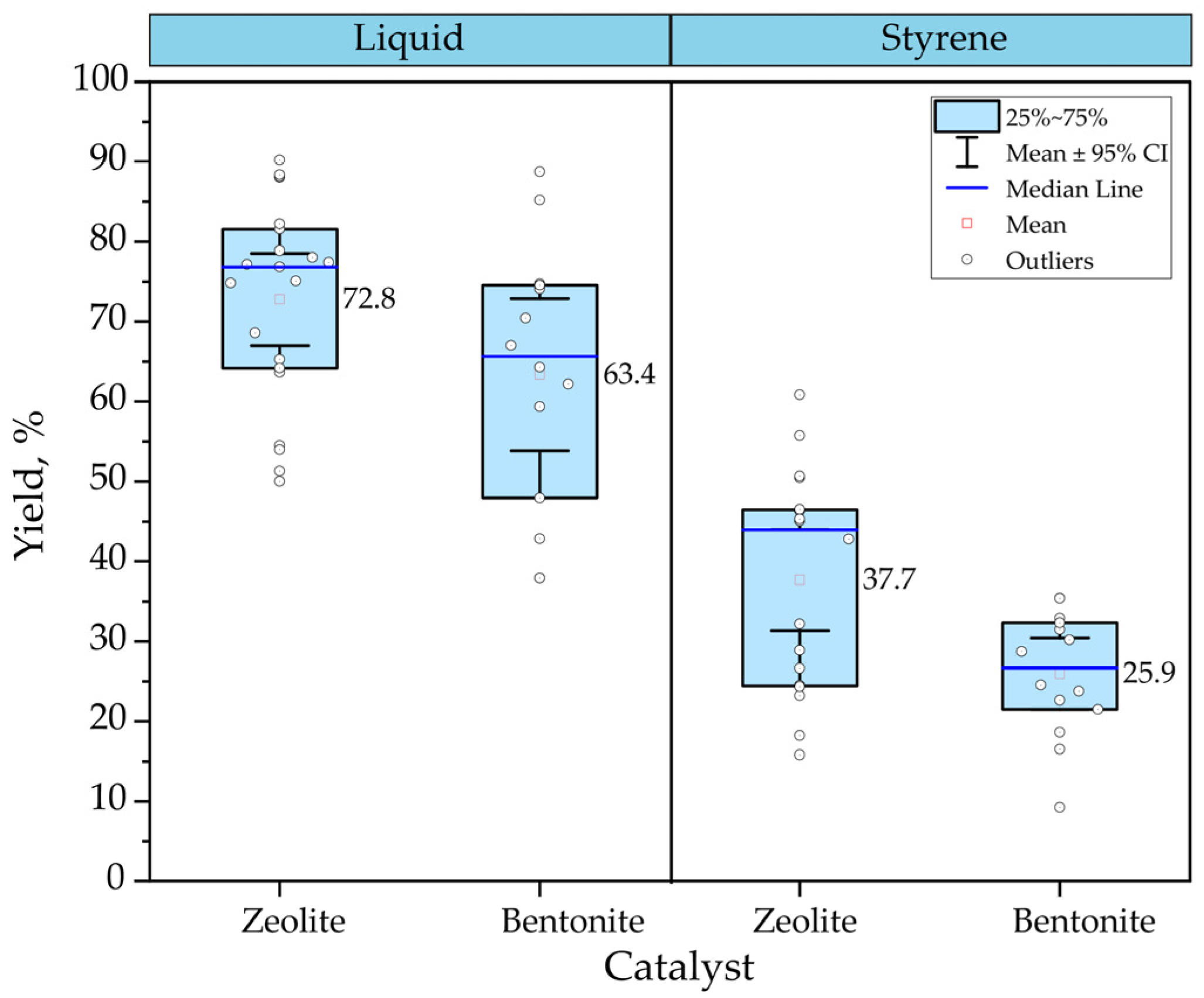
Figure 2. Comparison between zeolite and bentonite in the catalytic pyrolysis of PS.
Part of the data collected includes those reported by Dewangga et al. [104], in which they evaluated the behavior of bentonite and natural zeolite at different catalyst percentages, from 0 to 25%. Their results showed that, for both catalysts, the higher the catalyst percentage, the higher the liquid yield obtained. Specifically for zeolite, Rehan et al. [103] evaluated the performance of liquid yields with natural and synthetic zeolite in the catalytic pyrolysis of PS. Their results showed that using natural zeolites slightly favors the production of liquid hydrocarbon with 4% more than that obtained with synthetic zeolites. In contrast, gas production increases with synthetic zeolites, reaching approximately 10% more. Regarding styrene formation, the difference between the two types of zeolites is more notorious, obtaining about 60.80% and 15.80% for natural and synthetic zeolites.
5. Production of Fuels from PS Pyrolysis
Obtaining alternative fuels from plastic waste has received significant attention because it can solve the problems of the final disposal of this type of waste. In addition, the growing demand for energy worldwide, the depletion of oil resources, and the high cost of petroleum-based fuels have led to the development of alternative fuels from different types of plastic waste [106][107].
Despite that, PS pyrolytic oil has significant aromatic content, and its application as a fuel is questionable; however, its main advantage is its high calorific content. Therefore, studies on producing a combustible derived from PS thermo-catalytic pyrolysis have been developed [108][77][79]. Nisar et al. [97] evaluated the obtaining of fuel from the pyrolysis of PS waste; the experiments were carried out in a salt bath reactor using copper oxide (CuO) as a catalyst. They reported that using the catalyst reduced the temperature and time of the process and increased the liquid produced during pyrolysis. Liquid properties were compared with some standard values from fossil fuels, and finally, they concluded that the liquid obtained had a great potential to be a substitute for commercial fuels.
Budsaereechai et al. [109] produced oil from plastic packaging waste in a bench-scale fixed pyrolysis batch reactor. Among their objectives, they studied the influence of heating rate on oil production; the results showed that the liquid yield decreases as the heating rate increases. Moreover, they concluded that oil derived from catalytic pyrolysis with commercial clay bentonite resulted in greater engine power, comparable engine temperature, and lower carbon monoxide (CO) and carbon dioxide (CO2) emissions than uncatalyzed oils and commercial fuel in the gasoline range. Figure 3 shows the distribution of carbons from C5 to higher C13 for the pyrolytic oil derived from this research, compared to diesel and gasohol 91. It is remarkable to identify the high content of aromatic components (C5–C9) in the PS oil derived from thermal pyrolysis with a value of 60%. In comparison, diesel only contains 2.18%, evidencing the significant difference between them. PS oil contains short-chain (C5–C9) and long-chain (>C13) hydrocarbon chains, while medium-chain carbons characterize diesel.
Figure 3. Carbon distribution of PS pyrolysis oil and commercial fuels.
Table 1 summarizes the comparison of the described properties of commercial fuels with pyrolytic PS oils found in the literature.
Table 1. Summary of fuel properties from PS pyrolysis and commercial fuels.
|
Fuel properties |
PS Pyrolysis Oil |
Commercial Fuels |
|||||||
|
|
Temperatures [°C] |
Kerosene [97]
|
|||||||
|
150–379 [113] |
400 1 [97] |
500 [109] |
550 [79] |
~695 [108] |
~782 [108] |
||||
|
Density, g cm−3 |
0.95 |
0.79 |
0.85 |
0.92 |
0.95 |
0.98 |
0.72–0.78 |
0.80–0.87 |
0.78–0.82 |
|
Kinematic viscosity, mm2 s−1 |
0.92 |
1.34 |
1.71 |
0.88 |
0.97 |
1.26 |
1.08–1.17 |
1.90–5.30 |
1.54–2.20 |
|
Flashpoint, °C |
48 |
30.50 |
79 |
79 |
>42 |
>48 |
|
||
|
Pour point, °C |
<−35 |
19 |
−39 |
−39 |
−6 to 19 |
|
|||
|
Calorific value, MJ kg−1 |
43.55 |
40.89 |
40.02 |
46.95 |
|
||||
1 Mixed with CuO catalyst, 98:2.
The summary of fuel properties shows that the density of the liquid derived from PS at any temperature is relatively high, placing it out of the range of fuels such as gasoline, diesel, and kerosene. However, its value resembles marine application fuels, such as marine residual fuel (RMG-380) [79]. There is no trend in kinematic viscosity with pyrolysis temperatures; however, the values of 400, 500, and ~768 °C are within the established range for all commercial fuels compared. Gasoline and diesel must comply with a flash point above 42 °C, so pyrolytic oil, except for 550 °C, is compliant. Finally, one of the beneficial properties of PS pyrolytic oils is their high energy content. Due to the high aromatic content of PS oil, it is not recommended to be used directly as fuel. However, it is possible to blend it with other combustibles or biocombustibles to improve this property.
6. Co-Pyrolysis of PS and Biomass
Biomass is the biodegradable fraction of products, residues, and wastes of biological origin from agricultural activities, including substances of plant and animal origin, forestry and related industries, fishing, and aquaculture. Biomass is a biodegradable fraction of industrial and municipal waste of biological origin [114]. Biomass can produce alternative fuels, mitigating fossil fuel environmental and climate change impacts [115]. Moreover, co-pyrolysis refers to the process of thermo-catalytic pyrolysis in which different feedstocks are mixed, and the process is carried out. Several co-pyrolysis studies of biomass with waste plastics have been evaluated to enhance fuel production yields and improve fuel properties [116][117][118][119][120].
Regarding fuel energy content, another reason for blending PS pyrolytic oil with biomass or conducting co-pyrolysis of both feedstocks is the increase in the calorific value of the biofuels. Reshad et al. [121] evaluated the energy content behavior of biofuels derived from the pyrolysis of rubber seeds (RSC) with PS at different operating conditions. Their results showed that PS pyrolytic oil contained 42.10 MJ kg−1, and its high calorific value influenced the co-pyrolysis with the seeds. In the experiments with varied RSC: PS ratios, the results showed that the higher the amount of PS, the greater the increase in the calorific value of the biofuels, achieving up to 41.10 MJ kg−1. This same behavior can be observed in Table 2, except for Niger and Karanja seeds, where a seed:PS ratio of 2:1 maximizes the calorific value of the biofuels.
Table 2. Energy content of co-pyrolysis of PS and biomass seeds.
|
Type |
Seed: PS Ratio |
HHV [MJ kg−1] |
References |
|
Grape seeds |
100:0 |
35.49 |
[122] |
|
90:10 |
36.90 |
||
|
80:20 |
40.40 |
||
|
80:20 |
40.90 * |
[123] |
|
|
Coffee grounds |
100:0 |
25.91 |
[124] |
|
75:25 |
26.68 |
||
|
50:50 |
33.98 |
||
|
25:75 |
39.66 |
||
|
Rubber seeds |
1:0 |
32.25 |
[121] |
|
2:1 |
37.48 |
||
|
1:1 |
37.61 |
||
|
1:2 |
41.10 |
||
|
Karanja seeds |
1:0 |
37.65 |
[125] |
|
1:1 |
38.88 |
[126] |
|
|
2:1 |
42.18 |
||
|
4:1 |
29.02 |
||
|
8:1 |
26.53 |
||
|
Niger seed |
1:0 |
35.87 |
[127] |
|
1:1 |
32.15 |
[126] |
|
|
2:1 |
41.42 |
||
|
4:1 |
31.82 |
||
|
8:1 |
29.77 |
7. Conclusions
Statistical analysis showed that low temperatures are recommended if a pyrolytic liquid is required; in contrast, increasing temperatures enhance the production of the gaseous fraction. The PS pyrolytic liquid can contain in its composition high yields of styrene, benzene, toluene, xylene, and ethylbenzene, among others. Styrene has the highest yield, reaching up to 84.74% [86]. The application of PS pyrolytic oil as a fuel is questionable due to its high aromatic content, differentiating it from diesel; however, it has a similarity of about 60% with gasohol [109].
Furthermore, the mixture of PS with biomass in a co-pyrolysis process favors the liquid production that biomass by itself would not achieve and, in addition, improves its properties, such as increasing the heating value to values close to 41 MJ kg−1 and reducing up to 8.67% of its viscosity value [126][121]. It is concluded that the thermo-catalytic pyrolysis process is an effective recycling method to reduce a large amount of PS waste in the environment and convert it into high-value products for the chemical and petrochemical industry.
References
- Prajapati, R.; Kohli, K.; Maity, S.K.; Sharma, B.K. Potential Chemicals from Plastic Wastes. Molecules 2021, 26, 3175.
- Armenise, S.; SyieLuing, W.; Ramírez-Velázquez, J.M.; Launay, F.; Wuebben, D.; Ngadi, N.; Rams, J.; Muñoz, M. Plastic Waste Recycling via Pyrolysis: A Bibliometric Survey and Literature Review. J. Anal. Appl. Pyrolysis 2021, 158, 105265.
- Hasanzadeh, R.; Azdast, T.; Mojaver, M.; Park, C.B. High-Efficiency and Low-Pollutant Waste Polystyrene and Waste Polystyrene Foam Gasification: Comprehensive Comparison Analysis, Multi-Objective Optimization and Multi-Criteria Decision Analysis. Fuel 2022, 316, 123362.
- Wong, S.L.; Ngadi, N.; Abdullah, T.A.T.; Inuwa, I.M. Current State and Future Prospects of Plastic Waste as Source of Fuel: A Review. Renew. Sustain. Energy Rev. 2015, 50, 1167–1180.
- Jeswani, H.; Krüger, C.; Russ, M.; Horlacher, M.; Antony, F.; Hann, S.; Azapagic, A. Life Cycle Environmental Impacts of Chemical Recycling via Pyrolysis of Mixed Plastic Waste in Comparison with Mechanical Recycling and Energy Recovery. Sci. Total Environ. 2021, 769, 144483.
- Millican, J.M.; Agarwal, S. Plastic Pollution: A Material Problem? Macromolecules 2021, 54, 4455–4469.
- Klaimy, S.; Lamonier, J.-F.; Casseta, M.; Heymans, S.; Duquesne, S. Recycling of Plastic Waste Using Flash Pyrolysis—Effect of Mixture Composition. Polym. Degrad. Stab. 2021, 187, 109540.
- Sharuddin, S.D.A.; Abnisa, F.; Wan Daud, W.M.A.; Aroua, M.K. A Review on Pyrolysis of Plastic Wastes. Energy Convers. Manag. 2016, 115, 308–326.
- Al-Salem, S.M.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. A Review on Thermal and Catalytic Pyrolysis of Plastic Solid Waste (PSW). J. Environ. Manag. 2017, 197, 177–198.
- Plastics Europe Plastics-the Facts. 2022. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2022/ (accessed on 1 January 2023).
- Geyer, R.; Jamberck, J.R.; Law, K.L. Production, Use, and Fate of All Plastics Ever Made. Sci. Adv. 2017, 3, e1700782.
- Volk, R.; Stallkamp, C.; Steins, J.J.; Yogish, S.P.; Müller, R.C.; Stapf, D.; Schultmann, F. Techno-Economic Assessment and Comparison of Different Plastic Recycling Pathways: A German Case Study. J. Ind. Ecol. 2021, 25, 1318–1337.
- Uttaravalli, A.N.; Dinda, S.; Gidla, B.R. Scientific and Engineering Aspects of Potential Applications of Post-Consumer (Waste) Expanded Polystyrene: A Review. Process Safety Environ. Protec. 2020, 137, 140–148.
- Maafa, I.M. Pyrolysis of Polystyrene Waste: A Review. Polymers 2021, 13, 225.
- Nanda, S.; Berruti, F. Thermochemical Conversion of Plastic Waste to Fuels: A Review. Environ. Chem. Lett. 2021, 19, 123–148.
- Klemencová, K.; Grycová, B.; Inayat, A.; Lestinský, P. Thermo-Catalytic Degradation of Polystyrene over α-Fe2O3. In Proceedings of the Nanocon 2020, 12th International Conference on Nanomaterials—Research & Application, Brno, Czech Republic, 21–23 October 2020; pp. 267–271.
- Ramlo Sulong, N.H.; Mustapa, S.A.S.; Abdul Rashid, M.K. Application of Expanded Polystyrene (EPS) in Buildings and Constructions: A Review. J. Appl. Polym. Sci. 2017, 136, 47529.
- Aljabri, N.M.; Lai, Z.; Hadjichristidis, N.; Huang, K.-W. Renewable Aromatics from the Degradation of Polystyrene under Mild Conditions. J. Saudi Chem. Society 2017, 21, 983–989.
- Maaroufi, M.; Belarbi, R.; Abahri, K.; Benmahiddine, F. Full Characterization of Hygrothermal, Mechanical and Morphological Properties of a Recycled Expanded Polystyrene-Based Mortar. Constr. Building Mater. 2021, 301, 124310.
- Zhang, F.; Zhao, Y.; Wang, D.; Yan, M.; Zhang, J.; Zhang, P.; Ding, T.; Chen, L.; Chen, C. Current Technologies for Plastic Waste Treatment: A Review. J. Clean. Prod. 2021, 282, 124523.
- Hidalgo-Crespo, J.; Moreira, C.M.; Jervis, F.X.; Soto, M.; Amaya, J.L.; Banguera, L. Circular Economy of Expanded Polystyrene Container Production: Environmental Benefits of Household Waste Recycling Considering Renewable Energies. Energy Rep. 2022, 8, 306–311.
- Chen, X.; Wang, Y.; Zhang, L. Recent Progress in the Chemical Upcycling of Plastic Wastes. ChemSusChem 2021, 14, 4137–4151.
- Sun, K.; Themelis, N.J.; Bourtsalas, A.T.; Huang, Q. Selective Production of Aromatics from Waste Plastic Pyrolysis by Using Sewage Sludge Derived Char Catalyst. J. Clean. Prod. 2020, 268, 122038.
- Kosloski-Oh, S.C.; Wood, Z.A.; Manjarrez, Y.; De los Ríos, J.P.; Fieser, M.E. Catalytic Methods for Chemical Recycling or Upcycling of Commercial Polymers. Mater. Horiz. 2021, 8, 1084–1129.
- Roosen, M.; Mys, N.; Kusenberg, M.; Billen, P.; Dumoulin, A.; Dewulf, J.; Van Geem, K.M.; Ragaert, K.; De Meester, S. Detailed Analysis of the Composition of Selected Plastic Packaging Waste Products and Its Implications for Mechanical and Thermochemical Recycling. Environ. Sci. Technol. 2020, 54, 13282–13293.
- Jaafar, Y.; Abdelouahed, L.; El Hage, R.; El Samrani, A.; Taouk, B. Pyrolysis of Common Plastics and Their Mixtures to Produce Valuable Petroleum-like Products. Polym. Degrad. Stab. 2022, 195, 109770.
- Zeller, M.; Netsch, N.; Richter, F.; Leibold, H.; Stapf, D. Chemical Recycling of Mixed Plastic Wastes by Pyrolysis-Pilot Scale Investigations. Chem. Ing. Tech. 2021, 93, 1763–1770.
- Grigore, M.E. Methods of Recycling, Properties and Applications of Recycled Thermoplastic Polymers. Recycling 2017, 2, 24.
- Liu, S.; Kots, P.A.; Vance, B.; Danielson, A.; Vlachos, D.G. Plastic Waste to Fuels by Hydrocracking at Mild Conditions. Sci. Adv. 2021, 7, eabf8283.
- Samori, C.; Parodi, A.; Tagliavini, E.; Galletti, P. Recycling of Post-Use Starch-Based Plastic Bags through Pyrolysis to Produce Sulfonated Catalysts and Chemicals. J. Anal. Appl. Pyrolysis 2021, 155, 105030.
- Nzioka, A.M.; Yan, C.Z.; Kim, M.-G.; Sim, Y.-J.; Lee, C.-S.; Kim, Y.-J. Improvement of the Chemical Recycling Process of Waste Carbon Fibre Reinforced Plastics Using a Mechanochemical Process: Influence of Process Parameters. Waste Manag. Res. 2018, 36, 952–964.
- Coates, G.W.; Getzler, Y.D.Y.L. Chemical Recycling to Monomer for an Ideal, Circular Polymer Economy. Nature Rev. Mater. 2020, 5, 501–516.
- Dement’ev, K.I.; Palankoev, T.A.; Alekseeva, O.A.; Babkin, I.; Maksimov, A.L. Thermal Depolymerization of Polystyrene in Highly Aromatic Hydrocarbon Medium. J. Anal. Appl. Pyrolysis 2019, 142, 104612.
- Okan, M.; Aydin, H.M.; Barsbay, M. Current Approaches to Waste Polymer Utilization and Minimization: A Review. J. Chem. Technol. Biotech. 2019, 94, 8–21.
- Heidari, M.; Garnaik, P.P.; Dutta, A. The Valorization of Plastic Via Thermal Means: Industrial Scale Combustion Methods. In Plastics to Energy; Fuel, Chemicals, and Sustainability Implications; William Andrew: Norwich, NY, USA, 2019; Volume Plastics Design Library, pp. 295–312. Available online: https://www.sciencedirect.com/book/9780128131404/plastics-to-energy (accessed on 15 March 2023).
- Zhang, G.; Huang, X.; Liao, W.; Kang, S.; Ren, M.; Hai, J. Measurement of Dioxin Emissions from a Small-Scale Waste Incinerator in the Absence of Air Pollution Controls. Int. J. Environ. Res. Public Health 2019, 16, 1267.
- Kusenberg, M.; Eschenbacher, A.; Djokic, M.R.; Zayoud, A.; Ragaert, K.; De Meester, S.; Van Geem, K.M. Opportunities and Challenges for the Application of Post-Consumer Plastic Waste Pyrolysis Oils as Steam Cracker Feedstocks: To Decontaminate or Not to Decontaminate? Waste Manag. 2022, 138, 83–115.
- Lopez, G.; Artetxe, M.; Amutio, M.; Bilbao, J.; Olazar, M. Thermochemical Routes for the Valorization of Waste Polyolefinic Plastics to Produce Fuels and Chemicals. A Review. Renew. Sustain. Energy Rev. 2017, 73, 346–368.
- Kremer, I.; Tomic, T.; Katancic, Z.; Hrnjak-Murgic, Z.; Erceg, M.; Schneider, D.R. Catalytic Decomposition and Kinetic Study of Mixed Plastic Waste. Clean Technol. Environ. Policy 2021, 23, 811–827.
- Martín-Lara, M.A.; Piñar, A.; Ligero, A.; Blázquez, G.; Calero, M. Characterization and Use of Char Produced from Pyrolysis of Post-Consumer Mixed Plastic Waste. Water 2021, 13, 1188.
- Nwankwor, P.E.; Onuigbo, I.O.; Chukwuneke, C.E.; Yahaya, M.F.; Agboola, B.O.; Jahng, W.J. Synthesis of Gasoline Range Fuels by Catalytic Cracking of Waste Plastics Using Titanium Dioxide and Zeolite. Int. J. Energy Environ. Eng. 2021, 12, 77–86.
- Holubcik, M.; Klackova, I.; Durcansky, P. Pyrolysis Conversion of Polymer Wastes to Noble Fuels in Conditions of the Slovak Republic. Energies 2020, 13, 4849.
- Quesada, L.; Calero, M.; Martín-Lara, M.A.; Pérez, A.; Blázquez, G. Characterization of Fuel Produced by Pyrolysis of Plastic Film Obtained of Municipal Solid Waste. Energy 2019, 186, 115874.
- Quesada, L.; Calero, M.; Martín-Lara, M.Á.; Pérez, A.; Blázquez, G. Production of an Alternative Fuel by Pyrolysis of Plastic Wastes Mixtures. Energy Fuels 2020, 34, 1781–1790.
- Lerici, L.C.; Renzini, M.S.; Pierella, L.B. Chemical Catalyzed Recycling of Polymers: Catalytic Conversion of PE, PP and PS into Fuels and Chemicals over H-Y. Procedia Mater. Sci. 2015, 8, 297–303.
- Parku, G.K.; Collard, F.-X.; Görgens, J.F. Pyrolysis of Waste Polypropylene Plastics for Energy Recovery: Influence of Heating Rate and Vacuum Conditions on Composition of Fuel Product. Fuel Proc. Technol. 2020, 209, 106522.
- Riesco-Ávila, J.M.; Vera-Rozo, J.R.; Rodríguez-Valderrama, D.A.; Pardo-Cely, D.M.; Ramón-Valencia, B. Effects of Heating Rate and Temperature on the Yield of Thermal Pyrolysis of a Random Waste Plastic Mixture. Sustainability 2022, 14, 9026.
- Wyss, K.M.; Beckham, J.L.; Chen, W.; Luong, D.X.; Hundi, P.; Raghuraman, S.; Shahsavarri, R.; Tour, J.M. Converting Plastic Waste Pyrolysis Ash into Flash Graphene. Carbon 2021, 2021, 430–438.
- Wu, C.; Nahil, M.A.; Miskolczi, N.; Huang, J.; Williams, P.T. Production and Application of Carbon Nanotubes, as a Co-Product of Hydrogen from the Pyrolysis-Catalytic Reforming of Waste Plastic. Process Safety Environ. Protec. 2016, 103, 107–114.
- Wang, J.; Jiang, J.; Sun, Y.; Zhong, Z.; Wang, X.; Xia, H.; Liu, G.; Pang, S.; Wang, K.; Li, M.; et al. Recycling Benzene and Ethylbenzene from In-Situ Catalytic Fast Pyrolysis of Plastic Wastes. Energy Convers. Manag. 2019, 200, 112088.
- Zhou, J.; Qiao, Y.; Wang, W.; Leng, E.; Huang, J.; Yu, Y.; Xu, M. Formation of Styrene Monomer, Dimer and Trimer in the Primary Volatiles Produced from Polystyrene Pyrolysis in a Wire-Mesh Reactor. Fuel 2016, 182, 333–339.
- Park, K.-B.; Jeong, Y.-S.; Guzelciftci, B.; Kim, J.-S. Two-Stage Pyrolysis of Polystyrene: Pyrolysis Oil as a Source of Fuels or Benzene, Toluene, Ethylbenzene, and Xylenes. Appl. Energy 2020, 259, 114240.
- Jha, K.K.; Kannan, T.T.M. Recycling of Plastic Waste into Fuel by Pyrolysis—A Review. Mater. Today Proc. 2021, 37, 3718–3720.
- Nisar, J.; Ali, G.; Shah, A.; Iqbal, M.; Ali Khan, R.; Sirajuddin; Anwar, F.; Ullah, R.; Akhter, M.S. Fuel Production from Waste Polystyrene via Pyrolysis: Kinetics and Products Distribution. Waste Manag. 2019, 88, 236–247.
- Miandad, R.; Nizami, A.S.; Barakat, M.A.; Khan, M.I.; Mustafa, A.; Ismail, I.M.I.; Murphy, J.D. Influence of Temperature and Reaction Time on the Conversion of Polystyrene Waste to Pyrolysis Liquid Oil. Waste Manag. 2016, 58, 250–259.
- Soni, V.K.; Singh, G.; Vijayan, B.K.; Chopra, A.; Kapur, G.S.; Ramakumar, S.S.V. Thermochemical Recycling of Waste Plastics by Pyrolysis: A Review. Energy Fuels 2021, 35, 12763–12808.
- Orozco, S.; Alvarez, J.; Lopez, G.; Artetxe, M.; Bilbao, J.; Olazar, M. Pyrolysis of Plastic Wastes in a Fountain Confined Conical Spouted Bed Reactor: Determination of Stable Operating Conditions. Energy Convers. Manag. 2021, 229, 113768.
- Plastics Europe. An Analysis of European Plastics Production, Demand and Waste Data; Plastics—The Facts 2020; Plastics Europe: Brussels, Belgium, 2020.
- IUPAC. Polystyrene. Available online: https://pubchem.ncbi.nlm.nih.gov/#query=polystyrene (accessed on 15 January 2023).
- Gil-Jasso, N.D.; Segura-González, M.A.; Soriano-Giles, G.; Neri-Hipolito, J.; Lopez, N.; Mas-Hernández, E.; Barrera-Díaz, C.E.; Varela-Guerrero, V.; Ballesteros-Rivas, M.F. Dissolution and Recovery of Waste Expanded Polystyrene Using Alternative Essential Oils. Fuel 2019, 239, 611–616.
- Calder, J.; Roy, M.M.; Wang, W. Performance and Emissions of a Diesel Engine Fueled by Biodiesel-Diesel Blends with Recycled Expanded Polystyrene and Fuel Stabilizing Additive. Energy 2018, 149, 204–212.
- Wypych, G. Handbook of Polymers, 2nd ed.; Chemical Technology Publishing: Toronto, ON, Canada, 2016; ISBN 978-1-895198-92-8.
- Tamri, Z.; Yazdi, A.V.; Haghighi, M.N.; Abbas-Abadi, M.S.; Heidarinasab, A. Effect of Temperature, Heating Rate and Zeolite-Based Catalysts on the Pyrolysis of High Impact Polystyrene (HIPS) Waste to Produce Fuel-like Products. Polyolefins J. 2019, 6, 43–52.
- Nciri, N.; Shin, T.; Cho, N. Towards the Use of Waste Expanded Polystyrene as Potential Modifier for Flexible Road Pavements. Mater. Today Proc. 2020, 24, 763–771.
- Verma, A.; Sharma, S.; Pramanik, H. Pyrolysis of Waste Expanded Polystyrene and Reduction of Styrene via In-Situ Multiphase Pyrolysis of Product Oil for the Production of Fuel Range Hydrocarbons. Waste Manag. 2021, 120, 330–339.
- Ng, H.M.; Saidi, N.M.; Omar, F.S.; Ramesh, K.; Bashir, S. Thermogravimetric Analysis of Polymers. Encycl. Polym. Sci. Technol. 2018, 1–29. https://doi.org/10.1002/0471440264.pst667
- Ren, X.; Huang, Z.; Wang, X.-J.; Guo, G. Isoconversional Analysis of Kinetic Pyrolysis of Virgin Polystyrene and Its Two Real-World Packaging Wastes. J. Therm. Anal. Calorim. 2022, 147, 1421–1437. https://doi.org/10.1007/s10973-020-10411-9.
- Tamri, Z.; Yazdi, A.V.; Haghighi, M.N.; Abbas-Abadi, M.S.; Heidarinasab, A. Effect of Temperature, Heating Rate and Zeolite-Based Catalysts on the Pyrolysis of High Impact Polystyrene (HIPS) Waste to Produce Fuel-like Products. Polyolefins J. 2019, 6, 43–52. https://doi.org/10.22063/poj.2018.2189.1114.
- Fuentes, C.; Colman Lerner, J.; Vázquez, P.; Sambeth, J. Analysis of the Emission of PAH in the Thermal and Catalytic Pyrolysis of Polystyrene. Catal. Today 2021, 372, 175–182. https://doi.org/10.1016/j.cattod.2020.11.030.
- Lu, C.; Xiao, H.; Chen, X. Simple Pyrolysis of Polystyrene into Valuable Chemicals. e-Polymers 2021, 21, 428–432. https://doi.org/10.1515/epoly-2021-0037.
- Inayat, A.; Fasolini, A.; Basile, F.; Fridrichova, D.; Lestinský, P. Chemical Recycling of Waste Polystyrene by Thermo-Catalytic Pyrolysis: A Description for Different Feedstocks, Catalysts and Operation Modes. Polym. Degrad. Stab. 2022, 201, 109981. https://doi.org/10.1016/j.polymdegradstab.2022.109981.
- Nisar, J.; Khan, M.S.; Ali, G.; Shah, A.; Ali Khan, R.; Shah, F. Pyrolysis of Polystyrene: The Influence of Commercially Available Oxides as Catalysts. J. Chem. Soc. Pak. 2019, 41, 779.
- Hassan, E.B.; Elsayed, I.; Eseyin, A. Production High Yields of Aromatic Hydrocarbons through Catalytic Fast Pyrolysis of Torrefied Wood and Polystyrene. Fuel 2016, 174, 317–324. https://doi.org/10.1016/j.fuel.2016.02.031.
- Jiang, L.; Yang, X.-R.; Gao, X.; Xu, Q.; Das, O.; Sun, J.-H.; Kuzman, M.K. Pyrolytic Kinetics of Polystyrene Particle in Nitrogen Atmosphere: Particle Size Effects and Application of Distributed Activation Energy Method. Polymers 2020, 12, 421. https://doi.org/10.3390/polym12020421.
- Ding, L.; Zhao, J.; Pan, Y.; Guan, J.; Jiang, J.; Wang, Q. Insights into Pyrolysis of Nano-Polystyrene Particles: Thermochemical Behaviors and Kinetics Analysis. J. Therm. Sci. 2019, 28, 763–771. https://doi.org/10.1007/s11630-019-1123-7.
- Inayat, A.; Klemencova, K.; Grycová, B.; Sokolava, B.; Lestinský, P. Thermo-Catalytic Pyrolysis of Polystyrene in Batch and Semi-Batch Reactors: A Comparative Study. Waste Manag. Res. 2021, 39, 260–269. https://doi.org/10.1177/0734242X20936746.
- Nisar, J.; Ali, G.; Shah, A.; Iqbal, M.; Ali Khan, R.; Sirajuddin; Anwar, F.; Ullah, R.; Akhter, M.S. Fuel Production from Waste Polystyrene via Pyrolysis: Kinetics and Products Distribution. Waste Manag. 2019, 88, 236–247. https://doi.org/10.1016/j.wasman.2019.03.035.
- Miandad, R.; Nizami, A.S.; Barakat, M.A.; Khan, M.I.; Mustafa, A.; Ismail, I.M.I.; Murphy, J.D. Influence of Temperature and Reaction Time on the Conversion of Polystyrene Waste to Pyrolysis Liquid Oil. Waste Manag. 2016, 58, 250–259.
- Burra, K.R.G.; Liu, X.; Wang, Z.; Li, J.; Che, D.; Gupta, K. Quantifying the Sources of Synergistic Effects in Co-Pyrolysis of Pinewood and Polystyrene. Appl. Energy 2021, 302, 117562. https://doi.org/10.1016/j.apenergy.2021.117562.
- Amjad, U.-S.; Ishaq, M.; Rehman, H.U.; Ahmad, N.; Sherin, L.; Hussain, M.; Mustafa, M. Diesel and Gasoline like Fuel Pro-duction with Minimum Styrene Content from Catalytic Pyrolysis of Polystyrene. Environ. Prog. Sustain. Energy 2021, 40, e13493. https://doi.org/10.1002/ep.13493.
- Adnan; Shah, J.; Jan, M.R. Recovery of Valuable Hydrocarbons from Waste Polystyrene Using Zinc Supported Catalysts. J. Polym. Environ. 2017, 25, 759–769. https://doi.org/10.1007/s10924-016-0858-4.
- Gonzalez-Aguilar, A.M.; Cabrera-Madera, V.P.; Vera-Rozo, J.R.; Riesco-Ávila, J.M. Effects of Heating Rate and Temperature on the Thermal Pyrolysis of Expanded Polystyrene Post-Industrial Waste. Polymers 2022, 14, 4957. https://doi.org/10.3390/polym14224957.
- Klaimy, S.; Lamonier, J.-F.; Casseta, M.; Heymans, S.; Duquesne, S. Recycling of Plastic Waste Using Flash Pyrolysis—Effect of Mixture Composition. Polym. Degrad. Stab. 2021, 187, 109540. https://doi.org/10.1016/j.polymdegradstab.2021.109540.
- Jha, K.K.; Kannan, T.T.M. Recycling of Plastic Waste into Fuel by Pyrolysis—A Review. Mater. Today Proc. 2021, 37, 3718–3720. https://doi.org/10.1016/j.matpr.2020.10.181.
- Maafa, I.M. Pyrolysis of Polystyrene Waste: A Review. Polymers 2021, 13, 225. https://doi.org/10.3390/polym13020225.
- Hu, Q.; Tang, Z.; Yao, D.; Yang, H.; Shao, J.; Chen, H. Thermal Behavior, Kinetics and Gas Evolution Characteristics for the Co-Pyrolysis of Real-World Plastic and Tyre Wastes. J. Clean. Prod. 2020, 260, 121102. https://doi.org/10.1016/j.jclepro.2020.121102.
- Fogler, H.S. Elements of Chemical Reaction Engineering. Chem. Eng. Sci. 1987, 42, 2493. https://doi.org/10.1016/0009-2509(87)80130-6.
- Saad, J.M.; Nahil, M.A.; Williams, P.T. Influence of Process Conditions on Syngas Production from the Thermal Processing of Waste High Density Polyethylene. J. Anal. Appl. Pyrolysis 2015, 113, 35–40. https://doi.org/10.1016/j.jaap.2014.09.027.
- Aljabri, N.M.; Lai, Z.; Hadjichristidis, N.; Huang, K.-W. Renewable Aromatics from the Degradation of Polystyrene under Mild Conditions. J. Saudi Chem. Society 2017, 21, 983–989.
- Hussain, Z.; Imtiaz, M.; Naz, M.Y.; Khan, K.M.; AbdEl-Salam, N.M.; Ibrahim, K.A. Thermal and Clinker-Catalyzed Pyrolyses of Polystyrene Waste Using the Portland Cement Solid-Base Catalyst. Asia-Pac. J. Chem. Eng. 2021, 16, e2556. https://doi.org/10.1002/apj.2556.
- Palmay, P.; Nuñez, R.; Donoso, C.; Bruno, J. Influence of Temperature and Reaction Time on the Performance of Thermal Pyrolysis of Compact Polystyrene Waste. IOP Conf. Ser. Earth Environ. Sci. 2021, 728, 012005.
- Klemencová, K.; Grycová, B.; Inayat, A.; Lestinský, P. Thermo-Catalytic Degradation of Polystyrene over α-Fe2O3. In Proceedings of the Nanocon 2020, 12th International Conference on Nanomaterials—Research & Application, Brno, Czech Republic, 21–23 October 2020; pp. 267–271. https://doi.org/10.37904/nanocon.2020.3719.
- Sogancioglu, M.; Yel, E.; Ahmetli, G. Investigation of the Effect of Polystyrene (PS) Waste Washing Process and Pyrolysis Temperature on (PS) Pyrolysis Product Quality. Energy Procedia 2017, 118, 189–194. https://doi.org/10.1016/j.egypro.2017.07.029.
- Abdullah, N.A.; Novianti, A.; Hakim, I.I.; Putra, N.; Koestoer, R.A. Influence of Temperature on Conversion of Plastics Waste (Polystyrene) to Liquid Oil Using Pyrolysis Process. IOP Conf. Ser. Earth Environ. Sci. 2018, 105, 012033. https://doi.org/10.1088/1755-1315/105/1/012033.
- Çelikgöğüs, Ç.; Karaduman, A. Thermal-Catalytic Pyrolysis of Polystyrene Waste Foams in a Semi-Batch Reactor. Energy Sources Part A Recovery Util. Environ. Effect 2015, 37, 2507–2513. https://doi.org/10.1080/15567036.2011.626492.
- Shah, J.; Jan, M.R.; Adnan Tertiary Recycling of Waste Polystyrene Using Magnesium Impregnated Catalyst into Valuable Products. J. Anal. Appl. Pyrolysis 2015, 114, 163–171. https://doi.org/10.1016/j.jaap.2015.05.009.
- Nisar, J.; Ali, G.; Shah, A.; Ashiq, M.N.; Farooqi, Z.H.; Sharif, A.; Ahmed, E.; Iqbal, M.; Sherazi, S.T.H.; Shah, M.R. Pyrolysis of Polystyrene Waste for Recovery of Combustible Hydrocarbons Using Copper Oxide as Catalyst. Waste Manag. Res. 2020, 38, 1269–1277. https://doi.org/10.1177/0734242X20904403.
- Lopez, G.; Artetxe, M.; Amutio, M.; Bilbao, J.; Olazar, M. Thermochemical Routes for the Valorization of Waste Polyolefinic Plastics to Produce Fuels and Chemicals. A Review. Renew. Sustain. Energy Rev. 2017, 73, 346–368. https://doi.org/10.1016/j.rser.2017.01.142.
- Uttaravalli, A.N.; Dinda, S.; Gidla, B.R. Scientific and Engineering Aspects of Potential Applications of Post-Consumer (Waste) Expanded Polystyrene: A Review. Process Safety Environ. Protec. 2020, 137, 140–148. https://doi.org/10.1016/j.psep.2020.02.023.
- Kosloski-Oh, S.C.; Wood, Z.A.; Manjarrez, Y.; De los Ríos, J.P.; Fieser, M.E. Catalytic Methods for Chemical Recycling or Upcycling of Commercial Polymers. Mater. Horiz. 2021, 8, 1084–1129. https://doi.org/10.1039/d0mh01286f.
- Eze, W.U.; Madufor, I.C.; Onyeagoro, G.N.; Obasi, H.C.; Ugbaja, M.I. Study on the Effect of Kankara Zeolite‑Y‑based Catalyst on the Chemical Properties of Liquid Fuel from Mixed Waste Plastics (MWPs) Pyrolysis. Polym. Bull. 2021, 78, 377–398. https://doi.org/10.1007/s00289-020-03116-4.
- Abbas-Abadi, M.S. The Effect of Process and Structural Parameters on the Stability, Thermo‑mechanical and Thermal Degradation of Polymers with Hydrocarbon Skeleton Containing PE, PP, PS, PVC, NR, PBR and SBR. J. Therm. Anal. Calorim. 2021, 143, 2867–2882. https://doi.org/10.1007/s10973-020-09344-0.
- Rehan, M.; Miandad, R.; Barakat, M.A.; Ismail, I.M.I.; Almeelbi, T.; Gardy, J.; Hassanpour, A.; Khan, M.Z.; Demirbas, A.; Nizami, A.S. Effect of Zeolite Catalysts on Pyrolysis Liquid Oil. Int. Biodeter. Biodegrad. 2017, 119, 162–175. https://doi.org/10.1016/j.ibiod.2016.11.015.
- Dewangga, P.B.; Purnomo, C.W. Styrene Recovery from the Pyrolysis of Polystyrene Waste Using Bentonite and Natural Zeolite Catalyst. Key Eng. Mater. 2020, 849, 84–89. https://doi.org/10.4028/www.scientific.net/kem.849.84.
- Verma, A.; Sharma, S.; Pramanik, H. Pyrolysis of Waste Expanded Polystyrene and Reduction of Styrene via In-Situ Multiphase Pyrolysis of Product Oil for the Production of Fuel Range Hydrocarbons. Waste Manag. 2021, 120, 330–339. https://doi.org/10.1016/j.wasman.2020.11.035.
- Dogu, O.; Pelucchi, M.; Van de Vijver, R.; Van Steenberge, P.H.M.; D’hooge, D.R.; Cuoci, A.; Mehl, M.; Frassoldati, A.; Faravelli, T.; Van Geem, K.M. The Chemistry of Chemical Recycling of Solid Plastic Waste via Pyrolysis and Gasification: State-of-the-Art, Challenges, and Future Directions. Prog. Energy Comb. Sci. 2021, 84, 100901. https://doi.org/10.1016/j.pecs.2020.100901.
- Jahirul, M.I.; Faisal, F.; Rasul, M.G.; Schaller, D.; Khan, M.M.K.; Dexter, R.B. Automobile Fuels (Diesel and Petrol) from Plastic Pyrolysis Oil-Production and Characterisation. Energy Rep. 2022, 8, 730–735. https://doi.org/10.1016/j.egyr.2022.10.218.
- Park, K.-B.; Jeong, Y.-S.; Guzelciftci, B.; Kim, J.-S. Two-Stage Pyrolysis of Polystyrene: Pyrolysis Oil as a Source of Fuels or Benzene, Toluene, Ethylbenzene, and Xylenes. Appl. Energy 2020, 259, 114240. https://doi.org/10.1016/j.apenergy.2019.114240.
- Budsaereechai, S.; Hunt, A.J.; Ngernyen, Y. Catalytic Pyrolysis of Plastic Waste for the Production of Liquid Fuels for Engines. RSC Adv. 2019, 9, 5844. https://doi.org/10.1039/c8ra10058f.
- Pinto, F.; Costa, P.; Gulyurtlu, I.; Cabrita, I. Pyrolysis of Plastic Wastes. 1. Effect of Plastic Waste Composition on Product Yield. J. Anal. Appl. Pyrolysis 1998, 51, 39–55. https://doi.org/10.1016/S0165-2370(99)00007-8.
- Blazsó, M. Composition of Liquid Fuels Derived from the Pyrolysis of Plastics. In Feedstock Recycling and Pyrolysis of Waste Plastics: Converting Waste Plastics into Diesel and Other Fuels; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2006; ISBN 978-0-470-02152-1.
- Ahmad, I.; Khan, M.I.; Khan, H.; Ishaq, M.; Tariq, R.; Gul, K.; Ahmad, W. Pyrolysis Study of Polypropylene and Polyethylene Into Premium Oil Products. Int. J. Green Energy 2015, 12, 663–671. https://doi.org/10.1080/15435075.2014.880146.
- Martínez-Narro, G.; Pozos-Vázquez, C.; Núñez-Delgado, A.; Morán-Medellín, D.; Lara-Zárate, V.E. Heavy Crude Oil Viscosity Reduction with Hydrocarbons Obtained via Pyrolysis of Polypropylene and Polystyrene. Petroleum Sci. Technol. 2020, 38, 651–658. https://doi.org/10.1080/10916466.2020.1769654.
- What is Biomass? Available online: https://www.appa.es/appa-biomasa/que-es-la-biomasa/ (accessed on 15 December 2022).
- Dyer, A.C.; Nahil, M.A.; Williams, P.T. Biomass:Polystyrene Co-Pyrolysis Coupled with Metal-Modified Zeolite Catalysis for Liquid Fuel and Chemical Production. J. Mater. Cycles Waste Manag. 2022, 24, 477–490. https://doi.org/10.1007/s10163-021-01334-0.
- Muhammad, I.; Manos, G. Intensification of Co-Pyrolysis of Plastic with Biomass via Pretreatment. Process Safety Environ. Protec. 2021, 146, 586–598. https://doi.org/10.1016/j.psep.2020.11.042.
- Li, Z.; Zhong, Z.; Zhang, B.; Wang, W.; Seufitelli, G.V.S.; Resende, F.L.P. Catalytic Fast Co-Pyrolysis of Waste Greenhouse Plastic Films and Rice Husk Using Hierarchical Micro-Mesoporous Composite Molecular Sieve. Waste Manag. 2020, 102, 561–568. https://doi.org/10.1016/j.wasman.2019.11.012.
- Fekhar, B.; Zsinka, V.; Miskolczi, N. Thermo-Catalytic Co-Pyrolysis of Waste Plastic and Paper in Batch and Tubular Reactors for in-Situ Product Improvement. J. Environ. Manag. 2020, 268, 110741. https://doi.org/10.1016/j.jenvman.2020.110741.
- Akubo, K.; Nahil, M.A.; Williams, P.T. Co‑pyrolysis–Catalytic Steam Reforming of Cellulose/Lignin with Polyethylene/Polystyrene for the Production of Hydrogen. Waste Dispos. Sustain. Energy 2020, 2, 177–191. https://doi.org/10.1007/s42768-020-00047-8.
- Ryu, H.W.; Kim, D.H.; Jae, J.; Lam, S.S.; Park, E.D.; Park, Y.-K. Recent Advances in Catalytic Co-Pyrolysis of Biomass and Plastic Waste for the Production of Petroleum-like Hydrocarbons. Bioresour. Technol. 2020, 310, 123473. https://doi.org/10.1016/j.biortech.2020.123473.
- Reshad, A.S.; Tiwari, P.; Goud, V.V. Thermal and Co-Pyrolysis of Rubber Seed Cake with Waste Polystyrene for Bio-Oil Production. J. Anal. Appl. Pyrolysis 2019, 139, 333–343.
- Veses, A.; Sanahuja-Parejo, O.; Navarro, M.V.; López, J.M.; Murillo, R.; Callén, M.S.; García, T. From Laboratory Scale to Pilot Plant: Evaluation of the Catalytic Co-Pyrolysis of Grape Seeds and Polystyrene Wastes with CaO. Catal. Today 2021, 379, 87–95. https://doi.org/10.1016/j.cattod.2020.04.054.
- Muelas, Á.; Aranda, D.; Callén, M.S.; Murillo, R.; Veses, A.; Asrardel, M.; Ballester, J. Properties and Combustion Characteristics of Bio-Oils from Catalytic Co-Pyrolysis of Grape Seeds, Polystyrene, and Waste Tyres. Energy Fuels 2020, 34, 14190–14203. https://doi.org/10.1021/acs.energyfuels.0c02257.
- Nguyen, Q.V.; Choi, Y.-S.; Choi, S.-K.; Jeong, Y.-W.; Han, S.-Y. Co-Pyrolysis of Coffee-Grounds and Waste Polystyrene Foam: Synergistic Effect and Product Characteristics Analysis. Fuel 2021, 292, 120375. https://doi.org/10.1016/j.fuel.2021.120375.
- Shadangi, K.P.; Mohanty, K. Thermal and Catalytic Pyrolysis of Karanja Seed to Produce Liquid Fuel. Fuel 2014, 115, 434–442. https://doi.org/10.1016/j.fuel.2013.07.053.
- Shadangi, K.P.; Mohanty, K. Co-Pyrolysis of Karanja and Niger Seeds with Waste Polystyrene to Produce Liquid Fuel. Fuel 2015, 153, 492–498. https://doi.org/10.1016/j.fuel.2015.03.017.
- Shadangi, K.P.; Mohanty, K. Production and Characterization of Pyrolytic Oil by Catalytic Pyrolysis of Niger Seed. Fuel 2014, 126, 109–115. https://doi.org/10.1016/j.fuel.2014.02.035.




