
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Chiara Puricelli | -- | 8082 | 2023-04-05 05:44:06 | | | |
| 2 | Camila Xu | + 2 word(s) | 8084 | 2023-04-06 02:13:17 | | | | |
| 3 | Chiara Puricelli | -22 word(s) | 8062 | 2023-04-10 18:45:58 | | |
Video Upload Options
Platelets, traditionally known for their roles in hemostasis and coagulation, are the most prevalent blood component after erythrocytes (150,000–400,000 platelets/μL in healthy humans). However, only 10,000 platelets/μL are needed for vessel wall repair and wound healing. Increased knowledge of the platelet’s role in hemostasis has led to many advances in understanding that they are crucial mediators in many other physiological processes, such as innate and adaptive immunity. Due to their multiple functions, platelet dysfunction is involved not only in thrombosis, mediating myocardial infarction, stroke, and venous thromboembolism, but also in several other disorders, such as tumors, autoimmune diseases, and neurodegenerative diseases. On the other hand, thanks to their multiple functions, nowadays platelets are therapeutic targets in different pathologies, in addition to atherothrombotic diseases; they can be used as an innovative drug delivery system, and their derivatives, such as platelet lysates and platelet extracellular vesicles (pEVs), can be useful in regenerative medicine and many other fields. The protean role of platelets, from the name of Proteus, a Greek mythological divinity who could take on different shapes or aspects, is precisely the focus of this entry.
1. Introduction
1.1. Platelet Granules and Receptors
1.1.1. α-Granules
1.1.2. Dense Granules
2. Platelet Derivatives
| Growth Factor |
Source | Function | Refs. |
|---|---|---|---|
| BDNF | Platelets, neurons, heart, lung, liver, skeletal muscle | MSC survival and proliferation, angiogenesis, neuron activity, survival, and function | [40][41][42] |
| b-FGF | Platelets, macrophages, T lymphocytes, mast cells, endothelial cells, fibroblasts, chondrocytes, osteoblasts, mesenchymal cells |
Mitogenic effect on fibroblasts, endothelial cells, MSCs, chondroblasts, and osteoblasts, angiogenesis, bone regeneration, corneal tissue repair, hair growth, wound healing, soft dental tissue regeneration, embryogenesis | [34][35][37][39][40][43] |
| BMP | Platelets, bone, CNS and many other tissues |
Immune system regulation, cell maturation and differentiation, angiogenesis, cartilage and bone formation, fracture repair, tooth development, tissue fibrosis, cancer cell inhibition, CNS function | [37][40] |
| CTGF | Platelets after endocytosis from bone marrow extracellular environment | Found in platelets at a concentration that is 20-fold higher than any other PGF, involved in platelet adhesion, angiogenesis, cartilage regeneration, fibrosis, platelet adhesion, white blood cell migration, angiogenesis, regulation of collagen synthesis | [34][35][37][38][39] |
| EGF | Platelets, macrophages, monocytes | Endothelial chemotaxis, epithelial and mesenchymal mitogenesis, regulation of collagenase secretion, keratinocyte and fibroblast migration and proliferation, angiogenesis, re-epithelialization, corneal tissue repair | [34][35][37][39][40][43] |
| IGF-1 | Platelets, epithelial cells, endothelial cells, fibroblasts, smooth muscle cells, osteoblasts |
Differentiation of osteoblasts in bone and of myeloblastic tissue in muscle, proliferation, migration, angiogenesis, neuroprotection and re-myelination, bone regeneration, regulation of collagen production, synergistic effect with PDGF | [39][40][43] |
| PDGF | Platelets, endothelial cells, macrophages, monocytes, smooth muscle cells, osteoblasts, keratinocytes |
First growth factor to be released in a wound, mitogenesis, chemotaxis of macrophages and neutrophils, regulation of collagen synthesis and collagenase activity, matrix formation and remodeling, angiogenesis, neurogenesis, bone regeneration, re-epithelialization, wound healing, corneal tissue repair, synergistic effect with TGF-β | [34][35][37][39][40][43] |
| TGF (α-β) | Platelets, activated Th1 cells, NK cells, monocytes, macrophages, endothelial cells, neutrophils, keratinocytes, fibroblasts, muscle cells, bone and cartilage ECM |
MSC proliferation, mitogenesis of fibroblasts, osteoblasts, and endothelial cells, chemotaxis of endothelial cells, angiogenesis, regulation of collagen synthesis and collagenase secretion, extracellular matrix formation and connective tissue regeneration, regulation of mitogenesis mediated by other growth factors, bone formation and regeneration, re-epithelialization, wound healing, cancer metastasis, inhibition of macrophage and lymphocyte proliferation, synergistic effect with PDGF, possible anti-proliferative effect at high concentrations | [34][35][37][39][40][43] |
| VEGF | Platelets, endothelial cells, fibroblasts, keratinocytes | Differentiation and mitogenesis of endothelial cells, chemotaxis, angiogenesis, increased vascular permeability, vascularization, neuroprotection, bone regeneration | [34][35][37][39][40][43] |
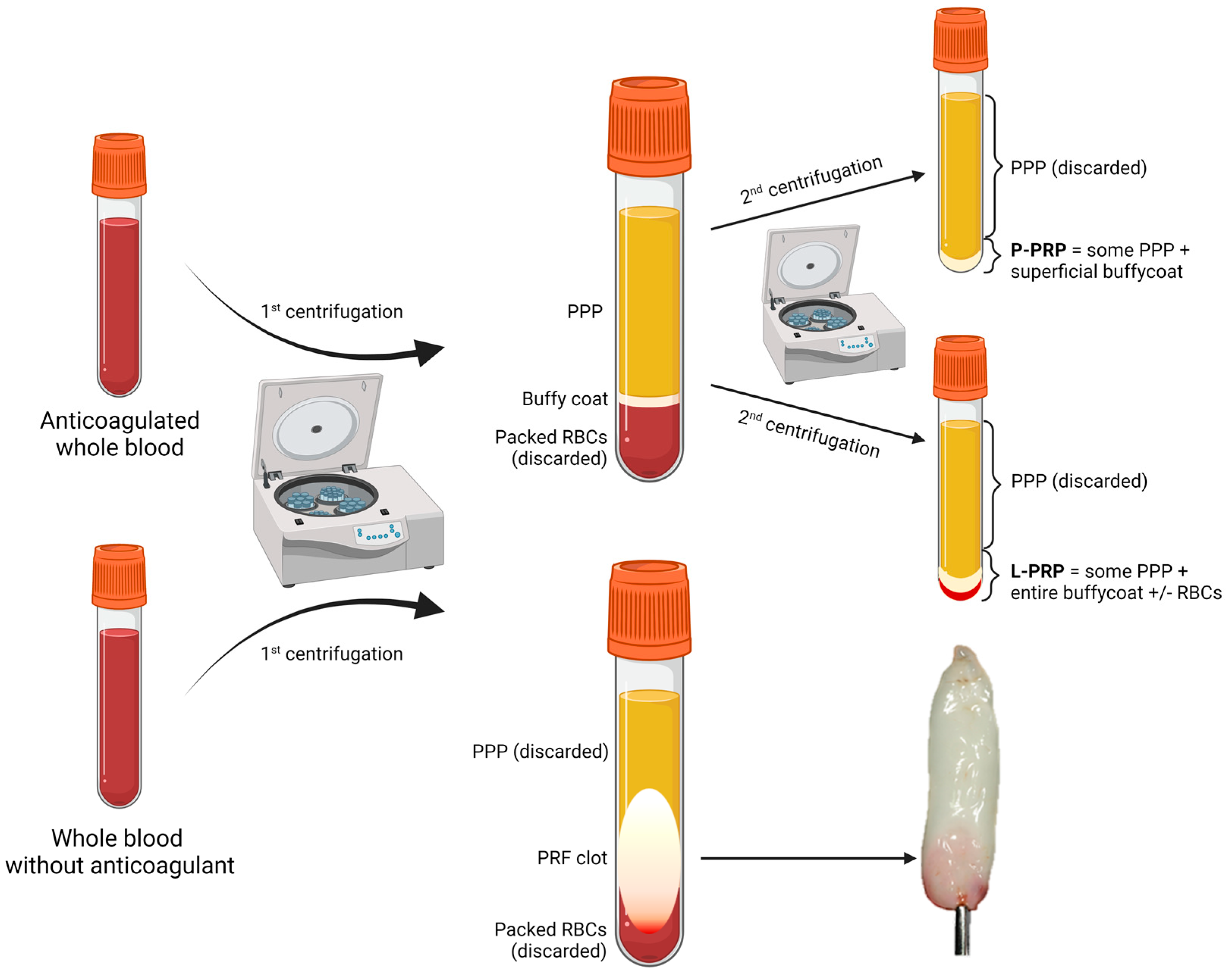
| Product | Source | Preparation Steps | Characteristics | Refs. |
|---|---|---|---|---|
| P-PRP L-PRP |
ACD-anticoagulated whole blood or apheresis | 1. Short soft-spin centrifugation to separate PPP, BC, and packed RBCs; 2. Collection of PPP and BC; 3. Long hard-spin centrifugation and discarding of the supernatant (PPP) to obtain P-PRP or L-PRP. |
|
[35][48][49] |
| PRF | ACD-anticoagulated whole blood or apheresis | Immediate centrifugation to obtain a flexible fibrin clot. |
|
[37][48][50][51][52][53][54][55] |
| PG | ACD-anticoagulated whole blood or apheresis | Same preparation as P-PRP or L-PRP but additional activation step to form a semi-solid (gelated) product by the action of calcium chloride or calcium gluconate with or without the addition of human thrombin, batroxobin, or synthetic TRAP (a thrombin receptor agonist), often added directly at the application site. |
|
[35][37][56][57][58][59] |
| PL | Whole blood or apheresis platelets | Platelet degranulation after freeze-thaw cycles, sonication, treatment with solvents and detergents, possibly followed by activation by thrombin or batroxobin just before application. |
|
[40] |
| E-S | Whole blood | Clotting at room temperature for 2–72 h, centrifugation to obtain serum and dilution with saline solution. |
|
[37][60][61] |
| E-PRP | Whole blood or apheresis platelets | Platelet degranulation after freeze-thaw cycles, dilution of the supernatant with saline solution. | Higher content of PGFs in a smaller volume, more suitable for intraocular instillation. | [37][56][62] |
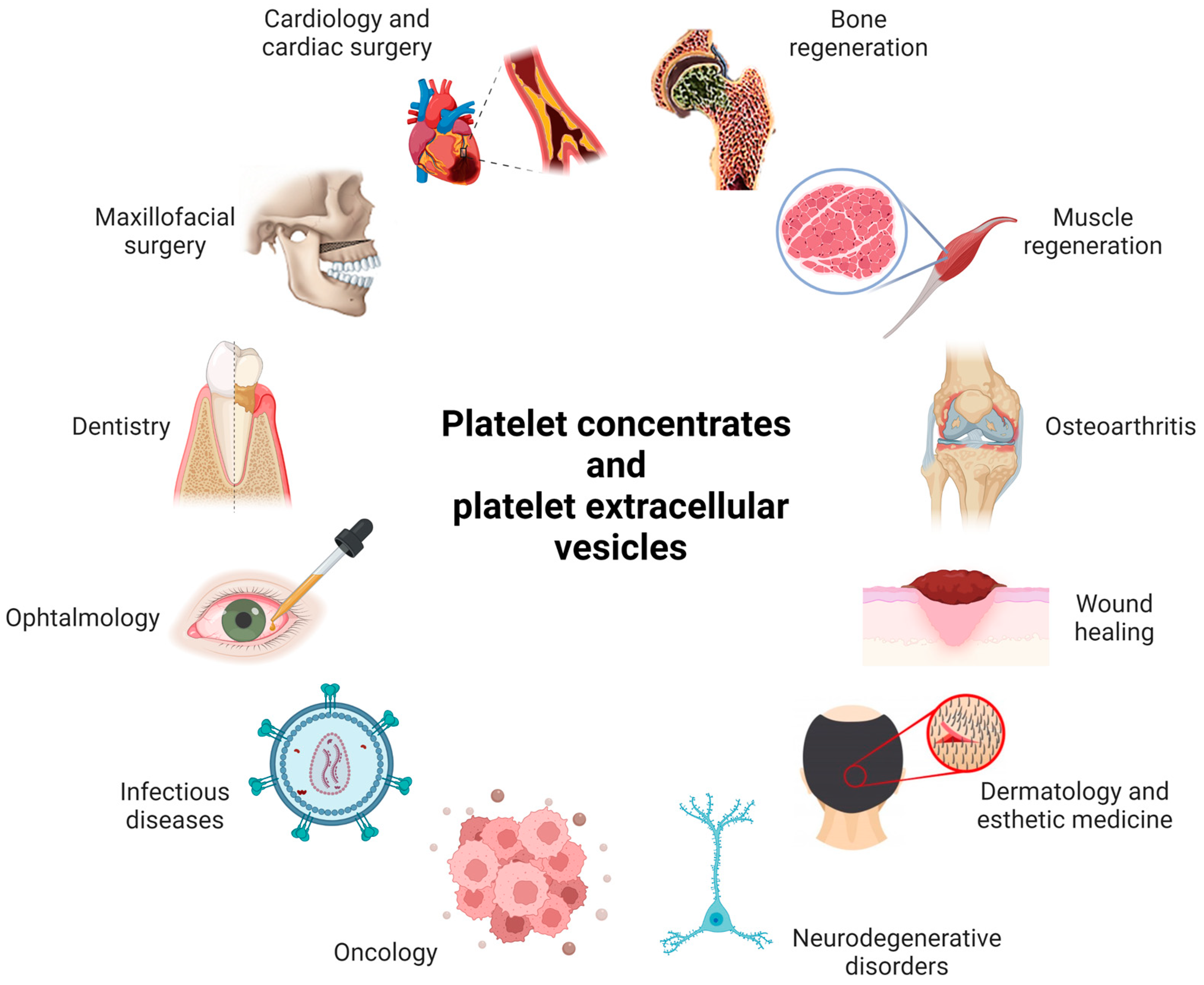
2.1. Examples of Medical Applications of PCs
2.2. Limitations in the Use of PCs
3. Platelet Role in Disease
3.1. Platelets and Atherosclerosis
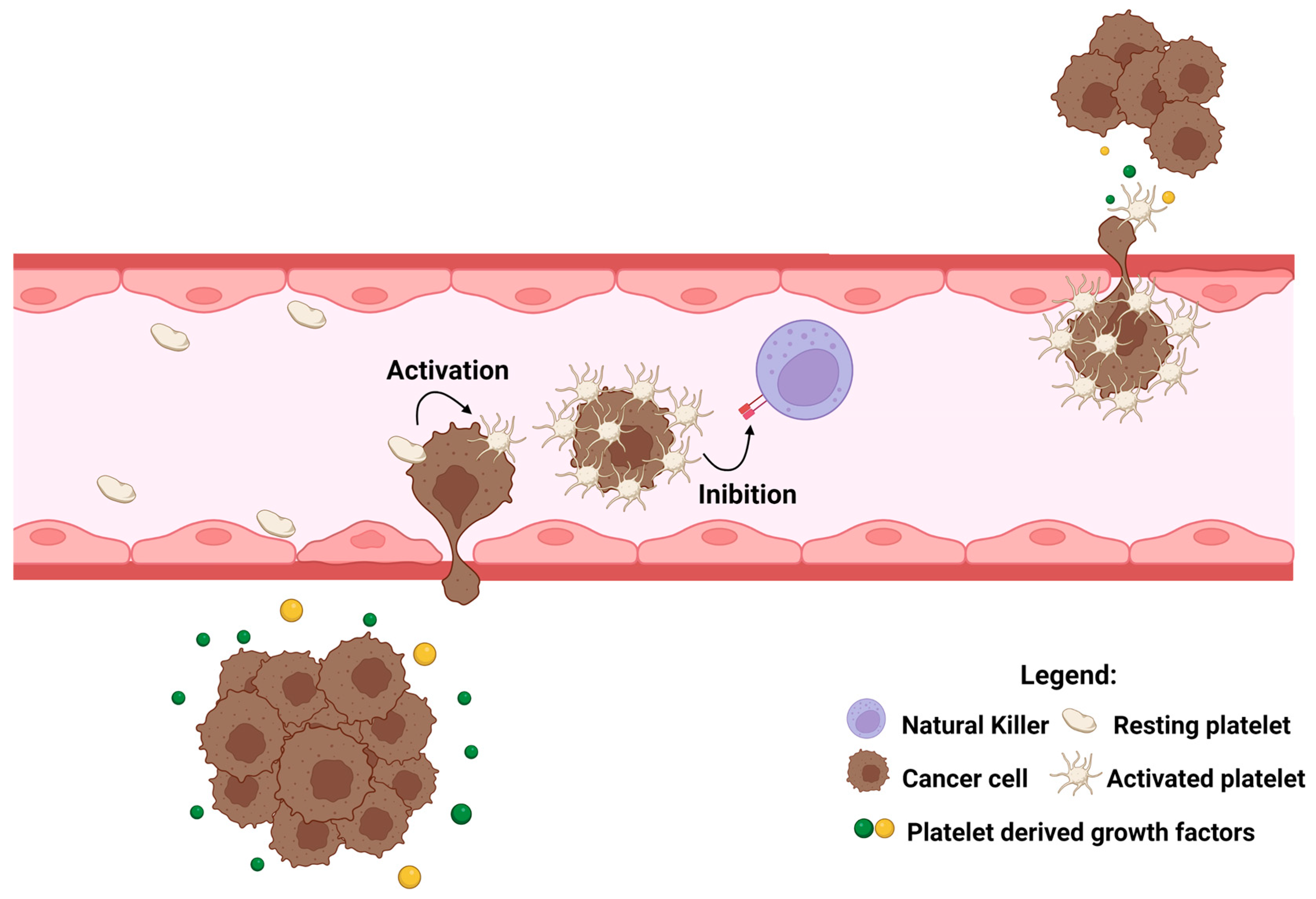
3.2. Platelets and Cancer
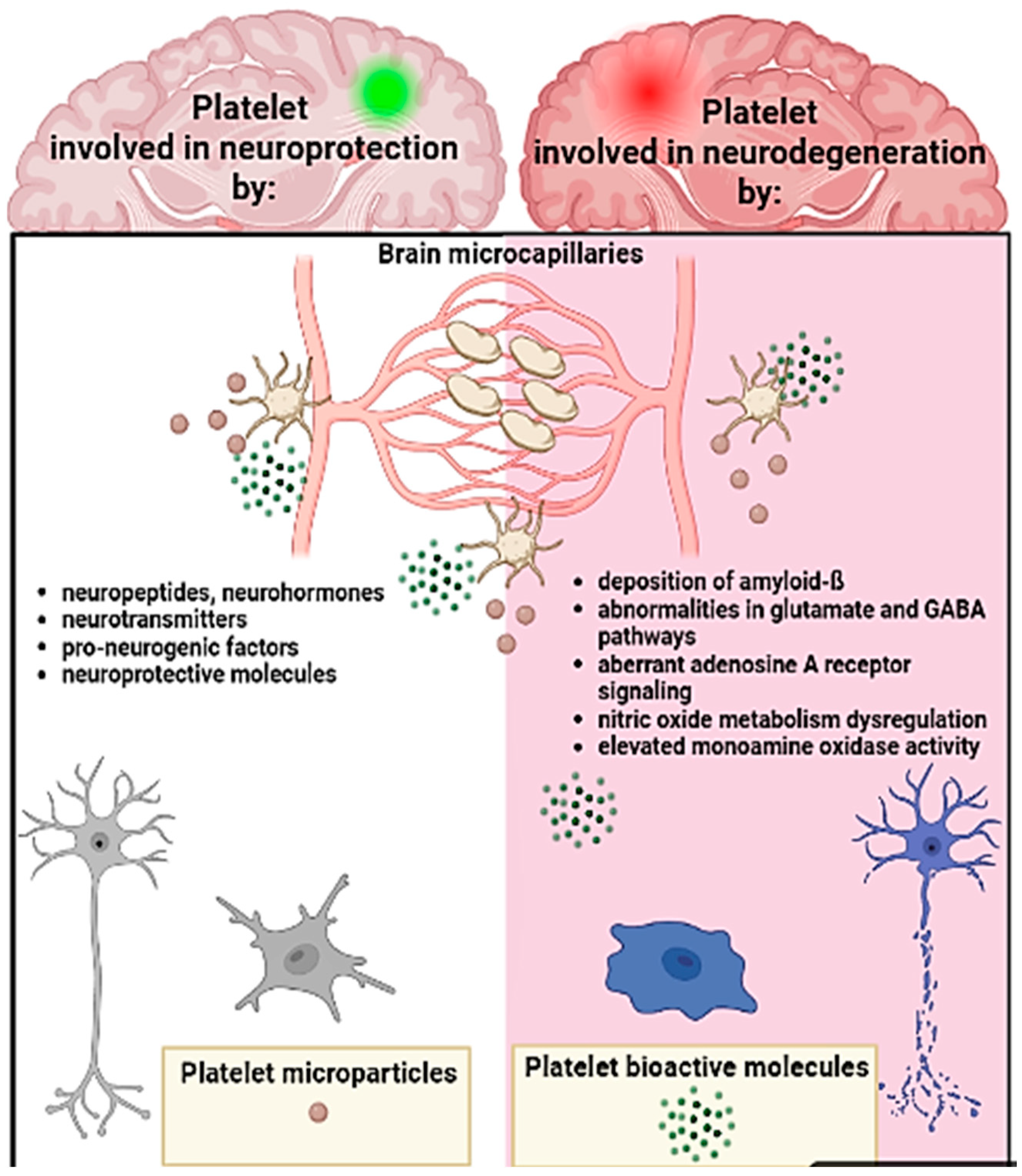
3.3. Platelets, the Brain, and Neurodegenerative Conditions
References
- Rolla, R.; Puricelli, C.; Bertoni, A.; Boggio, E.; Gigliotti, C.L.; Chiocchetti, A.; Cappellano, G.; Dianzani, U. Platelets: “multiple choice” effectors in the immune response and their implication in COVID-19 thromboinflammatory process. Int. J. Lab. Hematol. 2021, 43, 895–906.
- Diacovo, T.G.; Roth, S.J.; Buccola, J.M.; Bainton, D.F.; Springer, T.A. Neutrophil Rolling, Arrest, and Transmigration Across Activated, Surface-Adherent Platelets Via Sequential Action of P-Selectin and the &-Integrin CDllb/CDl8. Blood 1996, 88, 146–157.
- Shiraki, R.; Inoue, N.; Kawasaki, S.; Takei, A.; Kadotani, M.; Ohnishi, Y.; Ejiri, J.; Kobayashi, S.; Hirata, K.I.; Kawashima, S.; et al. Expression of Toll-like receptors on human platelets. Thromb. Res. 2004, 113, 379–385.
- Koupenova, M.; Clancy, L.; Corkrey, H.A.; Freedman, J.E. Circulating platelets as mediators of immunity, inflammation, and thrombosis. Circ. Res. 2018, 122, 337–351.
- Boilard, E.; Paré, G.; Rousseau, M.; Cloutier, N.; Dubuc, I.; Lévesque, T.; Borgeat, P.; Flamand, L. Influenza virus H1N1 activates platelets through FcγRIIA signaling and thrombin generation. Blood 2014, 123, 2854–2863.
- Yeung, J.; Tourdot, B.E.; Fernandez-Perez, P.; Vesci, J.; Ren, J.; Smyrniotis, C.J.; Luci, D.K.; Jadhav, A.; Simeonov, A.; Maloney, D.J.; et al. Platelet 12-LOX is essential for FcγRIIa-mediated platelet activation. Blood 2014, 124, 2271–2279.
- Holinstat, M. Normal platelet function. Cancer Metastasis Rev. 2017, 36, 198.
- McDonald, B.; Urrutia, R.; Yipp, B.G.; Jenne, C.N.; Kubes, P. Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host Microbe 2012, 12, 324–333.
- Ouseph, M.M.; Huang, Y.; Banerjee, M.; Joshi, S.; MacDonald, L.; Zhong, Y.; Liu, H.; Li, X.; Xiang, B.; Zhang, G.; et al. Autophagy is induced upon platelet activation and is essential for hemostasis and thrombosis. Blood 2015, 126, 1233.
- Yeung, J.; Li, W.; Holinstat, M. Platelet Signaling and Disease: Targeted Therapy for Thrombosis and Other Related Diseases. Pharmacol. Rev. 2018, 70, 526–548.
- Rendu, F.; Brohard-Bohn, B. The platelet release reaction: Granules’ constituents, secretion and functions. Platelets 2001, 12, 261–273.
- Maynard, D.M.; Heijnen, H.F.G.; Horne, M.K.; White, J.G.; Gahl, W.A. Proteomic analysis of platelet α-granules using mass spectrometry. J. Thromb. Haemost. 2007, 5, 1945–1955.
- Berger, G.; Masse, J.; Cramer, E. Alpha-granule membrane mirrors the platelet plasma membrane and contains the glycoproteins Ib, IX, and V. Blood 1996, 87, 1385–1395.
- Suzuki, H.; Murasaki, K.; Kodama, K.; Takayama, H. Intracellular localization of glycoprotein VI in human platelets and its surface expression upon activation. Br. J. Haematol. 2003, 121, 904–912.
- Coppinger, J.A.; Cagney, G.; Toomey, S.; Kislinger, T.; Belton, O.; McRedmond, J.P.; Cahill, D.J.; Emili, A.; Fitzgerald, D.J.; Maguire, P.B. Characterization of the proteins released from activated platelets leads to localization of novel platelet proteins in human atherosclerotic lesions. Blood 2004, 103, 2096–2104.
- Falati, S.; Liu, Q.; Gross, P.; Merrill-Skoloff, G.; Chou, J.; Vandendries, E.; Celi, A.; Croce, K.; Furie, B.C.; Furie, B. Accumulation of Tissue Factor into Developing Thrombi In Vivo Is Dependent upon Microparticle P-Selectin Glycoprotein Ligand 1 and Platelet P-Selectin. J. Exp. Med. 2003, 197, 1598.
- Kasper, B.; Brandt, E.; Bulfone-Paus, S.; Petersen, F. Platelet factor 4 (PF-4)–induced neutrophil adhesion is controlled by src-kinases, whereas PF-4–mediated exocytosis requires the additional activation of p38 MAP kinase and phosphatidylinositol 3-kinase. Blood 2004, 103, 1602–1610.
- Kasper, B.; Brandt, E.; Ernst, M.; Petersen, F. Neutrophil adhesion to endothelial cells induced by platelet factor 4 requires sequential activation of Ras, Syk, and JNK MAP kinases. Blood 2006, 107, 1768–1775.
- Nomura, S.; Ishii, K.; Kanazawa, S.; Inami, N.; Kamitsuji, Y.; Uoshima, N.; Ishida, H.; Yoshihara, T.; Kitayama, H.; Hayashi, K. Role of platelet-derived chemokines (RANTES and ENA-78) after stem cell transplantation. Transpl. Immunol. 2006, 15, 247–253.
- Smith, D.F.; Galkina, E.; Ley, K.; Huo, Y. GRO family chemokines are specialized for monocyte arrest from flow. Am. J. Physiol. Circ. Physiol. 2005, 289, H1976–H1984.
- Cha, J.K.; Jeong, M.H.; Bae, H.R.; Han, J.Y.; Jeong, S.J.; Jin, H.J.; Lim, Y.J.; Kim, S.H.; Kim, J.W. Activated platelets induce secretion of interleukin-1beta, monocyte chemotactic protein-1, and macrophage inflammatory protein-1alpha and surface expression of intercellular adhesion molecule-1 on cultured endothelial cells. J. Korean Med. Sci. 2000, 15, 278.
- Gleissner, C.A.; Von Hundelshausen, P.; Ley, K. Platelet chemokines in vascular disease. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1927.
- Lee, S.P.; Ataga, K.I.; Orringer, E.P.; Phillips, D.R.; Parise, L.V. Biologically Active CD40 Ligand Is Elevated in Sickle Cell Anemia. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1626–1631.
- Klesney-Tait, J.; Turnbull, I.R.; Colonna, M. The TREM receptor family and signal integration. Nat. Immunol. 2006, 7, 1266–1273.
- Haselmayer, P.; Grosse-Hovest, L.; von Landenberg, P.; Schild, H.; Radsak, M.P. TREM-1 ligand expression on platelets enhances neutrophil activation. Blood 2007, 110, 1029–1035.
- Au, A.E.; Josefsson, E.C. Regulation of platelet membrane protein shedding in health and disease. Platelets 2017, 28, 342–353.
- Ruiz, F.A.; Lea, C.R.; Oldfield, E.; Docampo, R. Human Platelet Dense Granules Contain Polyphosphate and Are Similar to Acidocalcisomes of Bacteria and Unicellular Eukaryotes. J. Biol. Chem. 2004, 279, 44250–44257.
- Müller, F.; Mutch, N.J.; Schenk, W.A.; Smith, S.A.; Esterl, L.; Spronk, H.M.; Schmidbauer, S.; Gahl, W.A.; Morrissey, J.H.; Renné, T. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell 2009, 139, 1156.
- Youssefian, T.; Massé, J.M.; Rendu, F.; Guichard, J.; Cramer, E.M. Platelet and Megakaryocyte Dense Granules Contain Glycoproteins Ib and IIb-IIIa. Blood 1997, 89, 4047–4057.
- Jenne, C.N.; Urrutia, R.; Kubes, P. Platelets: Bridging hemostasis, inflammation, and immunity. Int. J. Lab. Hematol. 2013, 35, 254–261.
- Mohammadi, M.; Zinkle, A. A threshold model for receptor tyrosine kinase signaling specificity and cell fate determination. F1000Research 2018, 7, 872.
- Zou, X.; Tang, X.Y.; Qu, Z.Y.; Sun, Z.W.; Ji, C.F.; Li, Y.J.; Guo, S.D. Targeting the PDGF/PDGFR signaling pathway for cancer therapy: A review. Int. J. Biol. Macromol. 2022, 202, 539–557.
- Huang, X.L.; Khan, M.I.; Wang, J.; Ali, R.; Ali, S.W.; Kazmi, A.; Lolai, A.; Huang, Y.L.; Hussain, A.; Bilal, M.; et al. Role of receptor tyrosine kinases mediated signal transduction pathways in tumor growth and angiogenesis—New insight and futuristic vision. Int. J. Biol. Macromol. 2021, 180, 739–752.
- Piccin, A.; Di Pierro, A.M.; Canzian, L.; Primerano, M.; Corvetta, D.; Negri, G.; Mazzoleni, G.; Gastl, G.; Steurer, M.; Gentilini, I.; et al. Platelet gel: A new therapeutic tool with great potential. Blood Transfus. 2017, 15, 333–340.
- Everts, P.A.M.; Knape, J.T.A.; Weibrich, G.; Schönberger, J.P.A.M.; Hoffmann, J.; Overdevest, E.P.; Box, H.A.M.; Van Zundert, A. Platelet-Rich Plasma and Platelet Gel: A Review. J. Extra. Corpor. Technol. 2006, 38, 187.
- Piccin, A.; Di Pierro, A.M.; Calabrese, L.; Fontanella, F.; Daves, M. Platelet gel: The “holy water” of regenerative medicine. La Riv. Ital. della Med. di Lab.-Ital. J. Lab. Med. 2018 144 2018, 14, 201–202.
- De Pascale, M.R.; Sommese, L.; Casamassimi, A.; Napoli, C. Platelet Derivatives in Regenerative Medicine: An Update. Transfus. Med. Rev. 2015, 29, 52–61.
- Cicha, I.; Garlichs, C.D.; Daniel, W.G.; Goppelt-Struebe, M. Activated human platelets release connective tissue growth factor. Thromb. Haemost. 2004, 91, 755–760.
- Dhurat, R.; Sukesh, M. Principles and Methods of Preparation of Platelet-Rich Plasma: A Review and Author’s Perspective. J. Cutan. Aesthet. Surg. 2014, 7, 197.
- Zamani, M.; Yaghoubi, Y.; Movassaghpour, A.; Shakouri, K.; Mehdizadeh, A.; Pishgahi, A.; Yousefi, M. Novel therapeutic approaches in utilizing platelet lysate in regenerative medicine: Are we ready for clinical use? J. Cell. Physiol. 2019, 234, 17172–17186.
- Chacón-Fernández, P.; Säuberli, K.; Colzani, M.; Moreau, T.; Ghevaert, C.; Barde, Y.A. Brain-derived Neurotrophic Factor in Megakaryocytes. J. Biol. Chem. 2016, 291, 9881.
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front. Cell. Neurosci. 2019, 13, 387.
- Chicharro-Alcántara, D.; Rubio-Zaragoza, M.; Damiá-Giménez, E.; Carrillo-Poveda, J.M.; Cuervo-Serrato, B.; Peláez-Gorrea, P.; Sopena-Juncosa, J.J. Platelet Rich Plasma: New Insights for Cutaneous Wound Healing Management. J. Funct. Biomater. 2018, 9, 10.
- Giusti, I.; D’Ascenzo, S.; Mancò, A.; Di Stefano, G.; Di Francesco, M.; Rughetti, A.; Dal Mas, A.; Properzi, G.; Calvisi, V.; Dolo, V. Platelet Concentration in Platelet-Rich Plasma Affects Tenocyte Behavior In Vitro. BioMed Res. Int. 2014, 2014, 630870.
- Dieudonné, S.C.; Foo, P.; Van Zoelen, E.J.J.; Burger, E.H. Inhibiting and stimulating effects of TGF-beta 1 on osteoclastic bone resorption in fetal mouse bone organ cultures. J. Bone Miner. Res. 1991, 6, 479–487.
- Weibrich, G.; Hansen, T.; Kleis, W.; Buch, R.; Hitzler, W.E. Effect of platelet concentration in platelet-rich plasma on peri-implant bone regeneration. Bone 2004, 34, 665–671.
- Zumarán, C.C.; Parra, M.V.; Olate, S.A.; Fernández, E.G.; Muñoz, F.T.; Haidar, Z.S. The 3 R’s for Platelet-Rich Fibrin: A “Super” Tri-Dimensional Biomaterial for Contemporary Naturally-Guided Oro-Maxillo-Facial Soft and Hard Tissue Repair, Reconstruction and Regeneration. Materials 2018, 11, 1293.
- Dohan Ehrenfest, D.M.; Rasmusson, L.; Albrektsson, T. Classification of platelet concentrates: From pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009, 27, 158–167.
- Del Fante, C.; Perotti, C.; Bonferoni, M.C.; Rossi, S.; Sandri, G.; Ferrari, F.; Scudeller, L.; Caramella, C.M. Platelet Lysate Mucohadesive Formulation to Treat Oral Mucositis in Graft Versus Host Disease Patients: A New Therapeutic Approach. AAPS PharmSciTech 2011, 12, 893–899.
- Passaretti, F.; Tia, M.; D’esposito, V.; De Pascale, M.; Del Corso, M.; Sepulveres, R.; Liguoro, D.; Valentino, R.; Beguinot, F.; Formisano, P.; et al. Growth-promoting action and growth factor release by different platelet derivatives. Platelets 2014, 25, 252–256.
- Choukroun, J.I.; Braccini, F.; Diss, A.; Giordano, G.; Doglioli, P.; Dohan, D.M. Influence of platelet rich fibrin (PRF) on proliferation of human preadipocytes and tympanic keratinocytes: A new opportunity in facial lipostructure (Coleman’s technique) and tympanoplasty? Rev. Laryngol. Otol. Rhinol. 2007, 128, 27–32.
- Diss, A.; Dohan, D.M.; Mouhyi, J.; Mahler, P. Osteotome sinus floor elevation using Choukroun’s platelet-rich fibrin as grafting material: A 1-year prospective pilot study with microthreaded implants. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008, 105, 572–579.
- Del Corso, M.; Vervelle, A.; Simonpieri, A.; Jimbo, R.; Inchingolo, F.; Sammartino, G.; M. Dohan Ehrenfest, D. Current Knowledge and Perspectives for the Use of Platelet-Rich Plasma (PRP) and Platelet-Rich Fibrin (PRF) in Oral and Maxillofacial Surgery Part 1: Periodontal and Dentoalveolar Surgery. Curr. Pharm. Biotechnol. 2012, 13, 1207–1230.
- Simonpieri, A.; Del Corso, M.; Vervelle, A.; Jimbo, R.; Inchingolo, F.; Sammartino, G.; M. Dohan Ehrenfest, D. Current knowledge and perspectives for the use of platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) in oral and maxillofacial surgery part 2: Bone graft, implant and reconstructive surgery. Curr. Pharm. Biotechnol. 2012, 13, 1231–1256.
- Braccini, F.; Dohan, D.M. The relevance of Choukroun’s platelet rich fibrin (PRF) during facial aesthetic lipostructure (Coleman’s technique): Preliminary results. Rev. Laryngol. Otol. Rhinol. 2007, 128, 255–260.
- Aprili, G.; Gandini, G.; Guaschino, R.; Mazzucco, L.; Salvaneschi, L.; Vaglio, S. SIMTI recommendations on blood components for non-transfusional use. Blood Transfus. 2013, 11, 622.
- Rodgers, G.M. Immune-mediated coagulopathy associated with topical bovine thrombin: Review of the pediatric literature. HJournal Pediatr. Hematol. 2011, 33, 86–88.
- Clark, J.; Crean, S.; Reynolds, M.W. Topical bovine thrombin and adverse events: A review of the literature. Curr. Med. Res. Opin. 2008, 24, 2071–2087.
- Harrison, S.; Vavken, P.; Kevy, S.; Jacobson, M.; Zurakowski, D.; Murray, M.M. Platelet activation by collagen provides sustained release of anabolic cytokines. Am. J. Sport. Med. 2011, 39, 729–734.
- Geerling, G.; MacLennan, S.; Hartwig, D. Autologous serum eye drops for ocular surface disorders. Br. J. Ophthalmol. 2004, 88, 1474.
- Quinto, G.G.; Campos, M.; Behrens, A. Autologous serum for ocular surface diseases. Arq. Bras. Oftalmol. 2008, 71, 47–54.
- Geremicca, W.; Fonte, C.; Vecchio, S. Blood components for topical use in tissue regeneration: Evaluation of corneal lesions treated with platelet lysate and considerations on repair mechanisms. Blood Transfus. 2010, 8, 112.
- Harmon, K.; Hanson, R.; Bowen, J.; Greenberg, S.; Magaziner, E.; Vandenbosch, J.; Harshfield, D.; Shiple, B.; Audley, D. Guidelines for the Use of Platelet Rich Plasma—Draft. Int. Cell. Med. Soc. 2011. Available online: https://www.scribd.com/document/159334949/206-ICMS-Guidelines-for-the-Use-of-Platelet-Rich-Plasma-Draftob-oasbonasdandbowndoww (accessed on 24 December 2022).
- Ferrari, M.; Zia, S.; Valbonesi, M.; Henriquet, F.; Venere, G.; Spagnolo, S.; Grasso, M.A.; Panzani, I. A new technique for hemodilution, preparation of autologous platelet-rich plasma and intraoperative blood salvage in cardiac surgery. Int. J. Artif. Organs 1987, 10, 47–50.
- Oz, M.C.; Jeevanandam, V.; Smith, C.R.; Williams, M.R.; Kaynar, A.M.; Frank, R.A.; Mosca, R.; Reiss, R.F.; Rose, E.A. Autologous fibrin glue from intraoperatively collected platelet-rich plasma. Ann. Thorac. Surg. 1992, 53, 530–531.
- Tawes, R.L.; Sydorak, G.R.; DuVall, T.B. Autologous fibrin glue: The last step in operative hemostasis. Am. J. Surg. 1994, 168, 120–122.
- Piccin, A.; Di Pierro, A.M.; Tagnin, M.; Russo, C.; Fustos, R.; Corvetta, D.; Primerano, M.; Magri, E.; Conci, V.; Gentilini, I.; et al. Healing of a soft tissue wound of the neck and jaw osteoradionecrosis using platelet gel. Regen. Med. 2016, 11, 459–463.
- San Sebastian, K.M.; Lobato, I.; Hernández, I.; Burgos-Alonso, N.; Gomez-Fernandez, M.C.; López, J.L.; Rodríguez, B.; March, A.G.; Grandes, G.; Andia, I. Efficacy and safety of autologous platelet rich plasma for the treatment of vascular ulcers in primary care: Phase III study. BMC Fam. Pract. 2014, 15, 211–218.
- Merchán, W.H.; Gómez, L.A.; Chasoy, M.E.; Alfonso-Rodríguez, C.A.; Muñoz, A.L. Platelet-rich plasma, a powerful tool in dermatology. J. Tissue Eng. Regen. Med. 2019, 13, 892–901.
- McCarrel, T.M.; Mall, N.A.; Lee, A.S.; Cole, B.J.; Butty, D.C.; Fortier, L.A. Considerations for the use of platelet-rich plasma in orthopedics. Sport. Med. 2014, 44, 1025–1036.
- L. Alio, J.; Arnalich-Montiel, F.; E. Rodriguez, A. The role of “eye platelet rich plasma” (E-PRP) for wound healing in ophthalmology. Curr. Pharm. Biotechnol. 2012, 13, 1257–1265.
- Ye, F.; Li, H.; Qiao, G.; Chen, F.; Tao, H.; Ji, A.; Hu, Y. Platelet-rich plasma gel in combination with Schwann cells for repair of sciatic nerve injury. Neural Regen. Res. 2012, 7, 2292.
- Chou, M.L.; Wu, J.W.; Gouel, F.; Jonneaux, A.; Tillerman, K.; Renn, T.Y.; Laloux, C.; Chang, H.M.; Lin, L.T.; Devedjian, J.C.; et al. Tailor-made purified human platelet lysate concentrated in neurotrophins for treatment of Parkinson’s disease. Biomaterials 2017, 142, 77–89.
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542.
- Krupski, W.; Reilly, L.; Perez, S.; Moss, K.; Crombleholme, P.; Rapp, J. A prospective randomized trial of autologous platelet-derived wound healing factors for treatment of chronic nonhealing wounds: A preliminary report. J. Vasc. Surg. 1991, 14, 526–532.
- Carter, M.J.; Fylling, C.P.; Parnell, L.K.S. Use of Platelet Rich Plasma Gel on Wound Healing: A Systematic Review and Meta-Analysis. Eplasty 2011, 11, e38.
- Alsousou, J.; Thompson, M.; Hulley, P.; Noble, A.; Willett, K. The biology of platelet-rich plasma and its application in trauma and orthopaedic surgery: A review of the literature. J. Bone Jt. Surgery. 2009, 91, 987–996.
- Forni, F.; Marzagalli, M.; Tesei, P.; Grassi, A. Platelet gel: Applications in dental regenerative surgery. Blood Transfus. 2013, 11, 102–107.
- Kim, M.; Won, J.Y.; Choi, S.Y.; Kim, M.; Ra, H.; Jee, D.; Kwon, J.W.; Kang, K.D.; Roh, Y.J.; Park, Y.G.; et al. Therapeutic efficacy of autologous platelet concentrate injection on macular holes with high myopia, large macular holes, or recurrent macular holes: A multicenter randomized controlled trial. J. Clin. Med. 2021, 10, 2727.
- Fox, R.I.; Chan, R.; Michelson, J.B.; Belmont, J.B.; Michelson, P.E. Beneficial effect of artificial tears made with autologous serum in patients with keratoconjunctivitis sicca. Arthritis Rheum. 1984, 27, 459–461.
- De Vos, R.J.; Windt, J.; Weir, A. Strong evidence against platelet-rich plasma injections for chronic lateral epicondylar tendinopathy: A systematic review. Br. J. Sport. Med. 2014, 48, 952–956.
- De Vos, R.J.; Weir, A.; Van Schie, H.T.M.; Bierma-Zeinstra, S.M.A.; Verhaar, J.A.N.; Weinans, H.; Tol, J.L. Platelet-rich plasma injection for chronic Achilles tendinopathy: A randomized controlled trial. JAMA 2010, 303, 144–149.
- Kuffler, D.P. Platelet-rich plasma and the elimination of neuropathic pain. Mol. Neurobiol. 2013, 48, 315–332.
- Malahias, M.A.; Johnson, E.O.; Babis, G.C.; Nikolaou, V.S. Single injection of platelet-rich plasma as a novel treatment of carpal tunnel syndrome. Neural Regen. Res. 2015, 10, 1856–1859.
- Centeno, C.; Markle, J.; Dodson, E.; Stemper, I.; Hyzy, M.; Williams, C.; Freeman, M. The use of lumbar epidural injection of platelet lysate for treatment of radicular pain. J. Exp. Orthop. 2017, 4, 38.
- Crovetti, G.; Martinelli, G.; Issi, M.; Barone, M.; Guizzardi, M.; Campanati, B.; Moroni, M.; Carabelli, A. Platelet gel for healing cutaneous chronic wounds. Transfus. Apher. Sci. 2004, 30, 145–151.
- Antich-Rosselló, M.; Forteza-Genestra, M.A.; Monjo, M.; Ramis, J.M. Platelet-Derived Extracellular Vesicles for Regenerative Medicine. Int. J. Mol. Sci. 2021, 22, 8580.
- Phipps, R.P. Atherosclerosis: The emerging role of inflammation and the CD40-CD40 ligand system. Proc. Natl. Acad. Sci. USA 2000, 97, 6930–6932.
- Huo, Y.; Ley, K.F. Role of Platelets in the Development of Atherosclerosis. Trends Cardiovasc. Med. 2004, 14, 18–22.
- Fitzgerald, D.J.; Roy, L.; Catella, F.; FitzGerald, G.A. Platelet activation in unstable coronary disease. N. Engl. J. Med. 1986, 315, 983–989.
- Steinhubl, S.R.; Badimon, J.J.; Bhatt, D.L.; Herbert, J.M.; Lüscher, T. Clinical evidence for anti-inflammatory effects of antiplatelet therapy in patients with atherothrombotic disease. Vasc. Med. 2007, 12, 113–122.
- Cohen Arazi, H.; Badimon, J.J. Anti-inflammatory effects of anti-platelet treatment in atherosclerosis. Curr. Pharm. Des. 2012, 18, 4311–4325.
- Iyengar, S.; Rabbani, L.R.E. Beyond platelet inhibition: Potential pleiotropic effects of ADP-receptor antagonists. J. Thromb. Thrombolysis 2009, 27, 300–306.
- Klinkhardt, U.; Bauersachs, R.; Adams, J.; Graff, J.; Lindhoff-Last, E.; Harder, S. Clopidogrel but not aspirin reduces P-selectin expression and formation of platelet-leukocyte aggregates in patients with atherosclerotic vascular disease. Clin. Pharmacol. Ther. 2003, 73, 232–241.
- Li, N.; Hu, H.; Hjemdahl, P. Aspirin treatment does not attenuate platelet or leukocyte activation as monitored by whole blood flow cytometry. Thromb. Res. 2003, 111, 165–170.
- Diehl, P.; Olivier, C.; Haischeid, C.; Helbing, T.; Bode, C.; Moser, M. Clopidogrel affects leukocyte dependent platelet aggregation by P2Y 12 expressing leukocytes. Basic Res. Cardiol. 2010, 105, 379–387.
- Patel, M.R.; Marso, S.P.; Dai, D.; Anstrom, K.J.; Shunk, K.A.; Curtus, J.P.; Brennan, J.M.; Sedrakyan, A.; Messenger, J.C.; Douglas, P.S. Comparative Effectiveness of Drug-Eluting Versus Bare-Metal Stents in Elderly Patients Undergoing Revascularization of Chronic Total Coronary Occlusions: Results From the National Cardiovascular Data Registry, 2005–2008. JACC Cardiovasc. Interv. 2012, 5, 1054–1061.
- Crimi, G.; Gritti, V.; Galiffa, V.A.; Scotti, V.; Leonardi, S.; Ferrario, M.; Ferlini, M.; De Ferrari, G.M.; Oltrona Visconti, L.; Klersy, C. Drug eluting stents are superior to bare metal stents to reduce clinical outcome and stent-related complications in CKD patients, a systematic review, meta-analysis and network meta-analysis. J. Interv. Cardiol. 2018, 31, 319–329.
- Piccolo, R.; Bonaa, K.H.; Efthimiou, O.; Varenne, O.; Baldo, A.; Urban, P.; Kaiser, C.; Remkes, W.; Räber, L.; de Belder, A.; et al. Drug-eluting or bare-metal stents for percutaneous coronary intervention: A systematic review and individual patient data meta-analysis of randomised clinical trials. Lancet 2019, 393, 2503–2510.
- Pickard, A.S.; Becker, R.C.; Schumock, G.T.; Frye, C.B. Clopidogrel-Associated Bleeding and Related Complications in Patients Undergoing Coronary Artery Bypass Grafting. Pharmacotherapy 2008, 28, 376–392.
- Li, J.; Li, W.; Zou, D.; Kou, F.; Hou, Y.; Yasin, A.; Zhang, K. Comparison of conjugating chondroitin sulfate A and B on amine-rich surface: For deeper understanding on directing cardiovascular cells fate. Compos. Part B Eng. 2022, 228, 109430.
- Han, Z.; Guo, H.; Zhou, Y.; Wang, L.; Zhang, K.; Li, J.A. Composite Coating Prepared with Ferulic Acid to Improve the Corrosion Resistance and Blood Compatibility of Magnesium Alloy. Metals 2022, 12, 545.
- Li, J.; Zhang, K.; Hou, Y. From selective cardiovascular cells adhesion to regulating spatiotemporal orderliness of function: Understanding based on biomaterials surface modification with functional molecules. Curr. Top. Med. Med. Res. 2020, 5, 155–161.
- Haemmerle, M.; Stone, R.L.; Menter, D.G.; Afshar-Kharghan, V.; Sood, A.K. The Platelet Lifeline to Cancer: Challenges and Opportunities. Cancer Cell 2018, 33, 965–983.
- Palacios-Acedo, A.L.; Mège, D.; Crescence, L.; Dignat-George, F.; Dubois, C.; Panicot-Dubois, L. Platelets, Thrombo-Inflammation, and Cancer: Collaborating With the Enemy. Front. Immunol. 2019, 10, 1811.
- Xu, X.R.; Yousef, G.M.; Ni, H. Cancer and platelet crosstalk: Opportunities and challenges for aspirin and other antiplatelet agents. Blood 2018, 131, 1777–1789.
- Schlesinger, M. Role of platelets and platelet receptors in cancer metastasis. J. Hematol. Oncol. 2018, 11, 125–139.
- Cappellano, G.; Raineri, D.; Rolla, R.; Giordano, M.; Puricelli, C.; Vilardo, B.; Manfredi, M.; Cantaluppi, V.; Sainaghi, P.P.; Castello, L.; et al. Circulating Platelet-Derived Extracellular Vesicles Are a Hallmark of SARS-CoV-2 Infection. Cells 2021, 10, 85.
- Olsson, A.K.; Cedervall, J. The pro-inflammatory role of platelets in cancer. Platelets 2018, 29, 569–573.
- Patrignani, P.; Patrono, C. Aspirin, platelet inhibition and cancer prevention. Platelets 2018, 29, 779–785.
- Mezouar, S.; Darbousset, R.; Dignat-George, F.; Panicot-Dubois, L.; Dubois, C. Inhibition of platelet activation prevents the P-selectin and integrin-dependent accumulation of cancer cell microparticles and reduces tumor growth and metastasis in vivo. Int. J. Cancer 2015, 136, 462–475.
- Bibbins-Domingo, K.; Grossman, D.C.; Curry, S.J.; Davidson, K.W.; Epling, J.W.; García, F.A.R.; Gillman, M.; Harper, D.M.; Kemper, A.R.; Krist, A.H.; et al. Aspirin Use for the Primary Prevention of Cardiovascular Disease and Colorectal Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann. Intern. Med. 2016, 164, 836–845.
- Kolandaivelu, K.; Bhatt, D. Novel antiplatelet therapies. In Platelets; Michelson, A.D., Ed.; Academic Press: Amsterdam, The Netherlands, 2013; pp. 1185–1213. ISBN 9780123878373/0123878373.
- Jain, S.; Russell, S.; Ware, J. Platelet glycoprotein VI facilitates experimental lung metastasis in syngenic mouse models. J. Thromb. Haemost. 2009, 7, 1713–1717.
- Xu, M.; Ma, L.; Carrim, N.; Yougbare, I.; Li, J.; Chen, P.; Zhu, G.; Ni, H. Platelet GPIba Is Important for Thrombopoietin Production and Thrombopoietin-Induced Platelet Generation. Blood 2015, 126, 12.
- Leiter, O.; Walker, T.L. Platelets: The missing link between the blood and brain? Prog. Neurobiol. 2019, 183, 101695.
- Gaertner, F.; Ahmad, Z.; Rosenberger, G.; Fan, S.; Nicolai, L.; Busch, B.; Yavuz, G.; Luckner, M.; Ishikawa-Ankerhold, H.; Hennel, R.; et al. Migrating Platelets Are Mechano-scavengers that Collect and Bundle Bacteria. Cell 2017, 171, 1368–1382.e23.
- Hayon, Y.; Dashevsky, O.; Shai, E.; Varon, D.; Leker, R.R. Platelet lysates stimulate angiogenesis, neurogenesis and neuroprotection after stroke. Thromb. Haemost. 2013, 110, 323–330.
- Sotnikov, I.; Veremeyko, T.; Starossom, S.C.; Barteneva, N.; Weiner, H.L.; Ponomarev, E.D. Platelets recognize brain-specific glycolipid structures, respond to neurovascular damage and promote neuroinflammation. PLoS ONE 2013, 8, e58979.
- Dukhinova, M.; Kuznetsova, I.; Kopeikina, E.; Veniaminova, E.; Yung, A.W.Y.; Veremeyko, T.; Levchuk, K.; Barteneva, N.S.; Wing-Ho, K.K.; Yung, W.H.; et al. Platelets mediate protective neuroinflammation and promote neuronal plasticity at the site of neuronal injury. Brain Behav. Immun. 2018, 74, 7–27.
- Wasielewska, J.M.; Grönnert, L.; Rund, N.; Donix, L.; Rust, R.; Sykes, A.M.; Hoppe, A.; Roers, A.; Kempermann, G.; Walker, T.L. Mast cells increase adult neural precursor proliferation and differentiation but this potential is not realized in vivo under physiological conditions. Sci. Rep. 2017, 7, 17759.
- Anjayani, S.; Wirohadidjojo, Y.W.; Adam, A.M.; Suwandi, D.; Seweng, A.; Amiruddin, M.D. Sensory improvement of leprosy peripheral neuropathy in patients treated with perineural injection of platelet-rich plasma. Int. J. Dermatol. 2014, 53, 109–113.
- Chen, N.F.; Sung, C.S.; Wen, Z.H.; Chen, C.H.; Feng, C.W.; Hung, H.C.; Yang, S.N.; Tsui, K.H.; Chen, W.F. Therapeutic effect of platelet-rich plasma in rat spinal cord injuries. Front. Neurosci. 2018, 12, 252.
- Borhani-Haghighi, M.; Mohamadi, Y. The therapeutic effect of platelet-rich plasma on the experimental autoimmune encephalomyelitis mice. J. Neuroimmunol. 2019, 333, 476958.
- Hayon, Y.; Dashevsky, O.; Shai, E.; Varon, D.; Leker, R.R. Platelet microparticles promote neural stem cell proliferation, survival and differentiation. J. Mol. Neurosci. 2012, 47, 659–665.
- Gouel, F.; Do Van, B.; Chou, M.L.; Jonneaux, A.; Moreau, C.; Bordet, R.; Burnouf, T.; Devedjian, J.C.; Devos, D. The protective effect of human platelet lysate in models of neurodegenerative disease: Involvement of the Akt and MEK pathways. J. Tissue Eng. Regen. Med. 2017, 11, 3236–3240.
- Abubaker, A.A.; Vara, D.; Visconte, C.; Eggleston, I.; Torti, M.; Canobbio, I.; Pula, G. Amyloid Peptide β 1-42 Induces Integrin α IIb β 3 Activation, Platelet Adhesion, and Thrombus Formation in a NADPH Oxidase-Dependent Manner. Oxid. Med. Cell. Longev. 2019, 2019, 1050476.
- Leiter, O.; Walker, T.L. Platelets in Neurodegenerative Conditions—Friend or Foe? Front. Immunol. 2020, 11, 760.
- Inyushin, M.Y.; Sanabria, P.; Rojas, L.; Kucheryavykh, Y.; Kucheryavykh, L. A β Peptide Originated from Platelets Promises New Strategy in Anti-Alzheimer’s Drug Development. BioMed Res. Int. 2017, 2017, 3948360.




