Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | José Pereira | -- | 7556 | 2023-04-06 13:06:30 | | | |
| 2 | Rita Xu | -10 word(s) | 7546 | 2023-04-07 04:53:35 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pereira, J.; Moita, A.; Moreira, A. 2D Nanofluids. Encyclopedia. Available online: https://encyclopedia.pub/entry/42844 (accessed on 24 January 2026).
Pereira J, Moita A, Moreira A. 2D Nanofluids. Encyclopedia. Available at: https://encyclopedia.pub/entry/42844. Accessed January 24, 2026.
Pereira, José, Ana Moita, António Moreira. "2D Nanofluids" Encyclopedia, https://encyclopedia.pub/entry/42844 (accessed January 24, 2026).
Pereira, J., Moita, A., & Moreira, A. (2023, April 06). 2D Nanofluids. In Encyclopedia. https://encyclopedia.pub/entry/42844
Pereira, José, et al. "2D Nanofluids." Encyclopedia. Web. 06 April, 2023.
Copy Citation
The homogeneous dispersions of 2D nanomaterials in heat transfer base fluids—so-called 2D nanofluids. The data compilation emerged from the critical overview of the findings of the published scientific articles regarding 2D nanofluids. The applicability of such fluids as promising alternatives to the conventional heat transfer and thermal energy storage fluids is comprehensively investigated. These are fluids that simultaneously possess superior thermophysical properties and can be processed according to innovative environmentally friendly methods and techniques.
Heat Transfer
2D Nanomaterials
Thermal Management
1. Introduction
Introductory studies on 1D nanostructures received immediate attention from researchers soon after their introduction by Iijima [1] on carbon nanotubes (CNTs). Following this, various types of 0D and 1D nanostructures were investigated. The recent advances in layered nanomaterials allow the industrial production of different 2D nanomaterials, where their atoms are combined in flat layers that can be stacked on top of each other. Current 2D nanomaterials are of great relevance in the nanotechnologies field of work and research [2], given that their properties, especially in heat transfer practical situations, hold greater potential compared with traditional nanoparticles [3]. Recently, 2D nanomaterials and nanofluids have become the focus of the scientific community because of their improved thermophysical properties [4]. These innovative nanomaterials and nanofluids include graphene (Gr), graphene oxide (GO), reduced graphene oxide (rGO), CNTs, fullerenes, transition metal dichalcogenides, graphitic carbon nitride, hexagonal boron nitride (h-BN), tungsten disulfide (WS2), and molybdenum disulfide (MoS2), MXene, among others, dispersed in adequate base fluids. In this kind of nanomaterial, the heat transfer, photons, and charge carriers will be limited in the 2D plane, leading to intense modifications in the electrical and optical features of such nanomaterials [5]. These materials will perform better than the traditional nanoparticles and corresponding nanofluids due to their large surface area to volume ratio [6]. Apart from three-dimensional nanomaterials, there are two-dimensional (2D), one-dimensional (1D), and zero-dimensional (0D) nanomaterials. The latter are those materials in which all three dimensions are beyond the micro/nanoscale under the form of core–shell nanoparticles, composite nanoparticles, and carbon and graphene quantum dots. One-dimensional nanomaterials are those in which two dimensions are at nanoscale and the remaining dimension is expressed (in most cases) in millimeters. Examples of such nanomaterials are CNTs, carbon nanowires, and graphene fibers. Finally, 2D nanomaterials possess one of their dimensions at nanoscale and the other two dimensions at a different scale. Examples of 2D nanomaterials are graphene nanomesh, nanosheets, and nanowires. In terms of thermal characteristics, such as thermal conductivity (TC) and heat transfer coefficient and rate, these are generally improved during the transfer from the OD nanomaterials to the 2D nanomaterials. However, other factors also influence predominantly the heat transfer capability of these materials, such as the chemical composition, surface-to-mass and volume ratios, and charge carrier mobility, among other factors. For instance, and considering only the heat transfer performance, carbon or graphene quantum dots (0D) are widely used in solar cells and solar light harvesting systems. Those nanomaterials have an improved absorption ability in the ultra-violet region, complementing the solar light harvesting in the visible region of the systems and enhancing their overall efficiencies. There are not used in search of very high TC values, for instance. On the other hand, 1D nanomaterials, such as carbon nanotubes, can achieve a TC of approximately 6000 W·m−1·K−1. Furthermore, 2D nanomaterials, such as graphene nanoplatelets, can reach 6500 W·m−1·K−1 [7] of TC, and graphene oxide nanosheets can achieve TC values of nearly 6800 W·m−1·K−1 [8], enhancing the heat transfer capability to its fullest. Figure 1 shows the materials and their possible forms in 0D, 1D, and 2D nanomaterials.

Figure 1. Types of 0D, 1D, and 2D nanomaterials.
One of the most common types of layered nanomaterial is Gr, and it has been widely studied for its superior characteristics and uses in different fields [9]. Two-dimensional nanomaterials can be a suitable option as nanofillers in traditional heat transfer fluids as they present a very high heat transfer area [10]. A common synthesis technique for these layered nanomaterials is exfoliation, where the single layers of the material are separated from each other by chemical or mechanical procedures [11]. It is worth mentioning that even though the exfoliation process can be performed mechanically on a small scale, liquid phase procedures are required for various ends, such as nanoelectronics, micro-electromechanical systems, nanoelectromechanical systems, and sensors, among others. These 2D materials can also be manufactured by chemical growth of the individual layers, which is the most common technique to produce Gr nanosheets [12], using chemical vapor deposition (CVD) onto the surface of catalysts, such as copper or silica, through heating at temperature values up to 1200 °C and passing a carbon-containing gas (e.g., methane) over the catalyst. Coleman et al. [2] demonstrated that it is possible to synthesize 2D materials, such as MoS2, WS2, and h-BN, using wet exfoliation procedures. The exfoliation process of 2D insulators, such as h-BN, would decrease its residual bulk conductance [13] and promote the surface-driven effects. This novel class of materials and fluids represent a broadened, unexploited part of 2D systems with superior thermophysical characteristics, high specific surface areas of relevance for thermal management, and thermal energy storage purposes. Furthermore, Gr nanofluids can be potentially investigated and utilized as a promising thermal fluid in many applications, including concentrated solar power (CSP) and PV/T (photovoltaic/thermal) systems due the following advantages:
These benefits offer the 2D nanomaterials and corresponding nanofluids the possibility to perform better in comparison with conventional nanomaterials and nanofluids. Figure 2 presents a schematic qualitative comparison of the main thermophysical characteristics and cost effectiveness to be considered in heat transfer enhancement applications of the main 2D nanofluids.
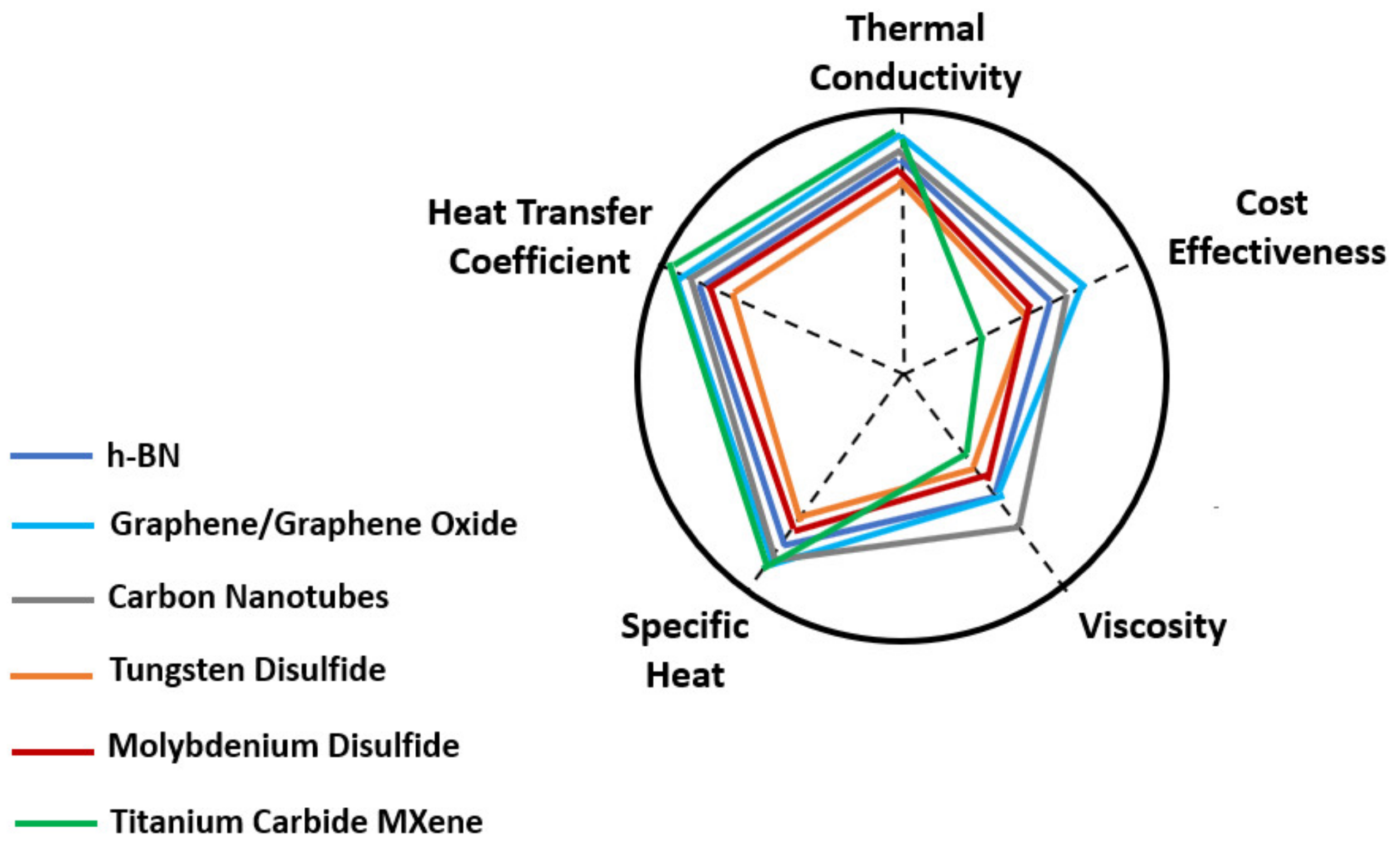
Figure 2. Schematic diagram of the qualitative comparison of the thermophysical properties of the main 2D nanofluids.
Moreover, hybrid nanofluids can be characterized by the incorporation of binary, ternary, and tetra [17] hybrid nanoparticles in base fluids and are very high TC heat transfer fluids when compared, for instance, with some mono-dispersed nanofluids. They exist as metal hybrid nanofluids, metal oxide hybrid nanofluids, and carbon-based hybrid nanofluids, among others. The hybrid nanofluids have composite nanoparticles and nanoparticle-decorated 2D nanostructures and can be synthesized according to various physical and chemical methodologies. The physical methodologies include atom beam splintering, laser induced heating, and ion implantation. Among the chemical synthesis routes, which are facile and inexpensive, there is the sol-gel process, sonochemical synthesis, hydrothermal synthesis, co-precipitation, and seed growth. The hybrid nanofluids are commonly employed to enhance the operating fluid thermophysical characteristics in solar thermal energy conversion and harvesting systems and equipment, such as flat plate collectors. Recent experimental works on innovative hybrid nanofluids [18][19] focused on the extremely high thermal transport capability of hybrid nanofluids and modified hybrid nanofluids flowing in rigid channels or in dilating/squeezing channels. Figure 3 presents the main potential applications of 2D nanofluids.
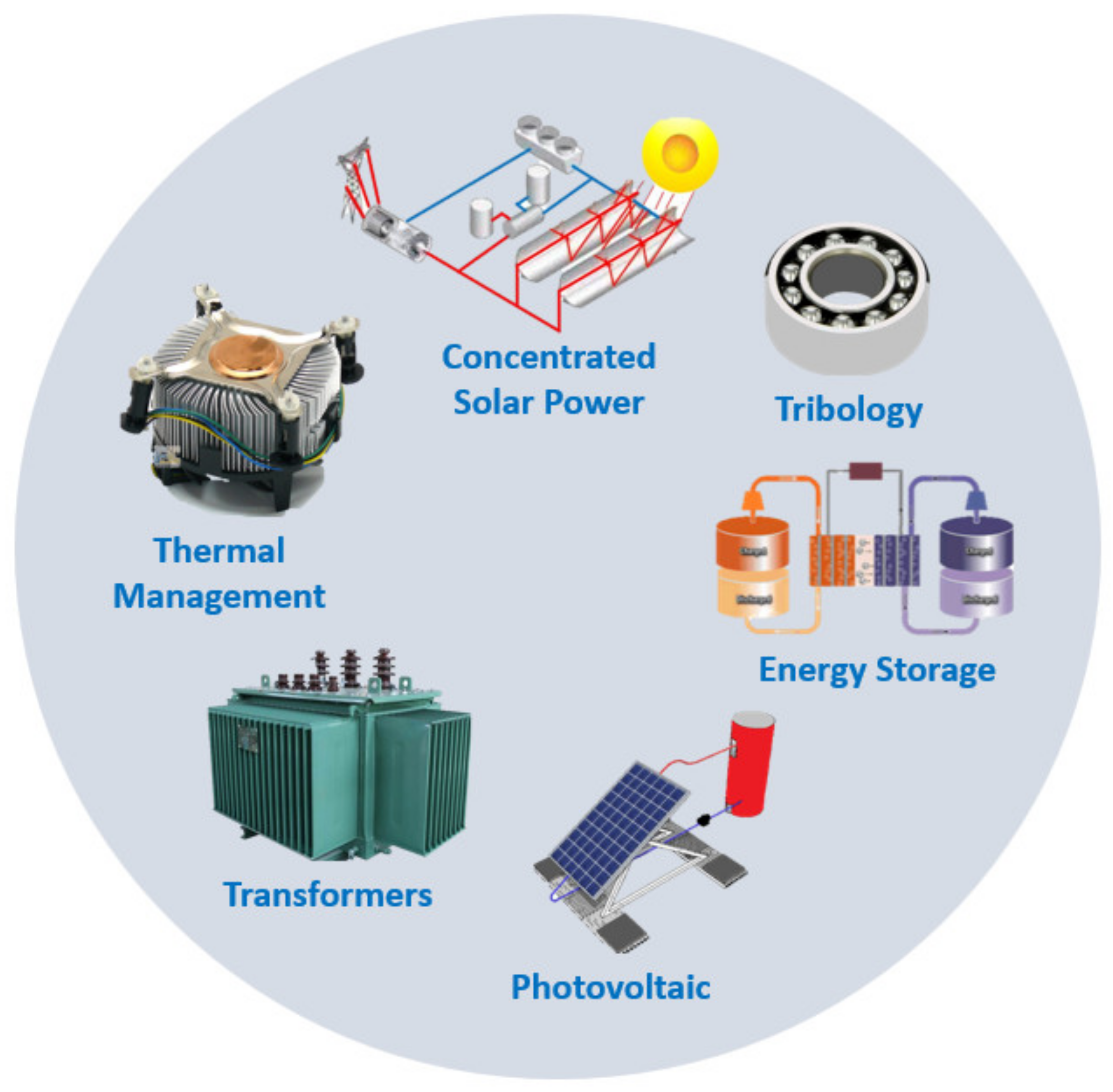
Figure 3. Main practical applications of 2D nanofluids.
2. Types of 2D Nanofluids
2.1. Hexagonal Boron Nitride
Other 2D ordered nanocrystals, different from those in the carbon nanomaterials family, are of great research and practical interest. These include h-BN nanosheets with a very high TC. This thermophysical property of h-BN is approximately 3400 W·m−1·k−1. The performant thermal characteristics and the low synthesis cost of the h-BN nanofluids compared to the Gr ones means that they can potentially be used in heat transfer and thermal energy storage. The h-BN innovative 2D material also has other noticeable characteristics, including mechanical stability and electrical resistivity. Furthermore, it is a solid lubricant with high efficiency in different areas, such as metal-working processes and wear-sealing of aerospace engines under high-temperature conditions. There exist many forms of BN, including carbon nanostructures possessing a variety of characteristics and functionalities. For instance, the honeycomb layered BN, also known as white graphite because of its structural similarity to that of graphite, is a common form of boron nitride that has a configuration similar to graphite with hexagonal ring layers separated by 3.33 Å, in which every boron atom is covalently bonded to three nitrogen atoms and vice versa. Between the layers, every boron interacts by van der Waals forces with a nitrogen atom. Thus, the strong B–N bond gives strength to the h-BN atomic layer and the individual BN layers could be isolated from the bulk h-BN crystals. Since it possesses an improved TC, the BN can overcome other fillers and is suitable for thermal performant hybrids. When synthesized through wet exfoliation, h-BN can offer a maximum exposure to the (002) lattice planes. It should be noted that h-BN is especially useful in thermal management situations where it can play the role of the thermal conductor and the electrical insulator. The application of h-BN in mineral oils, natural esters, and other fluids has already been examined. For instance, Taha-Tijerina et al. [20] demonstrated that h-BN changed the TC of mineral oil and acted as an electric insulator rather than a conductor. This is caused by the ability of the h-BN nanoparticles to trap moving electrons. Additionally, Salehirad and Nikje [21] incorporated h-BN nanostructures in a mineral oil and observed an enhanced TC, decreased viscosity, and improved insulation capability compared with those characteristics of the mineral oil alone. Moreover, Li et al. [22] synthesized ethylene glycol/BN nanofluids with different sized nanoparticles. The investigation team demonstrated that the nanofluids with larger nanoparticles exhibited greater TC enhancements when compared with those with smaller nanoparticles. Bhunia et al. [23] developed a 2D nanofluid with h-BN nanosheets and conventional transformer oil as the base fluid. The prepared nanofluids had stable dispersions with an enhanced dielectric breakdown strength and TC at 35 °C, 45 °C, and 55 °C. The synthesis method of the nanofluids is schematically described in Figure 4.
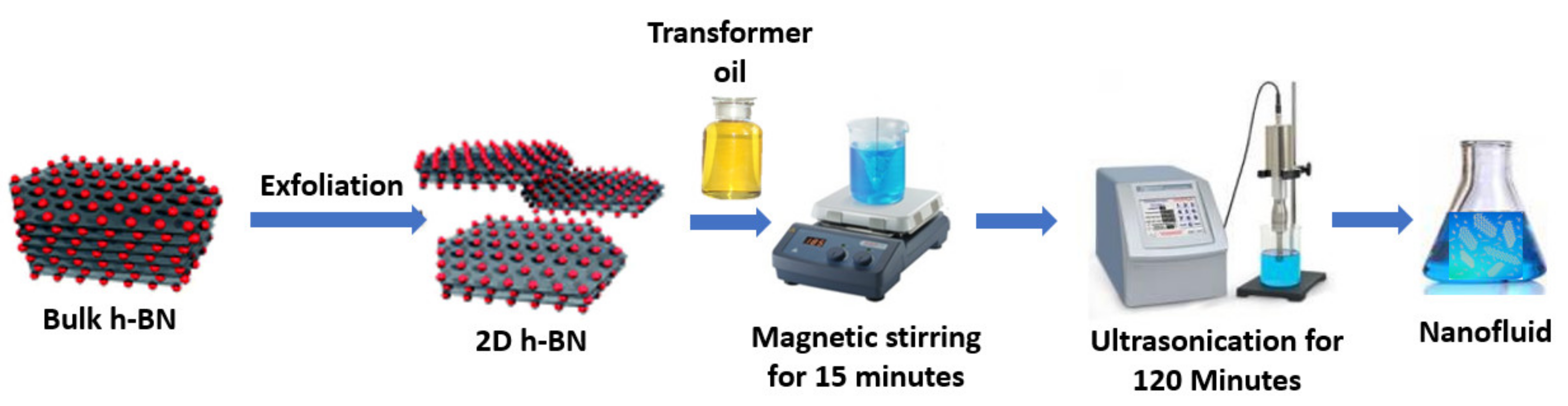
Figure 4. Schematic diagram of the preparation method of the 2D h-BN/transformer oil nanofluids.
The dispersed layered 2D h-BN has high specific area, enabling it to create a larger interfacial region. Due to its (002) plane dominance of the hexagonal phase, the BN possesses an improved TC above 600 W·m−1·K−1. The very thin and high specific surface h-BN nanosheets are lipophilic, allowing the existence of a perfect contact with the transformer oil base fluid. The intra-sheet propagation of heat introduces a free path to the phonon, which faces lower Kapitsa resistance and smaller thermal loss. Additionally, the clustering caused by a shorter inter-particle distance leads to the formation of a percolation network that acts as a conductive path for heat propagation. In cases where the clustering of sheets exists, an overlap of the interfacial regions occurs between the nanosheets leading to a continuous Kapitsa region with decreased vibrational density of states in phonons. The thermal imaging analysis of the nanofluids showed that the region of high temperature spreads with an increasing load of h-BN nanosheets, forming a percolation network that promotes the conduction of heat. With increasing temperature and fraction of the nanosheets, the quantity and velocity of the heat conduction phonons increased, and the overall thermal performance was improved. Nevertheless, above 0.05% wt. the rising concentration would promote the agglomeration of the nanostructures caused by the strong van der Waals forces in the vicinity of the nanostructures, provoking saturation. Such saturation is more prominent at higher temperature values and concentrations because of the intensified phonon scattering and eminent agglomeration. Furthermore, the authors found an appreciable increase in the AC breakdown voltage with the use of the nanofluids, which is large when compared with the BN nanoparticles. The referred increase can be interpreted by the extended nanosheets/oil interfacial regions, also known as Maxwell Garnet regions, of the 2D fillers in terms of charge trapping behavior. The faster heating/cooling rate and the enhanced TC is consistent with the Maxwell prediction model. The improved thermal performance can be explained through the augmented phonon transfer by the large interface between the nanosheets and the transformer oil. Furthermore, the natural electrostatic repulsion between the nanosheets at diluted concentrations promoted enhanced long-term and thermal stabilities compared with their nanoparticle counterpart. Han et al. [24] synthesized h-BN nanoparticles in water nanofluids and evaluated their TC.
2.2. Graphene/Graphene Oxide
Gr, GO, and rGO nanofluids possess very high heat transfer properties and they can be used in almost all heat transfer practical cases, including those related to solar thermal energy conversion and storage, PV/T systems, cooling of electronics, and thermal management solutions such as heat sinks and thermosyphons. Nevertheless, the synthesis of these nanofluids is not inexpensive and the use of Gr family nanofluids entails some important limitations that should be tackled in the future. These and other processing influencing factors, together with the improved thermophysical properties and field of application for Gr nanofluids. Gr is a carbon material originating from graphite in bulk state. It possesses the morphology of a two-dimensional, one-atom thick nanosheet (monolayer) and lattice of hexagonally arranged sp2 bonded carbon atoms. Its transverse sizes range from several nanometers to the macroscale. Gr was initially synthesized through the pioneering work of Novoselov et al. [25] using mechanical exfoliation by Scotch tape of the bulk graphite. Gr was categorized according to its structural arrangement, ranging from the 0D quantum dots, 1D nanofibers and nanoribbons, and 2D nanosheets, nanoplatelets, and rippled/wrinkled nanomesh. Regarding the 2D Gr nanosheets, a few proper production methods are commonly employed, including the laser-induced graphene production technique, exfoliation by mechanical means, silicon carbide sublimation, graphite fluid exfoliation, and CVD. These fabrication procedures synthesize solid Gr, excepting fluid exfoliation that produces Gr under the form of a colloidal suspension. The mechanical exfoliation method was the first approach to obtain Gr using Scotch tape to peel off a bulk pyrolytic graphite substrate, producing thin films of monolayer Gr attached to the tape. There are other, less common types of mechanical exfoliation, such as ball milling of graphite nanoparticles [26]. The laser-inducement route is usually conducted in ambient conditions through the application of a CO2 pulsed laser onto a carbon substrate. Such methodology combines bulk Gr fabrication and patterning in a single operating step without using any wet chemicals. Liquid exfoliation involves the application of a peeling force created by a horn ultrasonicator to separate the Gr nanosheets from the bulk graphite immersed in a, for instance, N-methyl-2-pyrrolidone solvent, but aqueous solutions can also be employed if a surfactant is included. The yield from the liquid exfoliation procedure is comparatively low and, consequently, it should be conducted at the centrifugation stage to obtain a significant monolayer and enough to create several layers of Gr flakes in the final dispersion. Moreover, the CVD technique uses hydrocarbon gases to induce the growth of Gr on high carbon solubility metallic substrates (e.g., nickel) by carbon diffusion and segregation. The improved features of Gr are derived mainly from the 2p orbitals that form the π-bonds, which are hybridized together to generate the π–π bands. Those bands are delocalized over the carbon platelet and produce Gr. Accordingly, Gr has excellent stiffness, enhanced TC, and great mobility of the charge carriers. Gr can be arranged according to the following possible configurations and nanomaterials:
-
Multi-layer Gr, which is a two-dimensional carbon nanomaterial composed of a small number of well-defined, stacked Gr layers.
-
Gr quantum dots, which contain one or few layers of Gr nanosheets mainly used in photoluminescence situations. In general, Gr quantum dots usually have transverse sizes of less than 10 nm.
-
Gr nanoplatelets, containing a two-dimensional honeycomb Gr lattice.
-
GO, which is a Gr modified chemically and produced by exfoliation and oxidation together with the general modification of the basal plane by oxidation. The GO reveals a monolayer material with an elevated content of oxygen.
-
rGO, which is a GO prepared through chemical, thermal, microwave, photo-thermal, and bacterial green methods for reducing the content of oxygen.
The use of Gr nanoparticles is an attractive option, given that they are less costly, and their production is simpler when compared with that of Gr nanosheets or CNTs. Furthermore, a homogeneous dispersion of Gr nanoplatelets is easier to obtain than those of Gr and CNTs since during dispersion, the single-layered Gr may be curled and the CNTs may be entangled. Moreover, Gr nanoparticles exhibit a much lower surface area compared with Gr nanosheets, but their surface area is still much greater than that of CNTs. It should be noted that the TC of Gr nanoparticles decreases as the number of layers increases [27]. Sadri et al. [28] synthesized covalently functionalized Gr nanoparticle colloidal suspensions that had enhanced stability and environmental benevolence. Gr nanoparticles were functionalized using clove buds through the one-pot method. Then, the clove-treated Gr nanoparticles were incorporated into the base fluid to synthesize the nanofluid using a two-step method. The hydrophilic functionalized groups were confirmed by UV–VIS spectroscopy and their presence resulted in good stability even two months after the as-prepared condition of the nanofluids. Dhar et al. [29] produced Gr nanofluids with the addition of Gr nanosheets, which were synthesized using a two-step preparation method. The oxidation process of the graphite nanopowder into GO nanosheets was performed using the modified Hummers method, which was followed by GO reduction into rGO. Ghozatloo et al. [30] and Park et al. [31] synthesized Gr nanoflakes. They used the CVD technique to create Gr nanosheets on copper foil by catalytic decomposition in a quartz tube furnace. Additionally, Safaei et al. [32] evaluated the heat transfer characteristics, pressure drop, and required pumping power for using an aqueous nanofluid with Gr/silver nanoparticles flowing in a conduit with a rectangular section. It was verified that the heat transfer features were enhanced with an increasing Reynolds number and concentration of the nanoparticles. However, when using the developed nanofluids, an increase in the required initial pumping power was also observed. Yarmand et al. [33] investigated the attributes of Gr nanoparticles and platinum dispersed in water. The prepared nanofluids had improved stability and any noticeable sedimentation was not observed after 22 days. The TC of the nanofluids showed a near 18% enhancement when compared with the TC of the water alone at 40 °C and at 0.1% wt. Since the synthesis of Gr is complex and costly, efforts have been made to develop cheaper procedures to synthesize and use Gr derivatives, such as GO. GO can be synthesized by exfoliating the GO into layered plates through ultrasonication or vigorous stirring and can be incorporated into base fluids, forming GO nanofluids. Vincely and Natarajan [34] conducted experiments using a solar flat plate collector and a GO nanofluid as the heat transfer fluid. The nanofluid was prepared via ultrasonication of GO in water. The enhancement of the collector efficiency was 7.3% at a flow rate of 0.0167 kg/s and a weight fraction of 0.02% wt. compared with that of the water alone. In addition, Yu et al. [35] determined the TC of the nanofluids composed by GO nanosheets and different base fluids. At a weight fraction of 5% wt., the enhancements of the TC were 30.2%, 62.3%, and 76.8% for water, propyl glycol, and liquid paraffin as base fluids, respectively. Owing to the enhanced thermal stability, charge transportation, TC, mechanical strength, and optical properties, rGO and its nanofluids can be used in the development of different types of innovative nanomaterials and thermal fluids in various applications, including solar energy conversion and harvesting systems, filtration, chemical batteries, and nanoscale electrodes. In this sense, Lin et al. [36] synthesized an innovative surfactant composed of two-dimensional charged zirconium phosphate nanoplatelets and applied it in the dispersion of rGO in a base fluid. The obtained results show that rGO nanofluids were altered from non-homogeneous, non-Newtonian nanofluids into homogeneous Newtonian nanofluids after exfoliation with the charged zirconium nanoplatelets. Zubir et al. [37] utilized an rGO nanofluid for improving the thermal performance of a closed duct structure. The authors produced the rGO using the reduction process with tannic acid from the chemically exfoliated GO. The heat transfer enhancement of the nanofluid was higher than the TC enhancement because of the considerable effects of the nanoparticles and turbulent induced flow features. Schlierf et al. [38] employed pyrene derivatives for stabilizing rGO dispersions due to the forces of repulsion and the π–π interaction between functionalized pyrene derivatives and the π systems of rGO. The chemical reduction of GO possesses the beneficial features of being a simple and inexpensive method, resulting in a greater yield of Gr with proper scalability for large-scale applications. The Gr family of nanofluids can be synthesized using the one-step route or two-step route. In the one-step route, the nanofluids are directly synthesized and are produced directly through chemical processes. In the two-step route, the Gr nanosheets are initially manufactured in nanopowder form by physical or chemical methods such as grinding, laser ablation, or the sol-gel method, and then are dispersed in a base fluid. Fan et al. [39] oxidized graphite nanoflakes using an ameliorated Hummer method. A mixture of phosphoric acid and sulfuric acid was incorporated into a mixture of Gr flakes and potassium permanganate. After vigorous stirring, the mixture was air cooled. When the mixture exhibited a yellow coloration after the addition of hydrogen peroxide, the deposit was centrifuged, and the remaining material was washed. The GO sheets were manufactured after air drying. Lee et al. [40] synthesized nanofluids with GO nanosheets dispersed in water. The investigation team prepared the GO nanosheets through CVD. The pH and zeta potential of the developed nanofluids were 3.58 and −31.5 mV, respectively, indicating a reasonable stability. Yu et al. [41] proposed an easy method for preparing ethylene glycol-based nanofluids, including Gr and GO nanosheets. The TC of the ethylene glycol increased up to 86% with the incorporation of the nanosheets. The TC of the GO nanofluid and Gr nanofluid were 4.9 W/mK and 6.8 W/mK, respectively. Lee and Rhee [42] produced ethylene glycol nanofluids incorporating Gr nanoplatelets, which exhibited similar TC enhancement at a weight fraction of 2% wt. and at temperature values between 10 °C and 90 °C. The TC of the nanofluids was much greater than that provided by the Hamilton–Crosser model [43], which may be due of the greater surface area and two-dimensional nanostructure of the Gr nanoplatelets. Akhavan-Zanjani et al. [44] measured the TC and viscosity of a nanofluid composed of Gr nanosheets dispersed in water. The experimental results revealed a considerable increase in the TC and a moderate increase in the viscosity with the incorporation of the Gr nanosheets. The maximum increases were of 4.95% and 10.30% for the viscosity and TC, respectively. Moreover, the authors Liu et al. [45] investigated the TC, specific heat capacity, viscosity, and density of the nanofluids based on Gr nanosheets suspended in an ionic base fluid. The TC of the nanofluid at 0.06 wt.% showed an enhancement within 15.2–22.9% in the base fluid itself in cases where the temperature values were raised from 25 °C to 200 °C. The viscosity of the prepared nanofluid was considerably decreased with increasing temperature. The specific heat capacity and density of the nanofluids suffered a minor reduction compared with those properties of the base fluid alone. Park and Kim [46] examined the evolution of the TC and viscosity of different types of Gr nanosheets dispersed in water, including those containing GO nanosheets and Gr nanosheets with diverse sizes. The enhancement of the TC in the GO nanofluid was the highest among all tested nanofluids, whereas the maximum increase in the viscosity was found for the Gr nanofluid with the largest nanosheets. Tesfai et al. [47] studied the rheological characteristics of aqueous suspensions in GO nanosheets. The suspensions exhibited a shear thinning behavior under small shear rates, which was followed by a shear-independent behavior. The shear thinning behavior became more intense with an increasing incorporated fraction of the nanosheets. Furthermore, the nanofluids possessed a considerable viscosity due to the high aspect ratio of the GO nanosheets. Ijam et al. [48] evaluated the viscosity of GO nanosheets dispersed in water and ethylene glycol. The nanofluids exhibited a shear thinning behavior under low shear rates and a Newtonian behavior under high shear rates. The viscosity of the nanofluid increased by up to 35% compared with that of the base fluid at 0.1% wt. and a temperature of 20 °C. The majority of experimental works on Gr and GO nanofluids have stated that the TC is temperature dependent; however, Sun et al. [49] reported inconsistent results, which was expected since a more in-depth understanding is required to establish precise correlations between the thermophysical characteristics of this type of nanofluid and many factors, including the size and incorporated fraction of the nanoparticles, rheological features, stability over time, the aspect ratio of the Gr nanosheets, pH regulation, zeta potential, and operating temperature. Naghash et al. [50] studied the HTC of nanofluids prepared from porous Gr nanosheets. The TC of the 0.1% wt. nanofluid remained almost unchanged with only a minor increase of 3.8%, while the HTC suffered a considerable enhancement of 34%. This confirmed that aside from TC enhancement, more factors may influence the heat transfer of Gr nanofluids, including the local order of the molecules of the base fluid present in the surroundings of the Gr nanosheets, parallel structure, and π–π stacking. Bahiraei et al. [51] examined the hydrothermal features of an eco-friendly Gr nanofluid flowing in a spiral heat exchanger. The results demonstrated that with an increasing Reynolds number or concentration, the overall HTC and heat transfer rate were increased. Furthermore, the pressure loss was enhanced with an increasing Reynolds number, and the nanofluid exhibited higher pressure losses compared with those using water, especially at high Reynolds numbers. The nanofluids with Gr nanosheets usually have a better convective heat transfer performance than GO nanosheet nanofluids. Indeed, GO nanosheets have sp2-hybridized and sp3-hybridized carbon atoms with epoxide and hydroxyl groups. The presence of the atoms of oxygen and saturated sp3 bonds in the GO nanosheets decreases the TC, promotes phonon scattering, and decreases the heat transfer of nanofluids in reference to the Gr nanosheets. Hu et al. [52] evaluated the pool boiling of Gr nanosheets dispersed in ethylene glycol and water. The authors observed that at lower concentrations, the heat transfer behavior was improved with the increasing concentration of the nanosheets, which was mainly due to the surface wettability enhancement induced by the deposition over time of the nanoparticles. Nonetheless, further enhancements in the concentration decreased the HTC caused by the sedimentation over time in the Gr nanosheets and the resulting blockage of the available nucleation sites. Moreover, at concentrations of Gr inferior to 0.02%, the critical heat flux was increased as the concentration increased. Zhang et al. [53] employed GO/water nanofluids to conduct flow boiling experiments in microchannels. The heat transfer capability was reduced as the concentration increased. The authors argued that the deposition process of GO nanoparticles was intensified with the increasing flow rate, operating temperature, and concentration of the nanoparticles.
Further studies on the decrease in the elevated cost of Gr nanomaterials and their derivatives and corresponding nanofluids are highly recommended since the current processing methodologies are very complex and demand the intensive use of chemicals and are otherwise energy consuming. A profound benefit–cost analysis should be performed to evaluate the use of Gr family nanofluids considering the balance between the obtained improvements in the thermophysical properties versus the high initial investment associated with such heat transfer solutions. More experimental works on the high temperature stability of Gr and GO nanofluids are required. In addition, there is a lack of studies on Gr and GO hybrid nanofluids in certain types of nanomaterials, such as metallic and metallic oxides. Innovative techniques based on the surface modification of Gr nanomaterials with hydrophilic groups should be further developed to ameliorate the stability over time of the Gr nanofluids. Additionally, further experimental investigation should be carried out to evaluate conclusively the trade-off between the extremely high values of TC inherent to Gr and derivative nanofluids and the increased density and viscosity leading to higher pressure drops, which are typical in this type of fluid. In this regard, there is a need to pursue the optimal concentration of Gr nanostructures that maximizes the TC of the thermal fluids and, simultaneously, minimizes the density and viscosity enhancements which, considering the published background on the subject, represents a significant challenge. Finally, more life cycle assessments of Gr nanomaterials are required to mitigate the negative impacts on the environment and human health derived from the production, use, and disposal of such materials.
2.3. Carbon Nanotubes
Nanofluids containing CNTs are very promising heat transfer operating fluids in several technological fields. For instance, the use of such nanofluids can improve the efficiency of different types of solar thermal energy collectors due to their enhanced light absorption ability and extremely high TC. The CNTs dispersed in base fluids can ameliorate the thermal performance of several types of thermal management equipment, such as heat exchangers, heat pipes, and thermosyphons to be used in a wide spectrum of applications, including, for instance, the cooling of electronics. CNTs are Gr nanosheets rolled into cylindrical tubes with a diameter of less than 1 nm with a half fullerene-capped end. Considering the number of consistent tubes, CNTs can be divided into single-walled carbon nanotubes (SWCNTs), double-walled carbon nanotubes (DWCNTs), and multi-walled carbon nanotubes (MWCNTs). SWCNTs are composed of only one tube, whereas DWCNTs and MWCNTs comprise of two and three or more tubes, respectively. The fundamental synthesis techniques of CNTs are laser ablation, arc discharge, and CVD. The preparation method based on laser ablation also uses the evaporation of a rod of graphite with a metallic-based catalyst to produce CNTs. The laser ablation synthesis procedure applies high-energy laser irradiation to heat a carbon substrate, leading to the transition from the solid to the gaseous phase, as the nanomaterial is deposited in a cold trap placed within the synthesis chamber. Using arc discharge methodology, doped graphite rods and catalysts are vaporized at temperature values of up to 5000 K in a closed chamber by an electric arc, resulting in a sediment of CNTs. On the other hand, the CVD decomposes carbonaceous gases on catalytic nanoparticles to fabricate CNTs. The employed catalytic nanoparticles are grown during the synthesis or, alternatively, are produced by a separate procedure. Moreover, the major benefit of the CVD is the improved controllability over the operating parameters, such as the carbon supply rate, temperature of growth, size of the catalytic nanoparticles, and nature of the CNTs growing substrate. The nanofluids with CNTs are used in different solar energy systems, such as PV/T collectors and water heating technology (it has been previously demonstrated that nanofluids with CNTs ameliorate the efficiency of the CSP systems in terms of energy and exergy standpoints). Their performance in solar energy harvesting may be affected by various parameters, such as the type of CNTs, operating conditions, concentration, and involved systems. CNTs have various beneficial features, such as their very high aspect ratio and improved thermal, mechanical, and optical characteristics. The TC of CNTs reaches 6000 W/m·K, which is a much higher value than that of the metals in bulk form. Liu et al. [54] observed that the suspension of CNTs at 1% vol. in ethylene glycol resulted in an enhancement of 12.4% in the TC. This increase was one of approximately 30% in the case of suspending CNTs in engine oil at 2% vol. Furthermore, Sadri et al. [55] evaluated the influence of the type of the surfactant on the TC of MWCNTs dispersed in water at 0.5% wt. The investigation team observed a maximum enhancement in the TC in the case of the nanofluids with MWCNTs of 22.3% compared with water itself. Kumar et al. [56] numerically investigated MWCNTs dispersed in water flowing in a double helically coiled tube heat exchanger, which resulted in an increase of 30% in the Nusselt number when compared with that of water. Apart from their extremely high TC, CNTs also possess favorable optical properties. This makes them suitable for application in solar systems, as the use of colloidal suspensions in CNTs in will result in a spectral absorptivity alteration over the entire solar range. It has been previously demonstrated that incorporating CNTs and their hybrid structures into thermal base fluids can reduce transmittance, which will improve their competence in solar energy systems [57]. For instance, Hjerrild et al. [58] stated that using a nanofluid with CNTs instead of using only water led to a reduction in transmittance between 5% and 10% in the long wavelength and visible spectra. The transmittance reduction was found to depend on the concentration of CNTs in the base fluid. In the experimental work conducted by Gorji et al. [59], absorption spectroscopy was used to determine and compare the radiation absorption of water and functionalized CNTs dispersed in water. The results revealed a higher absorption of radiation when the functionalized CNTs were incorporated into the water. Moreover, the authors confirmed that the increase in the working temperature of the nanofluid promoted a decrease in radiation absorption. The higher absorption characteristics of nanofluids in CNTs makes them favorable for application in solar thermal energy. These nanofluids are suitable in solar energy systems such as collectors, solar ponds, and PV/T devices and systems. Applying nanofluids to these technologies has resulted in their efficiency enhancement and size reduction. Furthermore, the dynamic viscosity of CNT nanofluids deserves special attention from researchers. The rheological properties of water-based carbon nanofluids have been comprehensively studied for heat transfer enhancement and lubricant applications [60]. Studies on rheology included measurements with diverse types of SWCNTs [61] and MWCNTs [62]. Additionally, viscosity measurements on covalently functionalized CNTs have been also reported [63]. CNT suspensions in water are non-Newtonian fluids at comparatively high concentrations. Nonetheless, such nanofluids present a behavior close to the Newtonian one in the region dominated by low concentrations [64]. The CNTs possess extreme aspect ratios, considerably exceeding those of metal rods, cylinders, and discs. The viscosity of dilute CNT dispersions has been correlated with the length of the CNTs [65]. Ansón-Casaos et al. [66] evaluated the viscosity of 1D diluted CNTs dispersed in water, performing viscosity measurements on the nanofluids of CNTs and functionalized CNTs. One type of functionalized CNT was directly suspended in water, and the other CNTs and functionalized CNTs were dispersed with the aid of surfactants. The authors evaluated the viscosity of the nanofluids based on the aspect ratio of the CNTs using prediction models. The CNT aspect ratio can be defined as the ratio between the size of the nanotube along a symmetry rotation axis and its perpendicular diameter. For CNTs, the aspect ratio parameter is the ratio between the nanotube length and its diameter and was found to be much larger than one. The authors concluded that the experimentally measured viscosity of the dilute CNTs could be fitted into the Maron–Pierce model [67] as a function of the concentration of the nanotubes and working temperature.
Further and conclusive experimental works on the inclusion of hybrid CNT formulations in solar thermal energy conversion and harvesting systems and in the nanofiltration of PV/T systems are welcome. There is a lack of studies on the use of CNT nanofluids in some specific heat transfer technologies, such as solar saline water desalination. Furthermore, research must prioritize the search for innovative synthesis procedures using new surfactants to mitigate the absence of the stability over time in the CNT nanofluids. Further studies regarding the impact of the size of CNTs on the thermophysical characteristics of the corresponding nanofluids are required. This factor should be comprehensively analyzed within the scope of the energy and exergy efficiencies of solar thermal energy systems, and not only with regard to its influence on heat transfer enhancement features.
2.4. Tungsten Disulfide
Tungsten disulfide nanostructures can be synthesized using different methods. These methods are presented in Figure 5.
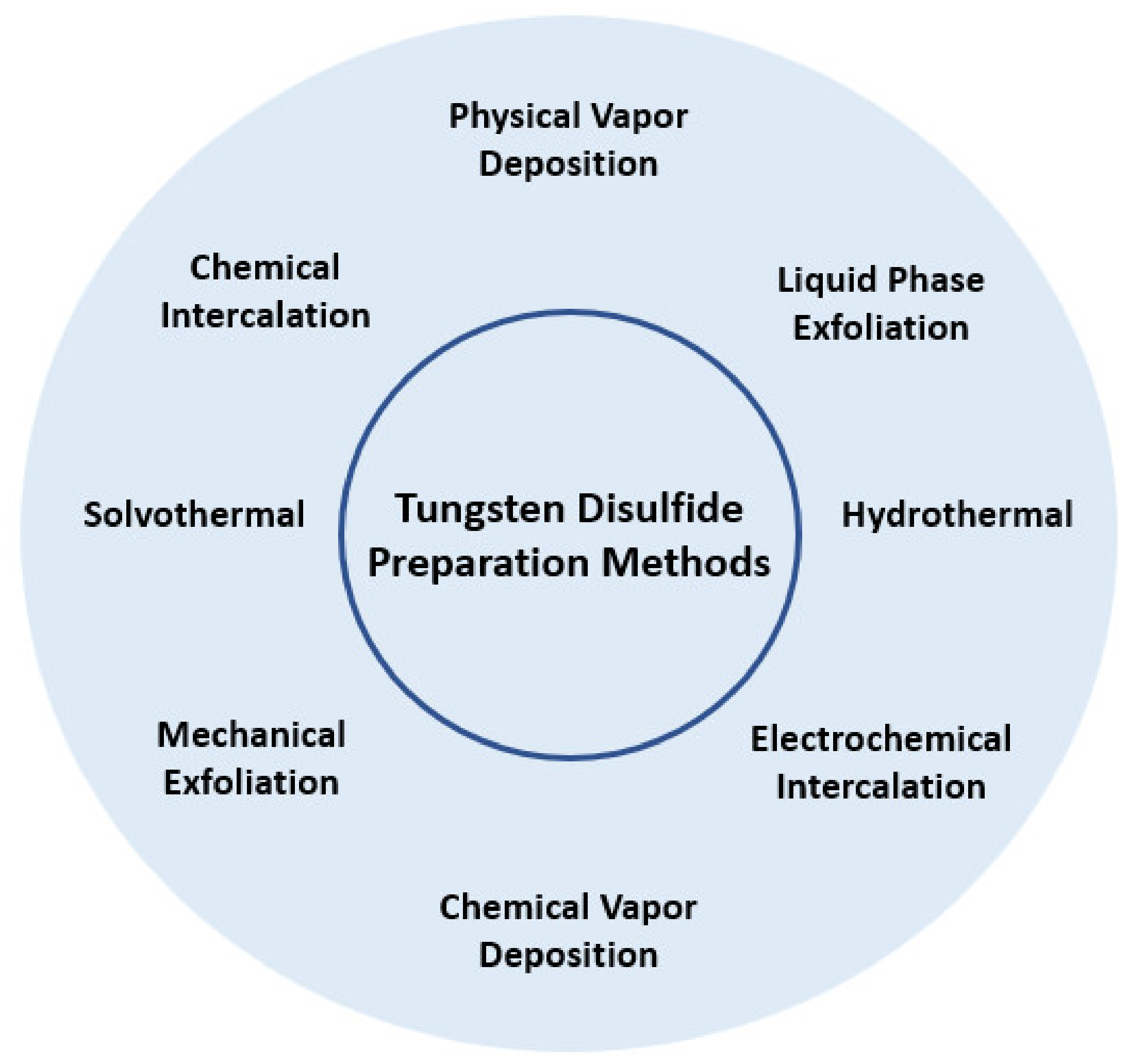
Figure 5. Main tungsten disulfide preparation methods.
WS2 nanofluids are promising in heat transfer applications within the CSP technological field. WS2 nanosheets are well-defined and highly stable, and do not significantly alter certain characteristics such as the surface tension, friction coefficient, viscosity, and Reynolds number of heat transfer systems. The incorporation of WS2 may improve the TC of synthetic oil base fluids by approximately 30%. Additionally, the authors Martínez-Merino et al. [68] prepared 2D WS2 nanosheet-based nanofluids with the oil commonly used as heat transfer fluid in CSP stations. The surfactants cetyl-trimethylammonium CTAB and polyethylene glycol PEG-200 were added to the nanofluids to improve the exfoliation procedure and the stability over time of the colloidal suspensions. The experimental results showed that an increase in the ultrasonication duration and the amount of the surfactant enhanced the stability over time of the nanofluids prepared with the surfactant CTAB. Furthermore, in the nanofluids synthesized with the surfactant PEG-200, the increase in the surfactant amount was the main factor contributing to the high concentration of stable nanomaterial suspended in the thermal oil. The size of the agglomerates in the stable nanofluids prepared with the addition of CTAB was between 200 nm and 250 nm, whereas in the PEG-200 nanofluids, the same measurement was approximately 180 nm. Furthermore, the viscosity of the oil base fluid increased to a maximum of only 1.5% with the nanosheets of WS2 and the surfactant. This minor increase would not result in considerable pressure-drop concerns in the circuits of the CSP plants. Moreover, the nanofluid with a concentration of 0.75% wt. of surfactant PEG-200 with four hours of ultrasonication (Case A) and the 0.01% wt. CTAB with eight hours of sonication (Case B) showed enhancements of 33% and 29% TC, respectively, compared with that of the thermal oil itself. Furthermore, the nanofluid in Case A was found to have a specific heat value 6.5% higher than that of the heat transfer fluid alone. The improvement in the specific heat and TC resulted in an enhancement in the HTC of 21% in the Case A nanofluid and of 17% in the Case B nanofluid when compared with that of the oil itself. On the other hand, a numerical analysis demonstrated that the efficiency of the CSP facilities was enhanced by up to 31% or 18% if the Case B or Case A nanofluids, respectively, are used compared with the commonly employed thermal oil. The predicted efficiency for a volumetric collector working with 2D WS2 nanofluids is a slightly lower than the efficiency of a surface collector operating with nanofluids; however, such efficiency is higher than that achieved using the surface collector with the thermal oil commonly used at the present time. All in all, the authors believe that the WS2-developed nanofluids are a promising alternative to the thermal oil commonly used in solar energy plants and would enable the adoption of volumetric collectors, which are less costly than traditional surface collectors. In the work conducted by Shah et al. [69], 2D nanofluids with WS2 volume fractions of 0.005% vol., 0.01% vol., and 0.02% vol., together with the surfactants sodium dodecyl sulfate (SDS), sodium dodecylbenzene sulfonate (SDBS), and CTAB surfactants at 0.05, 0.5%, 1%, and 2% were synthesized and characterized. The authors arrived at the following main conclusions:
-
The maximum enhancement in the agglomerate sizes were of 172%, 245%, and 261% with 0.005% WS2 and 2% SDS, 0.01% WS2 and 2% SDS, and 0.02% WS2 and 0.05% SDS, respectively. Collectively, the zeta potential saw an improvement of 554%, whereas the mean particle size presented an enhancement of up to 411% mainly caused by the adsorption of the surfactant molecules. This would entail a long-term agglomeration risk, resulting in the clustering and sedimentation of nanoparticles.
-
The maximum TC increases were approximately 2.8%, 1.9%, and 4.5% for combinations of 0.05% SDS and 0.005% WS2 at temperature values of 25 °C, 50 °C, and 70 °C, respectively. The authors argued that the TC reduced with increasing concentrations of the SDS. Alongside the 0.005% of WS2 filler concentration, the higher fractions of 0.01% and 0.02% of WS2 also exhibited higher TC enhancements with lower concentrations of surfactant.
-
The high-temperature condition revealed a pendular behavior concerning the TC. The reason behind this response may be the eventual detachment of the surfactant molecules and further interaction with the WS2 nanosheets.
-
The rheological analysis confirmed the formation of a nanostructured network inside the nanofluids with WS2 nanosheets and depicted the transition of the nanofluids from viscous to elastic behavior with the inclusion of surfactants. Such transition suggests that a higher pumping power was required to initiate the flow of the working fluid. The maximum decrease in viscosity of 8.2% was found at the minimum concentration of 0.005% vol. of WS2, confirming the superior fluidity of the developed nanofluids. This reduction was amplified at a concentration of 0.05% vol. of SDS, enlarging the referred value by approximately 10.5%.
-
The developed nanofluids evolved from non-Newtonian to Newtonian behavior under a 10 s−1 shear rate.
The most prominent conclusion is that surfactants, such as SDS, provide their steric hindrance effect to produce a more uniform dispersion of the WS2 nanosheets in the ethylene glycol base fluid and contribute to the interfacial tension reduction in the nanofluids, which may also promote a decrease in the viscosity of the system. On the other hand, the WS2 nanoparticles have already demonstrated [70] great potential as fillers in lubricating and engine oils. WS2 has shown improved lubricity due to the weak intermolecular interactions among the nanosheets, causing easy shearing with a fullerene-like structure. In lubrication, one of the main factors of the 2D nanofluids that contributes the most towards their lubricating ability is the amount of nanosheets present in the base fluid, as illustrated in Figure 6.
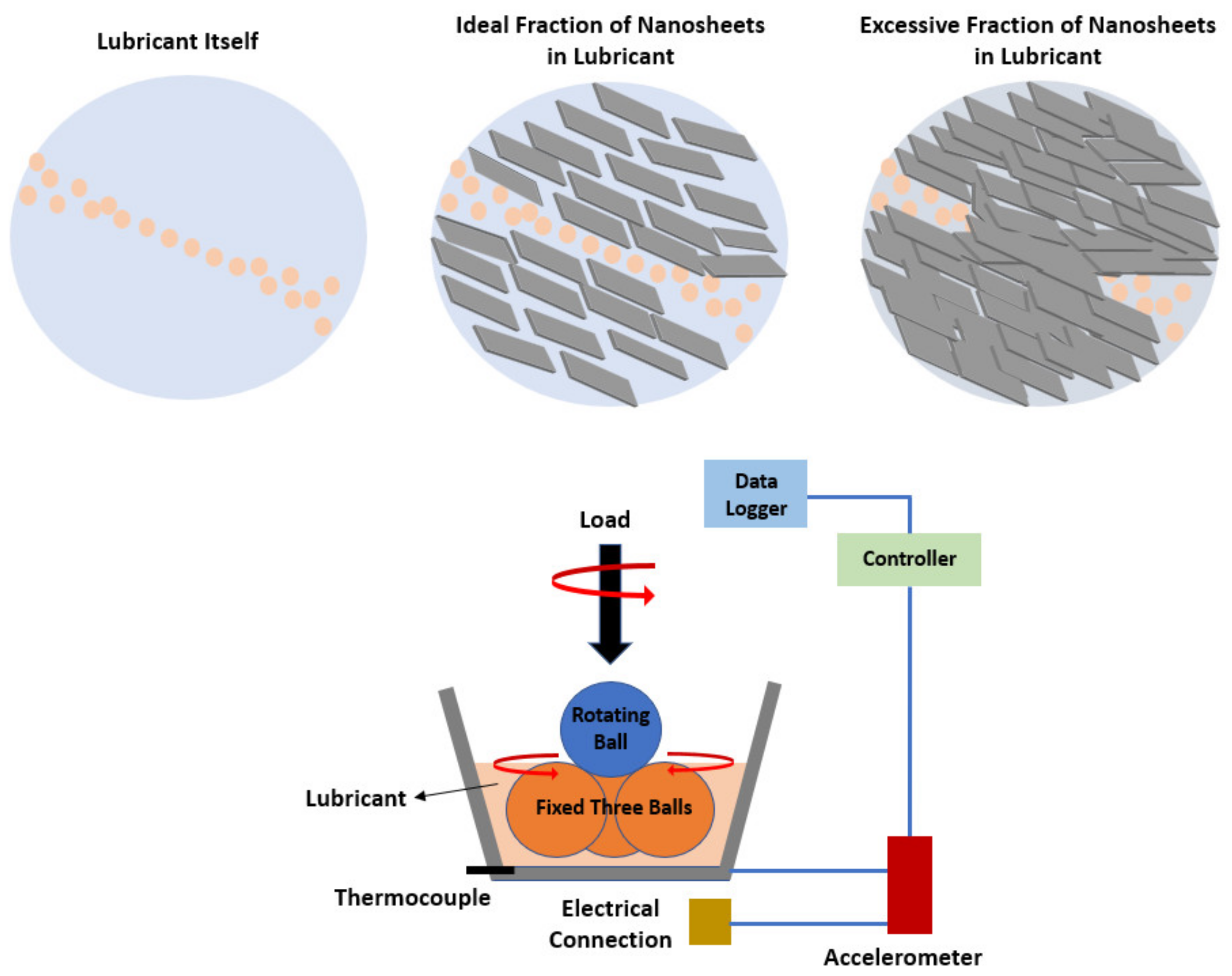
Figure 6. Schematic diagram of a four-ball tribometer and effect of the concentration of nanosheets.
However, more molecular dynamics are required for the WS2 nanostructures dispersed in the synthetic oils most commonly used in CSP plants, which are a mixture of biphenyl and diphenyl oxide, to provide useful insights into the heat transfer capability of WS2 nanofluids. The decoration of WS2 requires further theoretical and experimental confirmation, given that the decoration of the WS2 edge may induce considerable beneficial effects on the rheological and heat transfer characteristics of WS2 nanofluids. Furthermore, there is a lack of published studies considering the interactions between the surfactants, incorporated nanostructures, and liquid medium used in the liquid phase exfoliation of WS2 nanosheets. For instance, numeric analyses based on density functional theory determinations and electron localized functions are welcome to better understand the WS2 liquid phase exfoliation process.
2.5. Molybdenum Disulfide
MoS2 nanofluids have already proven to be an attractive option for application in, for instance, volumetric parabolic trough collectors. These nanofluids can improve by 5% or more the thermal efficiency of such collectors and, at the same time, reduce the initial required pumping power by around one-fifth. Furthermore, the use of MoS2 nanofluids diminishes the thermal stress on volumetric receivers and the overall required investment. Molybdenum disulfide is a 2D transition metal dichalcogenide with MX2 as chemical formula, where X denotes a chalcogen, such as sulfur, selenium, and tellurium, and M denotes the transition metal. MoS2 possesses improved thermal and chemical stabilities. It can be applied as a catalyst and lubricant because of its superior characteristics, including inertness, anisotropy, and photocorrosion resistance. MoS2 nanostructures have good thermophysical properties and great anti-friction properties, making it a promising material as reinforcement in the cooling process of nanofluids. Moreover, parameters including the type and concentration of nanoparticles, the nature of the base fluid, the synthesis procedure, and the stability/surfactant addition balance affect the thermophysical characteristics of the nanofluids, including the viscosity and thermal transport. Su et al. [71] investigated the thermal stability of MoS2 in water and oil nanofluids at weight fractions from 0.01% wt. to 0.5% wt. The authors found that with the enhancement in the amount of MoS2, the TC of the nanofluids was increased. The investigation team also reported that the TC improvement was higher in water-based nanofluids than in oil-based nanofluids. This fact was explained as the consequence of the improved dispersion and stability over time of the water nanofluids. Nagarajan et al. [72] prepared MoS2 nanosheets to be suspended in SAE 20W50 diesel engine oil to produce an effective lubricant. MoS2 nanoparticles were synthesized using a microwave hydrothermal reactor. The schematic diagram of the synthesis procedure is presented in Figure 7.
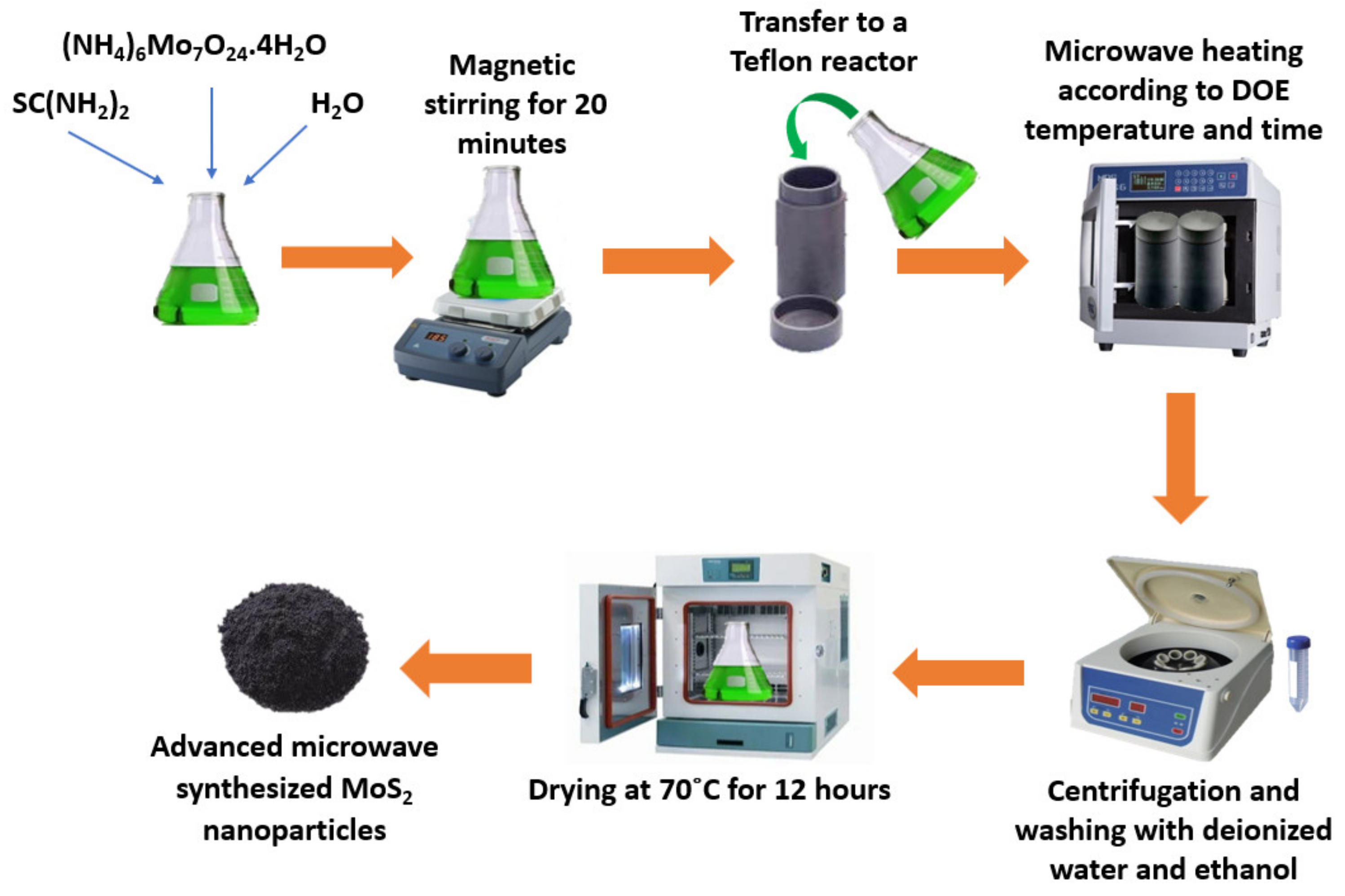
Figure 7. Schematic diagram of microwave-synthesized molybdenum disulfide nanoparticles.
The tribological and oxidation analysis showed that the lubricant with a weight fraction of 0.01% wt. of MoS2 nanoparticles provided better results than those of other concentrations and, hence, the nanofluid with this fraction was further evaluated for its TC. The authors reported an improvement in the TC of the 0.01% wt. nanofluid of approximately 10% compared with that of the base engine oil alone. Due to the lower amount of the MoS2 nanoparticles, the enhancement in the TC was due to the molecular collisions between the oil and the nanoparticles. The researchers argued that the TC behavior indicated that this enhancement was due to the Brownian motion of the nanosheets. The TC and heat transfer capability of a nanofluid depend on the Brownian motion of the incorporated nanoparticles. Indeed, the Brownian motion-driven convection and effective thermal conduction by the percolating paths mechanism of the nanoparticles are the most likely factors that promote the improved heat conduction in nanofluids. The percolation mechanism involves the formation of thermal conductive paths based on the enhanced thermal conductance that improves the overall TC. A schematic diagram of the percolation mechanism is presented in Figure 8. The Brownian motion allows the direct solid–solid transport of heat from one particle to the adjacent one, resulting in an increase in the thermal conductivity. The Brownian motion and resulting micro mixing of the nanoparticles and clusters, as well as aggregation kinetics of nanoparticles and clusters, are the main issues in the evaluation of the thermal conductivity of nanofluids. Any effort to understand the behavior of nanofluids is not complete without considering at the same time all these effects. Accordingly, the thermal transfer of the colliding nanoparticles increased the TC of the lubricant. This is why the TC of the lubricant increased more than that of the engine oil for temperature values higher than 60 °C, given that a more intense Brownian motion of the nanoparticles takes place.
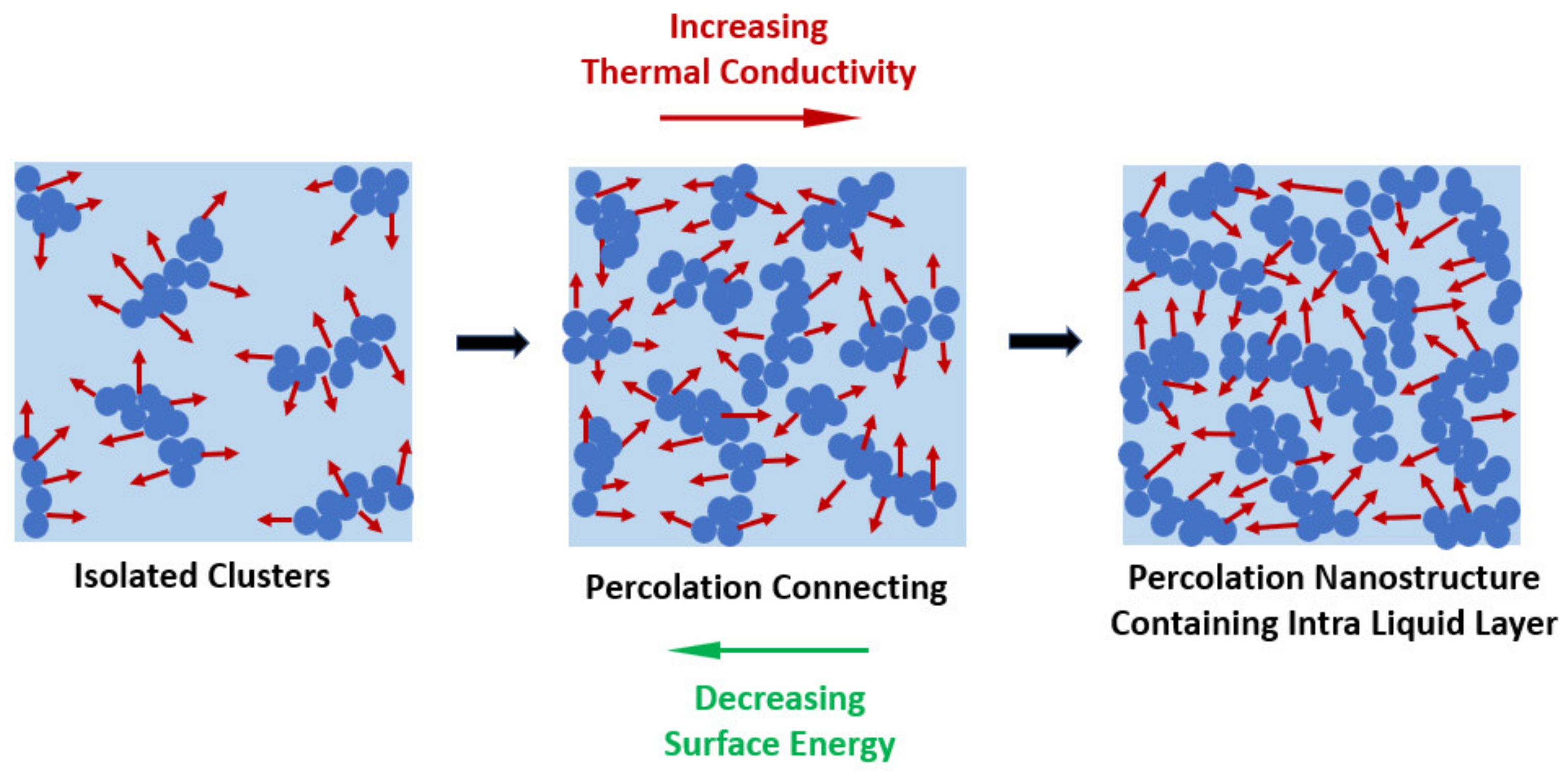
Figure 8. Schematic diagram of the percolation mechanism of nanofluids.
References
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58.
- Yang, P.-K.; Lee, C.-P. 2D Layered Nanomaterials for Energy Harvesting and Sensing Applications. In Applied Electrochemical Devices and Machines for Electric Mobility Solutions; Intechopen: London, UK, 2019.
- Khan, A.H.; Ghosh, S.; Pradhan, D.A.; Shreshta, L.K.; Acharya, S.; Ariga, K. Two-Dimensional (2D) Nanomaterials towards Electrochemical Nanoarchitectonics in Energy-Related Applications. Bull. Chem. Soc. Jpn. 2017, 90, 627–648.
- Zhang, H.; Chhowalla, M.; Liu, Z. 2D Nanomaterials: Graphene and transition metal dichalcogenides. Chem. Soc. Rev. 2018, 47, 3015–3017.
- Zhao, H.; Chen, X.; Wang, G.; Qiu, Y.; Guo, L. Two-dimensional amorphous nanomaterials: Synthesis and applications. 2D Mater. 2019, 6, 032002.
- Tan, C.; Cao, X.; Wu, X.-J.; He, Q.; Yang, J.; Zhang, X.; Chen, J.; Zhao, W.; Han, S.; Nam, G.-H.; et al. Recent Advances in Ultrathin Two-Dimensional Nanomaterials. Chem. Rev. 2017, 117, 6225–6331.
- Shahil, K.M.F.; Balandin, A.A. Graphene-Multilayer Graphene Nanocomposites as Highly Efficient Thermal Interface Materials. Nano Lett. 2012, 12, 861–867.
- Yu, A.; Ramesh, P.; Itkis, M.E.; Bekyarova, E.; Haddon, R.C. Graphite Nanoplatelet-Epoxy Composite Thermal Interface Materials. J. Phys. Chem. C 2007, 111, 7565–7569.
- Singh, S.; Hasan, M.R.; Sharma, P.; Narang, J. Graphene nanomaterials: The wondering material from synthesis to applications. Sens. Int. 2022, 3, 100190.
- Ali, B.; Qayoum, A.; Saleem, S.; Mir, F.Q. Effect of graphene/hydrofluoroether (HFE-7100) nanofluids on start-up and thermal characteristics of pulsating heat pipe. J. Therm. Anal. Calorim. 2023.
- Le, T.-H.; Oh, Y.; Kim, H.; Yoon, H. Exfoliation of 2D Materials for Energy and Environmental Applications. Chem. Eur. J. 2020, 26, 6360–6401.
- Guo, S.; Dong, S. Graphene nanosheet: Synthesis, molecular engineering, thin film, hybrids, and energy and analytical applications. Chem. Soc. Rev. 2011, 40, 2644–2672.
- Knobloch, T.; Illarionov, Y.Y.; Ducry, F.; Schleich, C.; Wachter, S.; Watanabe, K.; Taniguchi, T.; Mueller, T.; Waltl, M.; Lanza, M.; et al. The performance limits of hexagonal boron nitride as an insulator for scaled CMOS devices based on two-dimensional materials. Nat. Electron. 2021, 4, 98–108.
- Arshad, A.; Jabbal, M.; Yan, Y.; Reay, D. A review on graphene based nanofluids: Preparation, characterization and applications. J. Mol. Liq. 2019, 279, 444–484.
- Hamze, S.; Berrada, N.; Cabaleiro, D.; Desforges, A.; Ghanbaja, J.; Gleize, J.; Bégin, D.; Michaux, F.; Maré, T.; Vigolo, B.; et al. Few-Layer Graphene-Based Nanofluids with Enhnaced Thermal Conductivity. Nanomaterials 2020, 10, 1258.
- Das, S.; Giri, A.; Samanta, S.; Kanagaraj, S. Role of graphene nanofluids on heat transfer enhancement in thermosyphon. J. Sci. Adv. Mater. Dev. 2019, 4, 163–169.
- Adnan; Abbas, W.; Bani-Fwaz, M.Z.; Asogwa, K.K. Thermal efficiency of radiated tetra-hybrid nanofluid tetra under permeability effects over vertically aligned cylinder subject to magnetic field and combined convection. Sci. Prog. 2023, 106, 00368504221149797.
- Adnan; Alharbi, K.A.M.; Ashraf, W.; Eldin, S.M.; Yassen, M.F.; Jamshed, W. Applied heat transfer modelling in conventional hybrid (Al2O3-CuO)/C2H6O2 and modified hybrid nanofluids (Al2O3-CuO-Fe3O4)/C2H6O2 between slippery channel by using least square method (LSM). AIMS Math. 2022, 8, 4321–4341.
- Adnan; Alharbi, K.A.M.; Bani-Fwaz, M.Z.; Eldin, S.M.; Yassen, M.F. Numerical heat performance of TiO2/Glycerin under nanoparticles aggregation and nonlinear radiative heat flux in dilating/squeezing channel. Case Stud. Therm. Eng. 2023, 41, 102568.
- Taha-Tijerina, J.; Pena-Paras, L.; Narayanan, T.N.; Garza, L.; Lapray, C.; Gonzalez, J.; Palacios, E.; Molina, D.; García, A.; Maldonado, D.; et al. Multifunctional nanofluids with 2D nanosheets for thermal and tribological management. Wear 2013, 302, 1241–1248.
- Salehirad, M.; Nikje, M.M.A. Properties of Modified Hexagonal Boron Nitride as Stable Nanofluids for Thermal Management Applications. Russ. J. Appl. Chem. 2019, 92, 78–86.
- Li, Y.; Zhou, J.; Luo, Z.; Tung, S.; Schneider, E.; Wu, J.; Li, X. Investigation on two abnormal phenomena about thermal conductivity enhancement of BN/EG nanofluids. Nanoscale Res. Lett. 2011, 6, 443.
- Bhunia, M.M.; Chattopadhyay, K.K.; Chattopadhyay, P. Transformer oils nanofluids by two-dimensional hexagonal boron nitride nanofillers. Electr. Eng. 2022.
- Han, W.; Wang, L.; Zhang, R.; Ge, C.; Ma, Z.; Yang, Y.; Zhang, X. Water-Dispersible Boron Nitride Nanospheres with High Thermal Conductivity for Heat-Transfer Nanofluids. Eur. J. Inorg. Chem. 2017, 2017, 5466–5474.
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669.
- Panjiar, H.; Gakkhar, R.P.; Daniel, B.S.S. Strain-free graphite nanoparticle synthesis by mechanical milling. Powder Technol. 2015, 275, 25–29.
- Bahiraei, M.; Heshmatian, S. Graphene family nanofluids: A critical review and future research directions. Energy Convers. Manag. 2019, 196, 1222–1256.
- Sadri, R.; Hosseini, M.; Kazi, S.N.; Bagheri, S.; Abdelrazek, A.H.; Ahmadi, G.; Zubir, N.; Ahmad, R.; Abidin, N.I.Z. A facile, bio-based, novel approach for synthesis of covalently functionalized graphene nanoplatelet nano—Coolants toward improved thermos-physical and heat transfer properties. J. Colloid Interface Sci. 2018, 509, 140–152.
- Dhar, P.; Gupta, S.S.; Chakraborty, S.; Pattamatta, A.; Das, S.K. The role of percolation and sheet dynamics during heat conduction in poly-dispersed graphene nanofluids. Appl. Phys. Lett. 2013, 102, 163114.
- Ghozatloo, A.; Shariaty-Niasar, M.; Rashidi, A.M. Preparation of nanofluids from functionalized Graphene by new alkaline method and study on the thermal conductivity and stability. Int. Commun. Heat Mass Transf. 2013, 42, 89–94.
- Park, S.D.; Lee, S.W.; Kang, S.; Bang, I.C.; Kim, J.H.; Shin, H.S.; Lee, D.W.; Lee, D.W. Effects of nanofluids containing graphene/graphene oxide nanosheets on critical heat flux. Appl. Phys. Lett. 2010, 97, 023103.
- Safaei, M.R.; Ahmadi, G.; Goodarzi, M.S.; Shadloo, M.S.; Goshayeshi, H.R.; Dahari, M. Heat Transfer and Pressure Drop in Fully Developed Turbulent Flows of Graphene Nanoplatelets-Silver/Water Nanofluids. Fluids 2016, 1, 20.
- Yarmand, H.; Gharehkhani, S.; Shirazi, S.F.S.; Goodarzi, M.; Amiri, A.; Sarsam, W.S.; Alehashem, M.S.; Dahari, M.; Kazi, S.N. Study of synthesis, stability and thermophysical properties of graphene nanoplatelet/platinum hybrid nanofluid. Int. Commun. Heat Mass Transf. 2016, 77, 15–21.
- Vincely, D.A.; Natarajan, E. Experimental investigation of the solar FPC performance using graphene oxide nanofluid under forced circulation. Energy Convers. Manag. 2016, 117, 15.
- Yu, W.; Xie, H.; Bao, D. Enhanced thermal Conductivities of nanofluids containing graphene oxide nanosheets. Nanotechnology 2010, 21, 055705.
- Lin, P.; Yan, Q.; Chen, Y.; Li, X.; Cheng, Z. Dispersion and assembly of reduced graphene oxide in chiral nematic liquid crystals by charged two-dimensional nanosurfactants. Chem. Eng. J. 2018, 334, 1023–1033.
- Zubir, M.N.M.; Badarudin, A.; Kazi, S.N.; Ming, H.N.; Misran, M.; Sadeghinezdah, E.; Mehrali, M.; Syuhada, N.I.; Gharehkhani, S. Experimental investigation on the use of reduced graphene oxide and its hybrid complexes in improving closed conduit turbulent forced convective heat transfer. Exp. Therm. Fluid Sci. 2015, 66, 290–303.
- Schlierf, A.; Yang, H.; Gebremedhn, E.; Treossi, E.; Ortolani, L.; Chen, L.; Minoia, A.; Morandi, V.; Samori, P.; Casiraghi, C.; et al. Nanoscale insight into the exfoliation mechanism of graphene with organic dyes: Effect of charge, dipole and molecular structure. Nanoscale 2013, 5, 4205.
- Fan, D.; Li, Q.; Chen, W.; Zeng, J. Graphene nanofluids containing core-shell nanoparticles with plasmon resonance effect enhanced solar energy absorption. Sol. Energy 2017, 158, 31.
- Lee, S.W.; Kim, K.M.; Bang, I.C. Study on flow boiling critical heat flux enhancement of graphene oxide/water nanofluid. Int. J. Heat Mass Transf. 2013, 65, 348–356.
- Yu, W.; Xie, H.; Wang, X.; Wang, X. Significant thermal conductivity enhancement for nanofluids containing graphene nanosheets. Phys. Lett. 2011, 375, 1323–1328.
- Lee, G.-J.; Rhee, C.K. Enhanced thermal conductivity of nanofluids containing graphene nanoplatelets prepared by ultrasound irradiation. J. Mater. Sci. 2014, 49, 1506–1511.
- Hamilton, R.L.; Crosser, O.K. Thermal Conductivity of Heterogeneous Two-Components Systems. Ind. Eng. Chem. Fundamen. 1962, 1, 3.
- Akhavan-Zanjani, H.; Saffar-Avval, M.; Mansourkiaei, M.; Ahadi, M.; Sharif, F. Turbulent Convective Heat Transfer and Pressure Drop of Graphene-Water nanofluid Flowing Inside a Horizontal Circular Tube. J. Dispers. Sci. Technol. 2014, 35, 1230–1240.
- Liu, J.; Wang, F.; Zhang, L.; Fang, X.; Zhang, Z. Thermodynamic properties and thermal stability of ionic liquid-based nanofluids containing graphene as advanced heat transfer fluids for medium-to-high-temperature applications. Renew. Energy 2014, 63, 519–523.
- Park, S.S.; Kim, N.J. Influence of the oxidation treatment and the average particle diameter of graphene for thermal conductivity enhancement. J. Ind. Eng. Chem. 2014, 20, 1911–1915.
- Tesfai, W.; Singh, P.; Shatilla, Y.; Iqbal, M.Z. Rheology and microstructure of dilute graphene oxide suspension. J. Nanoparticle Res. 2013, 15, 1989.
- Ijam, A.; Saidur, R.; Ganesan, P.; Golsheikh, A.M. Stability, thermo-physical properties, and electrical conductivity of graphene oxide-deionized water/ethylene glycol based nanofluid. Int. J. Heat Mass Transf. 2015, 87, 92–103.
- Sun, Z.; Poller, S.; Huang, X.; Guschin, D.; Taetz, C.; Ebbinghaus, P.; Masa, J.; Erbe, A.; Kilzer, A.; Schuhmann, W.; et al. High-yield exfoliation of graphite in acrylate polymers: A stable few-layer graphene nanofluid with enhanced thermal conductivity. Carbon 2013, 64, 288–294.
- Naghash, A.; Sattari, S.; Rashidi, A. Experimental assessment of convective heat transfer coefficient enhancement of nanofluids prepared from high surface are nanoporous graphene. Int. Commun. Heat Mass Transf. 2016, 78, 127–134.
- Bahiraei, M.; Salmi, H.K.; Safaei, M.R. Effect of employing a new biological nanofluid containing functionalized graphene nanoplatelets on thermal and hydraulic characteristics of a spiral heat exchanger. Energy Convers. Manag. 2019, 180, 72–82.
- Hu, Y.; Li, H.; He, Y.; Wang, L. Role of nanoparticles on boiling heat transfer performance of ethylene glycol aqueous solution based graphene nanosheets nanofluid. Int. J. Heat Mass Transf. 2016, 96, 565–572.
- Zhang, H.; Wang, S.; Lin, Y.; Feng, M.; Wu, Q. Stability, thermal conductivity, and rheological properties of controlled reduced graphene oxide dispersed nanofluids. Appl. Therm. Eng. 2017, 119, 132–139.
- Liu, M.S.; Lin, M.C.C.; Wang, C.C. Enhancements of thermal conductivities with Cu, CuO, and carbon nanotube nanofluids and application of MWNT/water nanofluid on a water chiller system. Nanoscale Res. Lett. 2011, 6, 297.
- Sadri, R.; Ahmadi, G.; Togun, H.; Dahari, M.; Kazi, S.N.; Sadeghinezhad, E.; Zubir, N. An experimental study on thermal conductivity and viscosity of nanofluids containing carbon nanotubes. Nanoscale Res. Lett. 2014, 9, 151.
- Kumar, P.C.M.; Chandrasekar, M. CFD analysis on heat and flow characteristics of double helically coiled tube heat exchanger handling MWCNT/water nanofluids. Heliyon 2019, 5, e02030.
- Qu, J.; Zhang, R.; Wang, Z.; Wang, Q. Photo-thermal conversion properties of hybrid CuO-MWCNT/H2O nanofluids for direct solar thermal energy harvest. Appl. Therm. Eng. 2019, 147, 390–398.
- Hjerrild, N.E.; Mesgari, S.; Crisostomo, F.; Scott, J.A.; Amal, R.; Taylor, R.A. Hybrid PV/T enhancement using selectively absorbing Ag-SiO2/carbon nanofluids. Sol. Energy Mater. Sol. Cells 2016, 147, 281–287.
- Gorji, T.B.; Ranjbar, A.A.; Mirzababaei, S.N. Optical properties of carboxyl functionalized carbon nanotube aqueous nanofluids as direct solar thermal energy absorbers. Solar Energy 2015, 119, 332–342.
- Jabbari, F.; Saedodin, S.; Rajabpour, A. Experimental Investigation and Molecular Dynamics Simulations of CNT-Water Nanofluid at Different Temperatures and Volume Fractions of Nanoparticles. J. Chem. Eng. Data 2019, 64, 262–272.
- Sabiha, M.A.; Mostafizur, R.M.; Saidur, R.; Mekhilef, S. Experimental investigation on thermo physical properties of single walled carbon nanotube nanofluids. Int. J. Heat Mass Transf. 2016, 93, 862–871.
- Yu, L.; Bian, Y.; Liu, Y.; Xu, X. Experimental investigation on rheological properties of water based nanofluids with low MWCNT concentrations. Int. J. Heat Mass Transf. 2019, 135, 175–185.
- Hosseini, M.; Sadri, R.; Kazi, S.N.; Bagheri, S.; Zubir, N.; Teng, C.B.; Zaharinie, T. Experimental Study on Heat Transfer and Thermo-Physical Properties of Covalently Functionalized Carbon Nanotubes Nanofluids in an Annular Heat Exchanger: A Green and Novel Synthesis. Energy Fuels 2017, 31, 5635–5644.
- Halelfadl, S.; Estellé, P.; Aladag, B.; Doner, N.; Maré, T. Viscosity of carbon nanotubes water based nanofluids: Influence of concentration and temperature. Int. J. Therm. Sci. 2013, 71, 111–117.
- Tsentalovich, D.E.; Ma, A.W.K.; Lee, J.A.; Behabtu, N.; Bengio, E.A.; Choi, A.; Hao, J.; Luo, Y.; Headrick, R.J.; Green, M.J.; et al. Relationship of Extensional Viscosity and Liquid Crystalline Transition to Length Distribution in Carbon Nanotube Solutions. Macromolecules 2016, 49, 681–689.
- Ansón-Casaos, A.; Ciria, J.C.; Sanahuja-Parejo, O.; Victor-Román, S.; González-Dominguez, J.M.; Garcia-Bordejé, E.; Benito, A.M.; Maser, W.K. The viscosity of dilute carbon nanotube (1D) and graphene oxide (2D) nanofluids. Phys. Chem. Chem. Phys. 2020, 22, 11474.
- Maron, S.H.; Pierce, P.E. Application of ree-eyring generalized flow theory to suspensions of spherical particles. J. Colloid Sci. 1956, 11, 80–95.
- Martínez-Merino, P.; Estellé, P.; Alcántara, R.; Carrilo-Berdugo, I.; Navas, J. Thermal performance of nanofluids based on tungsten disulphide nanosheets as heat transfer fluids in parabolic trough solar collectors. Sol. Energy Mater. Sol. Cells 2022, 247, 111937.
- Shah, S.N.A.; Shahabuddin, S.; Sabri, M.F.M.; Salleh, M.F.M.; Said, S.M.; Khedher, K.M.; Sridewi, N. Two-Dimensional Tungsten Disulfide-based Ethylene Glycol Nanofluids: Stability, Thermal Conductivity, and Rheological Properties. Nanomaterials 2020, 10, 1340.
- Aldana, P.U. Tungsten Disulfide Nanoparticles as Lubricant Additives for the Automotive Industry. Ph.D. Thesis, Lyon University, Lyon, France, 2016.
- Su, Y.; Gong, L.; Li, B.; Chen, D. An experimental investigation on thermal properties of molybdenum disulfide nanofluids. In Proceedings of the International Conference on Materials, Environmental and Biological Engineering (MEBE), Guilin, China, 28–30 March 2015.
- Nagarajan, T.; Khalid, M.; Sridewi, N.; Jagadish, P.; Shahabuddin, S.; Muthoosamy, K.; Walvekar, R. Tribological, oxidation and thermal conductivity studies of microwave synthesized molybdenum disulfide (MoS2) nanoparticles as nano-additives in diesel based engine oil. Sci. Rep. 2022, 12, 14108.
More
Information
Subjects:
Engineering, Mechanical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
922
Revisions:
2 times
(View History)
Update Date:
07 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




