Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rayavarapu Prasada Rao | -- | 3809 | 2023-04-04 11:03:19 | | | |
| 2 | Catherine Yang | Meta information modification | 3809 | 2023-04-04 11:10:36 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Senthilkumar, S.H.; Ramasubramanian, B.; Rao, R.P.; Chellappan, V.; Ramakrishna, S. Advancements in Electrospun Anode Materials. Encyclopedia. Available online: https://encyclopedia.pub/entry/42773 (accessed on 07 February 2026).
Senthilkumar SH, Ramasubramanian B, Rao RP, Chellappan V, Ramakrishna S. Advancements in Electrospun Anode Materials. Encyclopedia. Available at: https://encyclopedia.pub/entry/42773. Accessed February 07, 2026.
Senthilkumar, Sri Harini, Brindha Ramasubramanian, Rayavarapu Prasada Rao, Vijila Chellappan, Seeram Ramakrishna. "Advancements in Electrospun Anode Materials" Encyclopedia, https://encyclopedia.pub/entry/42773 (accessed February 07, 2026).
Senthilkumar, S.H., Ramasubramanian, B., Rao, R.P., Chellappan, V., & Ramakrishna, S. (2023, April 04). Advancements in Electrospun Anode Materials. In Encyclopedia. https://encyclopedia.pub/entry/42773
Senthilkumar, Sri Harini, et al. "Advancements in Electrospun Anode Materials." Encyclopedia. Web. 04 April, 2023.
Copy Citation
Electronic devices commonly use rechargeable Li-ion batteries due to their potency, manufacturing effectiveness, and affordability. Electrospinning technology offers nanofibers with improved mechanical strength, quick ion transport, and ease of production, which makes it an attractive alternative to traditional methods. The electrospinning technique can be used to generate nanofibers for battery separators, the electrodes with the advent of flame-resistant core-shell nanofibers. The anode is the negative electrode of the electrochemical cell. There are three mechanisms of energy storage for the anode.
electrodes
separators
crosslinked nanofibers
needle-less
1. Anode Exhibiting Energy Storage by Insertion Mechanism
Graphite is the most commercially used anode for LIB batteries; however, for more advanced applications, features, such as fast charging, are not achievable with the current graphite-based anode, and the cyclic stability and coulombic efficiency must be increased to power large electrochemical energy storage stations. The graphite materials’ inherent drawbacks are low theoretical capacity and Li dendrite formation caused by intercalation and deintercalation through the inter basal plane in anisotropic graphite microstructure. To match the energy requirement for greater energy storage systems, such as electric vehicles, the intrinsic capacity of the most used anode; graphite (372 mAh/g) should be enhanced. There is always increasing research on graphite material to improve the rate capability, specific capacity, cycle stability, and safety of graphite anodes. However, it is challenging to improve the capacity since it is feasible by creating more space between the layers of graphite [1].
In the case of the materials exhibiting the insertion mechanism, the shortcoming of theoretical capacity can be enhanced by providing additional room for the Li ions; this can be achieved by increasing the surface-to-volume of the material by including carbon-based nanomaterials such as the carbon nanofiber (CNF) [2], carbon nanotube (CNT) [3]. In addition, if the Carbon material added is hollow or porous, it will lead to more Li-ion space, hence leading to increased interaction between the electrode and the electrolyte. Recently, an anode exhibiting an insertion mechanism was synthesized using precursors, such as polyacrylonitrile and preasphaltene; the use of presashaltene is novel since it is obtained from coal liquefication residue (CLR), which is an economical and sustainable source [4]. This precursor was fabricated into a nanofiber non-woven fabric. It was noted that electrospinning of non-woven fiber is the best method to utilize the CLR. Owing to the unique structure of the PA-based CF non-woven fabrics (PACF), which has a short diffusion path, disorder, and 3D interconnected conductive structure.
Titanium oxide-based material is also worthy of anode material; TiO2 and Li4Ti5O12 are used as they have excellent working potential and can suppress the SEI formation and the growth of lithium dendrites as well [5]. The TiO2 has a great specific capacity, and the spinel oxide structure is a zero-strain material; thus, both have distinct properties which are beneficial in its way. As such, excellent cycling stability and high-rate capability have been achieved with the design of nanofiber electrodes [6][7], and full cells based on the nanofiber electrodes exhibited excellent electrochemical properties. These TiO2-based anode materials’ shortcomings on low kinetics are solved by fabricating a one-dimensional nanofiber; this designed nanofiber structure through an electrospinning process exhibits high-rate capability and cyclic stability due to the structure. Further, the electrical conductivity is improved by forming a composite with the addition of conductive materials and through doping as well [8]. Tantalum-doped TiO2 was fabricated by electrospinning [9]. Tantalum is used as the dopant because it lowers the diffusion barrier, improves electrical conductivity by shifting the conduction band, and enhances specific surface area by phase transformation. Therefore, the Ta-doped TiO2/C nanofibers exhibit good electrochemical performance when made into an anode for LIB and KIB (Potassium Ion Batteries). The specific capacity of the constructed LIB with 5% rutile Ta doping was measured to be 399 mAh g−1 at 2 A g−1.
2. Anode Exhibiting Energy Storage by Conversion Mechanism
To further improve the capacity, anode material with greater storage capacity is explored, such as silicon, iron oxide, tin peroxide, and cupric oxide. Transition metal oxides possess properties, such as high specific capacity, inexpensiveness, and non-toxicity, which make them a potential candidate for the anode in LIB. These anodes were initially fabricated via the solvothermal and hydrothermal methods techniques forming a 3-D structure, such as core-shell nanospheres, which possess good thermodynamic stability, but pulverization of the active material takes place during the lithiation and de-lithiation cycles, in order to overcome the shortcoming the recent advancement focuses on fabricating a 1-Dimensional structure (Nanofiber, Nanorod, Nano Tubes); thus, the diffusion length is shorter, and movement of Li ions and electrons occurs faster. A quasi-anisotropic nano octahedron as well as nanofiber with nickel–cobalt–manganese oxide composite (NCM), which contains two ternary phases, was fabricated by Ling et al. [10]. To obtain the octahedron structure, the conductivity of the precursor polymer solution is altered. The added Ni helps in the formation of crystalline structure, and the specific capacity increases proportionally with the increase in the Ni Content. The methods and process parameters to fabricate the nanofiber and the nano octahedral structure are similar; the precursor solution is also the same, but in the case of nano octahedral structure, the metal salt precursors have smaller ion sizes than those of the acetate molecules, which is preferred so that the conductivity is enhanced favoring the octahedral structure formation. The nano-octahedron possesses better properties when compared to the nanofiber due to the synergistic effect.
A 1-Dimensional Transitional metal oxide along with a spinel phase was synthesized by Wang et al. [11], who fabricated MnCo2O4 (theoretical capacity 906 mAh g−1) hollow nanotubes where all the nanoparticles were well connected. This structure was responsible for the outstanding property of the material as it gave several active sites accelerating the rate of transportation and breakage of active material is mitigated. Since the fabrication technique is not tedious, this material finds application as an anode in LIB [12][13][14][15]. For high-performance lithium storage devices, ternary oxides are recommended as it has several electrochemically active components and greater theoretical specific capacity. When the ternary nickel cobaltite was considered, the capacity faded due to pulverization; thus, carbon materials are utilized as it resists the volume expansion and increases conductivity as well. By electrospinning followed by annealing, novel porous NiCoO2 nanofibers were fabricated by Wang et al. [16], and the rate of diffusion was fast as the lithium storage sites remained to expand after a continuous cyclic lithiation and delithiation cycle. Thereby the self-assembled nanoparticle exhibited superior electrochemical performance providing an excellent reversible lithium storage capacity (discharge capacity) [17].
3. Anode Exhibiting Energy Storage by Alloying Mechanism
High energy density battery is achieved by materials storing energy by alloying reaction of energy storage. The Li–Sn alloying exhibits good electrochemical performance; thus, Sn was incorporated into carbon materials and studied. However, the pulverization limits the application; thereby, voids were introduced in the electrospun fibers by fabricating coaxial bamboo-like composite of Sn@C nanoparticles in hollow CNF [18], a porous multi-channeled fiber with Sn nanoparticles reinforced by using single nozzle electrospinning, where PMMA poly(methyl methacrylate) aids the pore formation [19]. In another procedure, Poly Styrene was used to generate voids in the porous CNF incorporated with rattle-like tin nanoparticles [20]. Thus, through the electrospinning process, void engineering is also feasible, thereby providing beneficial electrochemical properties.
The most researched material exhibiting alloying-type energy storage is silicon since it can be used commercially, as it has an excellent specific capacity of (4212 mAh/g) and is economical as well. However, it suffers a volume expansion of 400% during the lithiation and delithiation process, leading to the pulverization of the active material [21]. Thereby improvements are included in the electrospinning process; the pulverization can be tackled by using (i) Si of nano dimension since the breakage is not expected to occur as the size of Si is already small, (ii) providing voids that can accommodate the volume change by using porous or hollow structures, (iii) creation of void spaces which can accommodate the volume change by carbon coating [22][23]. The carbon coating will be able to enhance the conductivity and block the direct contact of the electrolyte with the electrode. The schematic of techniques to overcome pulverization is shown in Figure 1. The oxide of Silicon also can be used to tackle the volume expansion, and it can maintain long-term stability because, during the alloying reaction, Li2O and Li4SiO4 from the SiO2 phase are formed [24], which reduces the electrical conductivity and mobility of the Li-ion leading to loss of irreversible capacity as well. When the mass ratio of the pore-forming element in the precursor solution is altered, the pore structure present in the electro-spun nanofiber can be optimized; thus, Si nanoparticles embedded porous CNF composite nanofiber was obtained, which exhibited superior performance owing to the porous structure. In a work by Tian et al. [25], they optimized the pore-forming with Polyethylene glycol (PEG) since it influences the pore formation. The cycling and rate capacities have significantly improved due to the formation of porous structures.
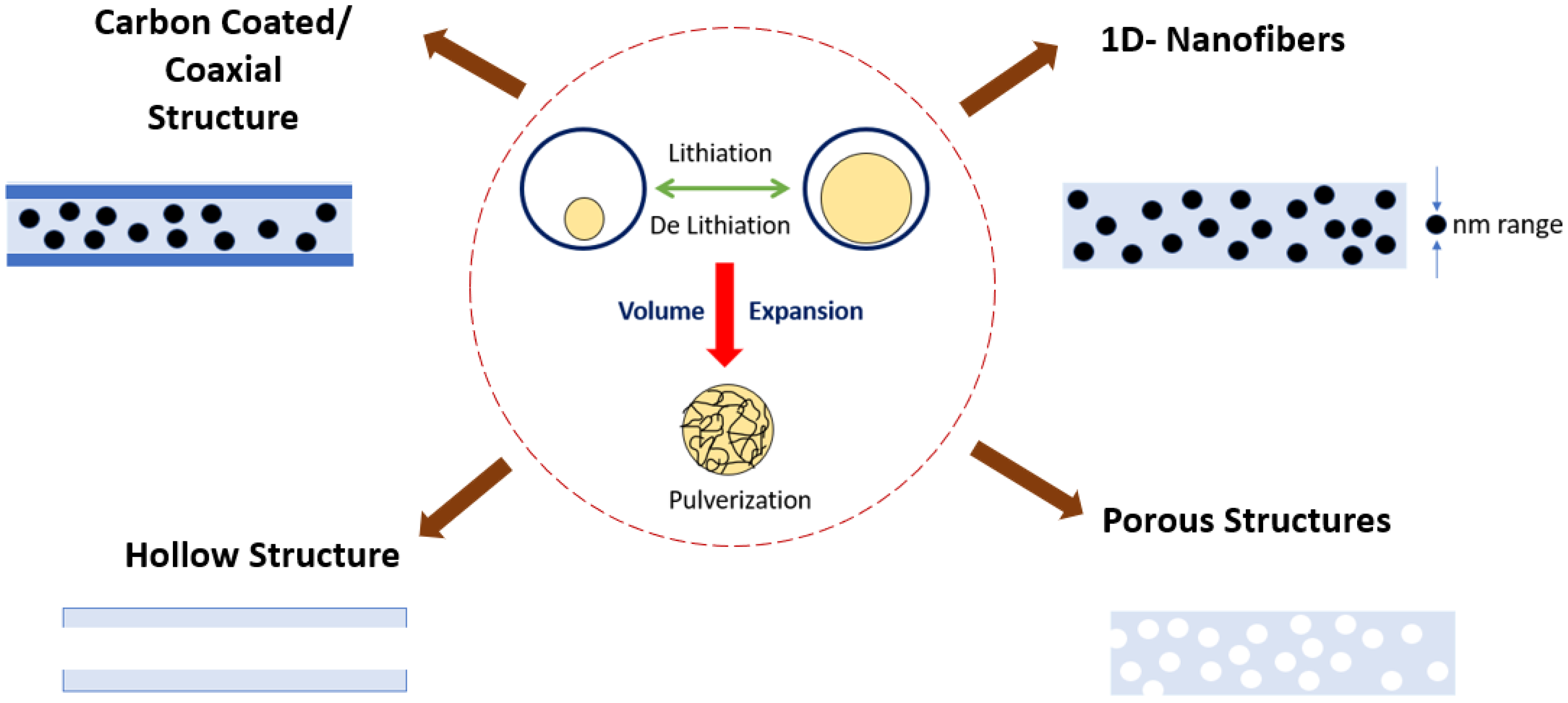
Figure 1. Strategies to overcome the pulverization of active material.
Other anode materials, such as phosphorous and germanium, exhibit phenomenal theoretical capacities of 2595 mAh/g and 1626 mAh/g for Li3P [26] and Li4.4Ge [27] phases, respectively. Phosphides can store energy by a dual electrochemical process when combined with metals, such as Sn, Sb, Bi, and Pb, out of which tin phosphides exhibit a greater capacity; thus, by combining electrospinning and solid-state synthesis, nano metal phosphides (SnP0.94) were combined with CNF to form a composite anode material [28]. Phosphorous has gained attention nowadays; apart from its high lithium storage capacity, it is available abundantly and can be recycled easily. There are two allotropes of Phosphorus, namely, black and red. Red is utilized as an anode instead of black because processing red needs a higher temperature.
4. Advanced Electro-Spun Anode Material
4.1. Composite
These materials with conversion-type storage mechanisms suffer greater volume change; thereby, the active anode material is encapsulated in a carbon-based structure forming composites. These composites are of greater interest as they have properties between the materials exhibiting the insertion and intercalation storage mechanism, as shown in Figure 2. These composites are fabricated via the electrospinning technique, and various structures can be achieved, which is advantageous for the performance as anode material. More active sites are generated because of doping using heteroatoms, such as B, S, and N, which enhance the capacity of lithium storage. Defects that cause the carbon structure to become disordered are incorporated, which makes this easier. Carbon’s electrical structure is altered by doping, which increases the number of electrochemically active sites that may be used to store lithium. Heteroatoms in CNF cause an expansion of interlayer distance, generating additional active sites and improving electrical conductivity.
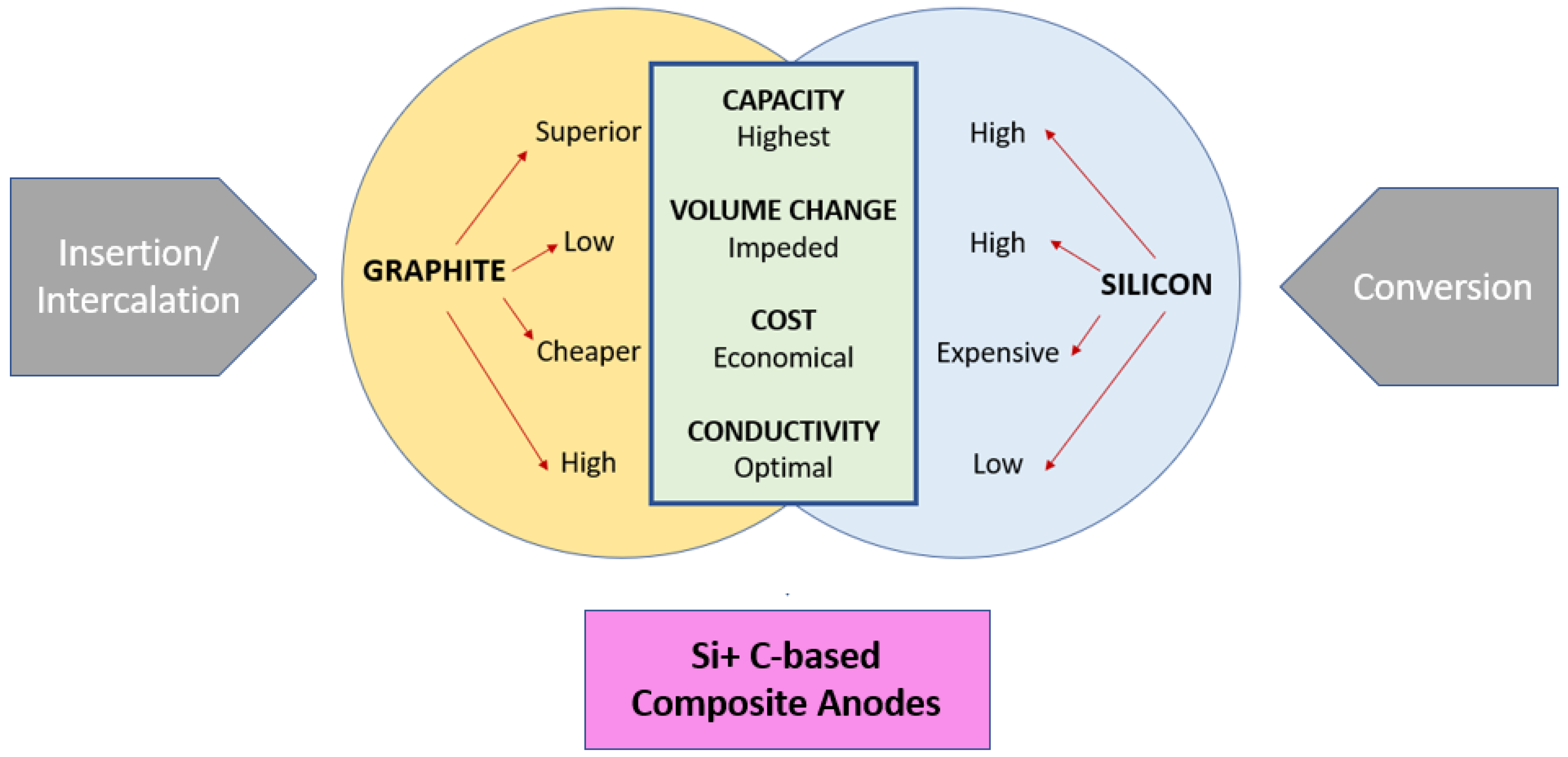
Figure 2. Composite energy storage mechanism.
To demonstrate the utilization of transition metals as an anode, to circumvent the low columbic efficiency and reversible capacity hindrance challenges presented by the N-doped carbon fiber alone, the author developed a composite nanofiber of TiO2@C/N [29], using a flexible film consisting of TiO2 nanoparticles [30] that were incorporated in nitrogen-doped carbon nanofiber. Even without the addition of a conductive binder or other chemicals, the nanofiber developed serves as the anode of the battery. Both the fiber’s performance in the electrochemical system and the nanofibers’ favorable shape for the purpose of electron conductivity have been confirmed. The performance of the charge cycle and the discharge were consistently attributed to the substantial lithium storage capacity of the electro-spun composite. The fibers also have a good rating capacity, and the electrical impedance also diminishes as the number of cycles climbs [31][32][33].
Electrospinning and electro-spraying were combined to develop a composite made of carbon nanofibers, along with carbon-coated SnO2 clustering, and SnO2 clusters adhered over the CNF [34] to achieve a stable hybrid structure that boosts electron transfer and ionic diffusion. Lithium transport was, therefore, seen to be enhanced when SnO2 was coated outside, which boosted battery efficiency [35][36][37]. A pseudo capacitor behavior is brought in the LIB by the synergistic effect when SnO2 (high theoretical capacity) and ZnO (high co-efficient of diffusion of Li ion) composite is coated with Sn and N-doped CNF hybrid material. In the study by L. Shang et al. [38], the mass ratio of SnO2 and ZnO precursor varied as 1:1, 3:1, and 1:3, along with the reference where the single precursor alone was only utilized. Among the different mass ratios, the material synthesized by equal proportions of both the precursor [Sn (Ac)4 for SnO2 and Zn (Ac)2 for ZnO] exhibited a comprehensive performance as LIB anode material.
The Li storage performance is attributed to the structure of the fiber, which also generates an efficient pathway of transport for all electrolyte penetration, Li ions, and electrons as well. When nanoparticles of FeCo were added to Nitrogen-doped CNF due to a synergistic effect, outstanding electrochemical properties were obtained [39]. A reversible capacity of 566.5 mAh g−1 was recorded at the end of 100 cycles at a current density of 100 mA g−1. These fibers could be used to produce batteries without the addition of any conducting or binding materials, and when evenly scattered FeCo nanoparticles restrict volume change and increase structural stability throughout charging and discharging cycles. The addition of FeCo increases the composite material’s Columbic efficiency while simultaneously acting as a catalyst for Li2O breakdown [40][41].
With the help of electrospinning and heat treatment, SnO2/TiO2 ultrafine particles with a molar ratio of 1.5:1 have been combined with carbon nanofiber. Here, SnO2 is used since it has a higher theoretical capacity and is easily obtainable [42], and TiO2 is used as it also provides greater electrochemical stability, and the significant volume change that occurs during the reaction is shown to be diminished to a greater extent, improving overall performance.
LIB is also used in wearable electronic devices; thus, it requires a flexible electrode that does not damage the active material present when it is bent; thereby flexible electrodes which do not compromise the electrochemical performance are areas of increasing demand; thus, a porous Sb2S3/TiO2/C nanofiber membrane was fabricated using a titanate coupling agent (titanium (IV) isopropoxide (TTIP)) as a precursor as it has the capacity to entangle with the polymer and, thus, bridging between the polymer chose and transition metal ion, providing an improved thermotolerant mechanical property [43]. Antimony sulfide (Sb2S3) was considered a potential material for the anode due to its low cost, higher theoretical capacity, and environmental friendliness. Using electrospinning and hydrothermal reaction to create a porous nanofiber membrane that was employed as a free-standing anode and has good cyclic stability and better capacity as well, the porous structure forbids a greater volume expansion. The fourfold folding of the fiber did not cause it to wrinkle, demonstrating mechanical flexibility [44][45][46].
In addition to the generally utilized methods, such as the restriction of particle size and dispersion of the active material in a conductive matrix, L. Jiao demonstrated the benefit of the inclusion of an inactive component that can attenuate the volume change. In his research, he employed Sn as the active substance and Co as the inactive substance, and nitrogen, which were doped in CNF. Co was chosen among the available inactive materials, such as Mn, Fe, Cu, Co, and Ni, to help maintain the electrode’s integrity during lithiation and de-lithiation. This metal also raises the usage efficiency of Li ions, given that it is immobile and does not consume Li. Additionally, the synergistic combination of N-doped CNF with the Co metal accelerates the rate of ionic diffusion and, as a result, the electron transport since it is electrically conductive. The energy density of the LIB is boosted since neither a binder nor a current collector is involved. The manufactured fiber with a diameter of 100 nm functioned well.
Metal and metal oxide are possible options to fulfill the growing need for LIB; nevertheless, even after frequent cycles of lithiation and delithiation, these materials also fracture. J. Li et al. reported on the process of manufacturing a free-standing and flexible membrane made of Sn@C [47] nanofiber because the carbon matrix is one-dimensional and contains Sn nanoparticles that are enclosed inside the structure. This electro-spun structure has an excessive current density of 10 A g−1 and a capacity of 668 mAh g−1 at 1 A g−1. The confinement brings such extraordinary qualities. The energy density is enhanced by omitting binders or collectors, which may be made without the need for additional slurry coating. The carbon coating can absorb a sizable volume change without causing the active material to be destroyed, which would otherwise result in fracture during the lithiation and delithiation cycle. As a result, it provides the potential for LIB industry growth in the anode [47][48].
A nanostructured carbon-based anode material with red phosphorous was fabricated by Liberale et al. [49]. This structure helps to overcome the limitation of phosphorous (high volume expansion, low electronic conductivity) as the C/P bonds allow to shorten Li+ diffusion path and stabilize P during cycling [50][51]. The amorphous composite fabricated by electrospinning the CNF followed by drop casting the phosphorus to obtain uniform dispersion possessed excellent electrochemical properties as well [52][53][54].
4.2. MOF Derived
To overcome the volume change, a new material was introduced, namely, MOF-derived metal oxides [55][56]. These materials have the capacity to adjust to the volume change; thus, it overcomes the existing issue faced by all materials discussed earlier [57][58]. Apart from volume change, the MOF–derived material has more structural stability and improved electrochemical performance as this porous metal oxide decreases the diffusion pathway and provides more active sites. The distinctive structure and morphology contribute to the outstanding electrochemical performance because the MOF precursor, during the peroxidation and pyrolysis process, forms more Li-ion reservoirs as nitrogen-doped or carbon-coated metal oxide is generated during the fabrication process.
Z. Li et al. fabricated Fe2O3@ Polyacrylonitrile (PAN) and ZnO@PAN [59] composite nanofibers using an electrospinning technique followed by subsequent pyrolysis of the precursor film, they exhibited a specific capacity of 1571.4 and 1053.8 mAh g−1 at 50 mA g−1, respectively, and the reversible capacity of the respective composites after 500 cycles at 1000 mA g−1 were 506.6 and 455.4 mAh g−1, which is primarily due to the interconnection of the CNF with the metal oxides [60]. Carbon nanofibers embedded with cobalt were fabricated by Y. Liang et al. [61] via pyrolysis of electro-spun fiber in a nitrogen atmosphere. This spindle-shaped nanofiber formed after mild oxidation exhibited superior properties due to the synergistic effect between the carbon shells and the Co present. This Co3O4@CNFs anode discharge specific capacity of 1404 mAh g−1 and 500 mAh g−1 after 100 cycles and 100 mA g−1 current density and after 500 cycles at 2000 mA g−1.
Although the 2D nanofiber sheets do not share the mechanical and thermal conductivity shortcomings of the electro-spun 1D nanofibers, they certainly possess their own downsides. As a result, a hybrid structure, as shown in Figure 3, with both 1D and 2D nanomaterials, was created, and the synergistic engineering was able to offer remarkable electrochemical capabilities. Electrospinning may be used gradually to create a hybrid structure, offering a new development path. The hybrid structure is also made by techniques such as physical blending or in situ growth [62]. A consolidated summary of the advanced electrospun anode materials is provided in Table 1.
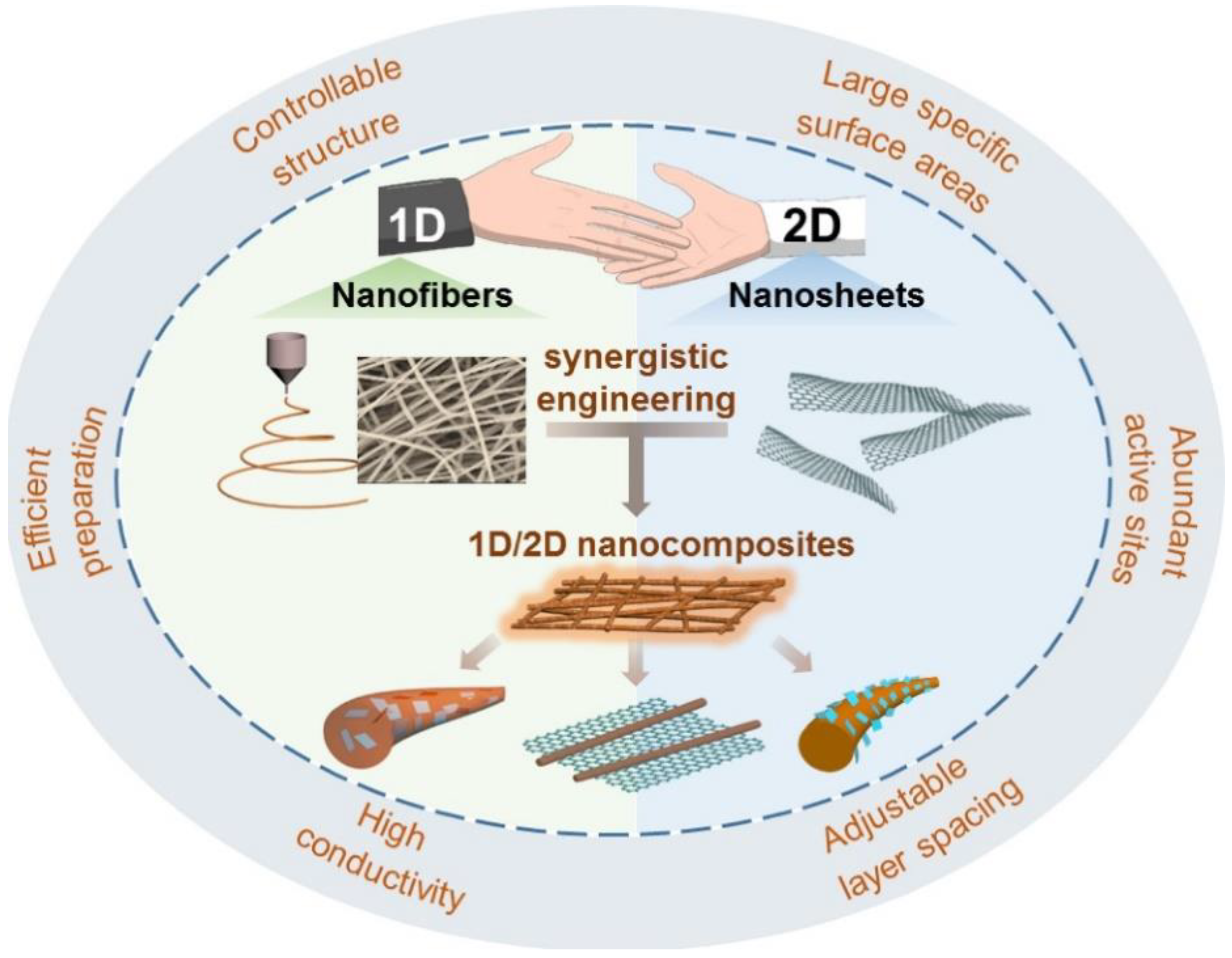
Figure 2. Hybrid Nanocomposites exhibiting outstanding properties brought by Synergistic engineering (reused with copyrights from Ref. [62] 2020, Elsevier).
Table 1. Electrochemical properties of advanced anode material.
| Material | Specific Capacity | Nanofiber Diameter (nm) | Coulombic Efficiency | Discharge Capacity | Reference |
|---|---|---|---|---|---|
| TiO2@C/N composite NF | 388 mAh g−1 after 400 cycles. | 800 | 563 mAh g−1 at 0.1 A g−1 | [29] | |
| Carbon-coated SnO2@carbon nanofibers | 500 mAh g−1 after 50 cycles | 400 | 63.24%. | 1425 mAh g−1 at 100 mA g−1 | [34] |
| Sn particle-coated composite of SnOx/ZnO and N-doped CNF | 1131 mAh/g | 350 | 73.2% at 0.5 A/g | 588.7 mAh/g after 100 cycles at 0.5 A/g (Reversible capacity) |
[38] |
| Nitrogen-doped carbon coated MnO nano peapods | 775.4 mAh g−1 at 100 mA g−1 | 125 | rate capacity of 559.7 mAh g−1 at 1000 mA g−1 s | [63] | |
| FeCo nanoparticles encapsulated in N-doped carbon nanofibers | 566.5 mAh g−1 at 100 mA hg−1 after 100 cycles. (Reversible Capacity) |
130 | 99.6% | [39] | |
| Ultra-fine SnO2/TiO2 particles into carbon nanofibers | 766.1 mAh/g. | 600 | 71%, and after 200 cycles | 1494.8 mAh/g Charge-1061.2 mAh/g, |
[42] |
| Sb2S3/TiO2/C nanofiber | 261.6 mAh g−1, 100 cycles, 50 mA g−1. | ||||
| Co/Co3SnC0.7@N-CNFs | 320 mAh g−1 at 500 mA g−1 even after 900 cycles. | ||||
| Red Phosphorous decorated carbon anode. | [49] | ||||
| MOF derived | |||||
| Fe2O3@ Polyacrylonitrile (PAN) | 1571.4 mAh g−1 | [59] | |||
| ZnO@PAN composite nanofibers | 1053.8 mAh g−1 | ||||
| Co3O4@CNFs | 1404 mAh g−1 (100 cycles and 100 mA g−1) | 100 | [61] | ||
| Bio-nitrogen (N)-doped composite carbon nanocomposites mat using chitosan and natural cellulose | 327 mAh g−1 at 100 mA g−1 | 316 | [64] | ||
4.3. Eco-Friendly, Bio-Derived Nanofibers
The future aims to make this electrospinning a much more sustainable process, which can be achieved by the following: (i) making the electrospinning process environmentally friendly by eliminating the possibility of any hazards, such as adopting needless electrospinning techniques, optimizing the process by making it energy-efficient by powering the equipment with rechargeable batteries, which can also be reused to harness its secondary lifetime; (ii) using precursors, which can be replaced with alternative nontoxic chemicals, precursors derived from biomass. The carbon, which is used widely for the electrode, can be derived from bio sources [65][66], such as coffee ground waste [67], shrimp waste [68], marine chitin [69][70], and cellulose [69][71][72].
The electrode is a crucial part that significantly affects the LIB’s overall performance. Conventional graphite electrodes contain volatile N-methyl-pyrrolidone (NMP), which is employed as a solvent, and poisonous polyvinylidene fluoride (PVDF), which serves as a binder. As a result, K. Xu et al. created a unique energy storage technique employing an affordable, eco-friendly carbon electrode material that also has high electrochemical properties. They developed a bio-nitrogen (N)-doped composite carbon nanocomposite mat using chitosan and natural cellulose [73]. The chitosan-based mat was first manufactured via the pyrolysis method, but because of its poor porosity and resolvability, additional fabrication techniques, such as hydrothermal carbonization and direct carbonization, were also employed. Later, with cellulose and chitosan mass ratios of 10:0, 7:3, 5:5, 3:7, and 0:10, the electrospinning technique was used in the single and coaxial nozzle to prepare a porous carbon with good specific surface area and interconnectivity. Better performance was recorded in a mass ratio of 5:5, which exhibited a high specific capacity of 327 mAh g−1 at 100 mA g−1, good rate performance with a specific capacity of 399 mAh g−1 at 30 mA g−1, and 210 mA g−1 at 1000 mA g−1; the stability after 300 cycles was also commendable [64][69][74].
The electrospinning process can be made more energy-efficient by adopting the following strategies: (i) use of direct-current electrospinning instead of traditional high voltage electrospinning as a comparatively lesser voltage is used making the process energy efficient [75][76]; (ii) powering the electrospinning equipment using renewable energy resources, such as solar panels and wind turbines, which can reduce the carbon footprint of the electrospinning process [77][78]; (iii) usage of renewable or biodegradable materials to prepare the fibers as these materials can be broken down in the environment, reducing the amount of energy needed for disposal [79][80]; (iv) to achieve appropriate end-of-life disposal of the electrospun fibers by recycling or composting the fibers as it can reduce the amount of energy required for disposal; (v) reusing the syringe after flushing the polymer melt left after processing using the appropriate solvent [81]; (vi) optimize the process by reducing the waste generated or recycling excess material that is not used in the fiber-formation process [82].
References
- Zhang, H.; Yang, Y.; Ren, D.; Wang, L.; He, X. Graphite as Anode Materials: Fundamental Mechanism, Recent Progress and Advances. Energy Storage Mater. 2021, 36, 147–170.
- Kim, C.; Yang, K.S.; Kojima, M.; Yoshida, K.; Kim, Y.J.; Kim, Y.A.; Endo, M. Fabrication of Electrospinning-Derived Carbon Nanofiber Webs for the Anode Material of Lithium-Ion Secondary Batteries. Adv. Funct. Mater. 2006, 16, 2393–2397.
- Li, W.; Li, M.; Wang, M.; Zeng, L.; Yu, Y. Electrospinning with Partially Carbonization in Air: Highly Porous Carbon Nanofibers Optimized for High-Performance Flexible Lithium-Ion Batteries. Nano Energy 2015, 13, 693–701.
- Li, X.; Sun, N.; Tian, X.; Yang, T.; Song, Y.; Xu, B.; Liu, Z. Electrospun Coal Liquefaction Residues/Polyacrylonitrile Composite Carbon Nanofiber Nonwoven Fabrics as High-Performance Electrodes for Lithium/Potassium Batteries. Energy Fuels 2020, 34, 2445–2451.
- Liu, J.; Song, K.; van Aken, P.A.; Maier, J.; Yu, Y. Self-Supported Li4Ti5O12-C Nanotube Arrays as High-Rate and Long-Life Anode Materials for Flexible Li-Ion Batteries. Nano Lett. 2014, 14, 2597–2603.
- Chen, Y.; Lu, Z.; Zhou, L.; Mai, Y.W.; Huang, H. In Situ Formation of Hollow Graphitic Carbon Nanospheres in Electrospun Amorphous Carbon Nanofibers for High-Performance Li-Based Batteries. Nanoscale 2012, 4, 6800–6805.
- Chen, Y.; Li, X.; Zhou, X.; Yao, H.; Huang, H.; Mai, Y.W.; Zhou, L. Hollow-Tunneled Graphitic Carbon Nanofibers through Ni-Diffusion-Induced Graphitization as High-Performance Anode Materials. Energy Environ. Sci. 2014, 7, 2689–2696.
- Lee, B.S. A Review of Recent Advancements in Electrospunanode Materials to Improve Rechargeable Lithium Battery Performance. Polymers 2020, 12, 2035.
- Su, D.; Liu, L.; Liu, Z.; Dai, J.; Wen, J.; Yang, M.; Jamil, S.; Deng, H.; Cao, G.; Wang, X. Electrospun Ta-Doped TiO2/C Nanofibers as a High-Capacity and Long-Cycling Anode Material for Li-Ion and K-Ion Batteries. J. Mater. Chem. A Mater. 2020, 8, 20666–20676.
- Ling, J.; Karuppiah, C.; Das, S.; Singh, V.K.; Misnon, I.I.; Ab Rahim, M.H.; Peng, S.; Yang, C.C.; Jose, R. Quasi-Anisotropic Benefits in Electrospun Nickel–Cobalt–Manganese Oxide Nano-Octahedron as Anode for Lithium-Ion Batteries. New J. Chem. 2022, 46, 9799–9810.
- Zhu, L.; Li, F.; Yao, T.; Liu, T.; Wang, J.; Li, Y.; Lu, H.; Qian, R.; Liu, Y.; Wang, H. Electrospun MnCo2O4Nanotubes as High-Performance Anode Materials for Lithium-Ion Batteries. Energy Fuels 2020, 34, 11574–11580.
- Kotalgi, K.; Kanojiya, A.; Tisekar, A.; Salame, P.H. Electronic Transport and Electrochemical Performance of MnCo2O4 Synthesized Using the Microwave-Assisted Sonochemical Method for Potential Supercapacitor Application. Chem. Phys. Lett. 2022, 800, 139660.
- Cheng, C. Hierarchical MnCo2O4 Micro/Nano Fibres as a High-Performance Anode of Lithium-Ion Battery. Mater. Lett. 2022, 324, 132770.
- Liu, Y.; Du, X.; Li, Y.; Bao, E.; Ren, X.; Chen, H.; Tian, X.; Xu, C. Nanosheet-Assembled Porous MnCo2O4.5 Microflowers as Electrode Material for Hybrid Supercapacitors and Lithium-Ion Batteries. J. Colloid Interface Sci. 2022, 627, 815–826.
- Gonçalves, J.M.; Silva, M.N.T.; Naik, K.K.; Martins, P.R.; Rocha, D.P.; Nossol, E.; Munoz, R.A.A.; Angnes, L.; Rout, C.S. Multifunctional Spinel MnCo2O4based Materials for Energy Storage and Conversion: A Review on Emerging Trends, Recent Developments and Future Perspectives. J. Mater. Chem. A Mater. 2021, 9, 3095–3124.
- Wang, J.; Xie, S.; Li, L.; Li, Z.; Asiri, A.M.; Marwani, H.M.; Han, X.; Wang, H. Electrospinning Synthesis of Porous NiCoO2 Nanofibers as High-Performance Anode for Lithium-Ion Batteries. Part. Part. Syst. Charact. 2019, 36, 1900109.
- Li, L.; Peng, S.; Lee, J.K.Y.; Ji, D.; Srinivasan, M.; Ramakrishna, S. Electrospun Hollow Nanofibers for Advanced Secondary Batteries. Nano Energy 2017, 39, 111–139.
- Yu, Y.; Gu, L.; Wang, C.; Dhanabalan, A.; van Aken, P.A.; Maier, J. Encapsulation of Nanoparticles in Bamboo-like Hollow Carbon Nanofibers as an Anode Material in Lithium-Based Batteries. Angew. Chem. Int. Ed. Engl. 2009, 48, 6485–6489.
- Yu, Y.; Gu, L.; Zhu, C.; van Aken, P.A.; Maier, J. Tin Nanoparticles Encapsulated in Porous Multichannel Carbon Microtubes: Preparation by Single-Nozzle Electrospinning and Application as Anode Material for High-Performance Li-Based Batteries. J. Am. Chem. Soc. 2009, 131, 15984–15985.
- Lee, J.H.; Oh, S.H.; Jeong, S.Y.; Kang, Y.C.; Cho, J.S. Rattle-Type Porous Sn/C Composite Fibers with Uniformly Distributed Nanovoids Containing Metallic Sn Nanoparticles for High-Performance Anode Materials in Lithium-Ion Batteries. Nanoscale 2018, 10, 21483–21491.
- Kim, J.; Kim, C.; Jang, I.; Park, J.; Kim, J.; Paik, U.; Song, T. Si Nanoparticles Embedded in Carbon Nanofiber Sheathed with Li6PS5Cl as an Anode Material for All-Solid-State Batteries. J. Power Sources 2021, 510, 230425.
- Li, Y.; Xu, G.; Yao, Y.; Xue, L.; Yanilmaz, M.; Lee, H.; Zhang, X. Coaxial Electrospun Si/C–C Core–Shell Composite Nanofibers as Binder-Free Anodes for Lithium-Ion Batteries. Solid State Ion 2014, 258, 67–73.
- Lu, W.; Guo, X.; Luo, Y.; Li, Q.; Zhu, R.; Pang, H. Core-Shell Materials for Advanced Batteries. Chem. Eng. J. 2019, 355, 208–237.
- Sivonxay, E.; Aykol, M.; Persson, K.A. The Lithiation Process and Li Diffusion in Amorphous SiO2 and Si from First-Principles. Electrochim. Acta 2020, 331, 135344.
- Tian, X.; Xu, Q.; Cheng, L.; Meng, L.; Zhang, H.; Jia, X.; Bai, S.; Qin, Y. Enhancing the Performance of a Self-Standing Si/PCNF Anode by Optimizing the Porous Structure. ACS Appl. Mater. Interfaces 2020, 12, 27219–27225.
- Zhi, H.; Yu, X. Recent Progress in Phosphorus Based Anode Materials for Lithium/Sodium Ion Batteries. Energy Storage Mater. 2019, 16, 290–322.
- Mo, R.; Rooney, D.; Sun, K. Yolk-Shell Architecture with Precision Expansion Void Control for Lithium Ion Batteries. iScience 2018, 9, 521–531.
- Yadav, P.; Yadav, P.; Yadav, P.; Malik, W.; Dwivedi, P.K.; Dwivedi, P.K.; Jones, L.A.; Shelke, M.V.; Shelke, M.V. Electrospun Nanofibers of Tin Phosphide (SnP0.94) Nanoparticles Encapsulated in a Carbon Matrix: A Tunable Conversion-Cum-Alloying Lithium Storage Anode. Energy Fuels 2020, 34, 7648–7657.
- Hu, J.; Wang, H.; Qin, C.; Li, Y.; Yang, Y. Fabrication of TiO2@C/N Composite Nanofibers and Application as Stable Lithium-Ion Battery Anode. Mater. Lett. 2020, 279, 128491.
- Paul, S.; Rahman, M.A.; Sharif, S.B.; Kim, J.H.; Siddiqui, S.E.T.; Hossain, M.A.M. TiO2 as an Anode of High-Performance Lithium-Ion Batteries: A Comprehensive Review towards Practical Application. Nanomaterials 2022, 12, 2034.
- Shen, J.; Hu, W.; Li, Y.; Li, L.; Lv, X.J.; Zhang, L. Fabrication of Free-Standing N-Doped Carbon/TiO2hierarchical Nanofiber Films and Their Application in Lithium and Sodium Storages. J. Alloys Compd. 2017, 701, 372–379.
- Ren, S.; Tang, L.; Sun, Q.; Li, Z.; Yang, H.; Zhao, J. The Structure, Oxygen Vacancies and Magnetic Properties of TiOx (0 < x < 2) Synthesized by Plasma Assisted Chemical Vapor Deposition and Reduction. Mater. Lett. 2018, 228, 212–215.
- Seok, S.; Choi, M.; Lee, Y.; Jang, D.; Shin, Y.; Kim, Y.H.; Jo, C.; Park, S. Ni Nanoparticles on Ni Core/N-Doped Carbon Shell Heterostructures for Electrocatalytic Oxygen Evolution. ACS Appl. Nano Mater. 2021, 4, 9418–9429.
- Wang, W.; Liang, Y.; Kang, Y.; Liu, L.; Xu, Z.; Tian, X.; Mai, W.; Fu, H.; Lv, H.; Teng, K.; et al. Carbon-Coated SnO2@carbon Nanofibers Produced by Electrospinning-Electrospraying Method for Anode Materials of Lithium-Ion Batteries. Mater. Chem. Phys. 2019, 223, 762–770.
- Jang, B.; Koo, J.; Choi, S.; Kim, J. Formation of SiOx Shell on Si Nanoparticles and Its Effects on Electrochemical Properties as a Li-Ion Battery’s Anode. Mater. Chem. Phys. 2018, 215, 11–19.
- Zolin, L.; Nair, J.R.; Beneventi, D.; Bella, F.; Destro, M.; Jagdale, P.; Cannavaro, I.; Tagliaferro, A.; Chaussy, D.; Geobaldo, F.; et al. A Simple Route toward Next-Gen Green Energy Storage Concept by Nanofibres-Based Self-Supporting Electrodes and a Solid Polymeric Design. Carbon 2016, 107, 811–822.
- Aravindan, V.; Sundaramurthy, J.; Kumar, E.N.; Kumar, P.S.; Ling, W.C.; von Hagen, R.; Mathur, S.; Ramakrishna, S.; Madhavi, S. Does Carbon Coating Really Improves the Electrochemical Performance of Electrospun SnO2 Anodes? Electrochim. Acta 2014, 121, 109–115.
- Ao, L.; Wu, C.; Xu, Y.; Wang, X.; Jiang, K.; Shang, L.; Li, Y.; Zhang, J.; Hu, Z.; Chu, J. A Novel Sn Particles Coated Composite of SnOx/ZnO and N-Doped Carbon Nanofibers as High-Capacity and Cycle-Stable Anode for Lithium-Ion Batteries. J. Alloys Compd. 2020, 819, 153036.
- Li, X.; Xiang, J.; Zhang, X.; Li, H.; Yang, J.; Zhang, Y.; Zhang, K.; Chu, Y. Electrospun FeCo Nanoparticles Encapsulated in N-Doped Carbon Nanofibers as Self-Supporting Flexible Anodes for Lithium-Ion Batteries. J. Alloys Compd. 2021, 873, 159703.
- Luo, Y.; Sun, L.; Xu, F.; Wei, S.; Wang, Q.; Peng, H.; Chen, C. Cobalt(II) Coordination Polymers as Anodes for Lithium-Ion Batteries with Enhanced Capacity and Cycling Stability. J. Mater. Sci. Technol. 2018, 34, 1412–1418.
- Kwon, T.G.; Park, H.; Jo, O.H.; Chun, J.; Kang, B.G. Facile Preparation of Magnetite-Incorporated Polyacrylonitrile-Derived Carbons for Li-Ion Battery Anodes. ACS Appl. Energy Mater. 2022, 5, 1262–1270.
- Mou, H.; Chen, S.; Xiao, W.; Miao, C.; Li, R.; Xu, G.; Xin, Y.; Nie, S. Encapsulating Homogenous Ultra-Fine SnO2/TiO2 Particles into Carbon Nanofibers through Electrospinning as High-Performance Anodes for Lithium-Ion Batteries. Ceram. Int. 2021, 47, 19945–19954.
- Xia, J.; Zhang, X.; Yang, Y.; Wang, X.; Yao, J. Electrospinning Fabrication of Flexible, Foldable, and Twistable Sb2S3/TiO2/C Nanofiber Anode for Lithium Ion Batteries. Chem. Eng. J. 2021, 413, 127400.
- Huang, Z.; Gao, H.; Yang, Z.; Jiang, W.; Wang, Q.; Wang, S.; Ju, J.; Kwon, Y.U.; Zhao, Y. Improved Capacity and Cycling Stability of SnO2 Nanoanode Induced by Amorphization during Cycling for Lithium Ion Batteries. Mater. Des. 2019, 180, 107973.
- Tan, F.; Guo, H.; Wang, Z.; Niu, X.; Li, X.; Yan, G.; Wang, J.; Peng, W.; Hu, Q. Electrospinning-Enabled SiOx@TiO2/C Fibers as Anode Materials for Lithium-Ion Batteries. J. Alloys Compd. 2021, 888, 161635.
- Wen, L.; Li, F.; Cheng, H.M. Carbon Nanotubes and Graphene for Flexible Electrochemical Energy Storage: From Materials to Devices. Adv. Mater. 2016, 28, 4306–4337.
- Li, J.; Zou, P.; Wang, R.; Yang, C. Electrospinning Nanofibers for High-Performance Flexible Lithium Ion Battery Anodes. IOP Conf. Ser. Earth Environ. Sci. 2019, 300, 042021.
- Kim, I.S.; Blomgren, G.E.; Kumta, P.N. Sn/C Composite Anodes for Li-Ion Batteries. Electrochem. Solid-State Lett. 2004, 7, A44.
- Liberale, F.; Fiore, M.; Ruffo, R.; Bernasconi, R.; Shiratori, S.; Magagnin, L. Red Phosphorus Decorated Electrospun Carbon Anodes for High Efficiency Lithium Ion Batteries. Sci. Rep. 2020, 10, 13233.
- Jin, H.; Huang, Y.; Wang, C.; Ji, H. Phosphorus-Based Anodes for Fast Charging Lithium-Ion Batteries: Challenges and Opportunities. Small Sci. 2022, 2, 2200015.
- Yuan, D. Amorphous Red Phosphorous Embedded in Carbon Nanotubes Scaffold as Promising Anode Materials for Lithium-Ion Batteries. J. Power Sources 2016, 301, 131–137.
- Kim, Y. An Amorphous Red Phosphorus/Carbon Composite as a Promising Anode Material for Sodium Ion Batteries. Adv. Mater. 2013, 25, 3045–3049.
- Hu, R.; Ouyang, Y.; Liang, T.; Wang, H.; Liu, J.; Chen, J.; Yang, C.; Yang, L.; Zhu, M. Stabilizing the Nanostructure of SnO2 Anodes by Transition Metals: A Route to Achieve High Initial Coulombic Efficiency and Stable Capacities for Lithium Storage. Adv. Mater. 2017, 29, 1605006.
- Li, D. Flexible Phosphorus Doped Carbon Nanosheets/Nanofibers: Electrospun Preparation and Enhanced Li-Storage Properties as Free-Standing Anodes for Lithium Ion Batteries. J. Power Sources 2018, 384, 27–33.
- Zhang, H.; Nai, J.; Yu, L.; Lou, X.W.D. Metal-Organic-Framework-Based Materials as Platforms for Renewable Energy and Environmental Applications. Joule 2017, 1, 77–107.
- Ramasubramanian, B.; Chinglenthoiba, C.; Huiqing, X.; Xiping, N.; Hui, H.K.; Valiyaveettil, S.; Ramakrishna, S.; Chellappan, V. Sustainable Nanocomposite Electrode for Supercapacitor. Surf. Interfaces 2022, 34, 102397.
- Qu, Q.; Gao, T.; Zheng, H.; Li, X.; Liu, H.; Shen, M.; Shao, J.; Zheng, H. Graphene Oxides-Guided Growth of Ultrafine Co3O4 Nanocrystallites from MOFs as High-Performance Anode of Li-Ion Batteries. Carbon 2015, 92, 119–125.
- Cai, D.; Zhan, H.; Wang, T. MOF-Derived Porous ZnO/ZnFe2O4 Hybrid Nanostructures as Advanced Anode Materials for Lithium Ion Batteries. Mater. Lett. 2017, 197, 241–244.
- Li, Z.; Hu, X.; Shi, Z.; Lu, J.; Wang, Z. MOFs-Derived Metal Oxides Inlayed in Carbon Nanofibers as Anode Materials for High-Performance Lithium-Ion Batteries. Appl. Surf. Sci. 2020, 531, 147290.
- Zheng, S.; Li, X.; Yan, B.; Hu, Q.; Xu, Y.; Xiao, X.; Xue, H.; Pang, H. Transition-Metal (Fe, Co, Ni) Based Metal-Organic Frameworks for Electrochemical Energy Storage. Adv. Energy Mater. 2017, 7, 1602733.
- Meng, D.; Zhang, C.; Liang, Y.; Qiu, W.; Kong, F.; He, X.; Chen, M.; Liang, P.; Zhang, Z. Electrospun Cobalt Prussian Blue Analogue-Derived Nanofibers for Oxygen Reduction Reaction and Lithium-Ion Batteries. J. Colloid. Interface Sci. 2021, 599, 280–290.
- Liu, J.; Zhang, F.; Hou, L.; Li, S.; Gao, Y.; Xin, Z.; Li, Q.; Xie, S.; Wang, N.; Zhao, Y. Synergistic Engineering of 1D Electrospun Nanofibers and 2D Nanosheets for Sustainable Applications. Sustain. Mater. Technol. 2020, 26, e00214.
- Ding, Y.; Chen, L.; Pan, P.; Du, J.; Fu, Z.; Qin, C.; Wang, F. Nitrogen-Doped Carbon Coated MnO Nanopeapods as Superior Anode Materials for Lithium Ion Batteries. Appl. Surf. Sci. 2017, 422, 1113–1119.
- Zhang, C.; Xie, Z.; Yang, W.; Liang, Y.; Meng, D.; He, X.; Liang, P.; Zhang, Z. NiCo2O4/Biomass-Derived Carbon Composites as Anode for High-Performance Lithium Ion Batteries. J. Power Sources 2020, 451, 227761.
- Wang, J.; Nie, P.; Ding, B.; Dong, S.; Hao, X.; Dou, H.; Zhang, X. Biomass Derived Carbon for Energy Storage Devices. J. Mater. Chem. A Mater. 2017, 5, 2411–2428.
- Ryu, D.J.; Oh, R.G.; Seo, Y.D.; Oh, S.Y.; Ryu, K.S. Recovery and Electrochemical Performance in Lithium Secondary Batteries of Biochar Derived from Rice Straw. Environ. Sci. Pollut. Res. 2015, 22, 10405–10412.
- Luna-Lama, F.; Rodríguez-Padrón, D.; Puente-Santiago, A.R.; Muñoz-Batista, M.J.; Caballero, A.; Balu, A.M.; Romero, A.A.; Luque, R. Non-Porous Carbonaceous Materials Derived from Coffee Waste Grounds as Highly Sustainable Anodes for Lithium-Ion Batteries. J. Clean. Prod. 2019, 207, 411–417.
- Mondal, A.K.; Kretschmer, K.; Zhao, Y.; Liu, H.; Fan, H.; Wang, G. Naturally Nitrogen Doped Porous Carbon Derived from Waste Shrimp Shells for High-Performance Lithium Ion Batteries and Supercapacitors. Microporous Mesoporous Mater. 2017, 246, 72–80.
- Wang, Y.Y.; Hou, B.H.; Lü, H.Y.; Wan, F.; Wang, J.; Wu, X.L. Porous N-Doped Carbon Material Derived from Prolific Chitosan Biomass as a High-Performance Electrode for Energy Storage. RSC Adv. 2015, 5, 97427–97434.
- Ding, B.; Huang, S.; Pang, K.; Duan, Y.; Zhang, J. Nitrogen-Enriched Carbon Nanofiber Aerogels Derived from Marine Chitin for Energy Storage and Environmental Remediation. ACS Sustain. Chem. Eng. 2018, 6, 177–185.
- Park, T.J.; Jung, Y.J.; Choi, S.W.; Park, H.; Kim, H.; Kim, E.; Lee, S.H.; Kim, J.H. Native Chitosan/Cellulose Composite Fibers from an Ionic Liquid via Electrospinning. Macromol. Res. 2011, 19, 213–215.
- Ramasubramanian, B.; Sundarrajan, S.; Rao, R.P.; Reddy, M.V.; Chellappan, V.; Ramakrishna, S. Novel Low-Carbon Energy Solutions for Powering Emerging Wearables, Smart Textiles, and Medical Devices. Energy Environ. Sci 2022, 15, 4928–4981.
- Chen, W.; Xu, D.; Chen, Y.; Tang, T.; Kuang, S.; Fu, H.; Zhou, W.; Yu, X. In Situ Electrospinning Synthesis of N-Doped C Nanofibers with Uniform Embedding of Mn Doped MFe1−xMnxPO4 (M = Li, Na) as a High Performance Cathode for Lithium/Sodium-Ion Batteries. Adv. Mater. Interfaces 2020, 7, 2000684.
- Kandeeban, R.; Brindha, R.; Manojkumar, K.; Batoo, K.M.; Raslan, E.H.; Hadi, M.; Imran, A.; Saminathan, K. Revealing the Synergetic Electrocatalyst Behaviour of Kish Graphite Recovered from Polyethylene Plastics. Mater. Lett. 2021, 297, 129740.
- Mikeš, P.; Baker, D.A.; Uhlin, A.; Lukáš, D.; Kuželová-Košťáková, E.; Vidrich, A.; Valtera, J.; Kopřivová, B.; Asatiani, N.; Salmén, L.; et al. The Mass Production of Lignin Fibres by Means of Needleless Electrospinning. J. Polym. Environ. 2021, 29, 2164–2173.
- Bölgen, N.; Demir, D.; Aşık, M.; Sakım, B.; Vaseashta, A. Introduction and Fundamentals of Electrospinning. In Electrospun Nanofibers; Springer: Cham, Switzerland, 2022; pp. 3–34.
- Anisiei, A.; Oancea, F.; Marin, L. Electrospinning of chitosan-based nanofibers: From design to prospective applications. Rev. Chem. Eng. 2023, 39, 31–70.
- Kang, S.; Hou, S.; Chen, X.; Yu, D.G.; Wang, L.; Li, X.; Williams, G.R. Energy-Saving Electrospinning with a Concentric Teflon-Core Rod Spinneret to Create Medicated Nanofibers. Polymers 2020, 12, 2421.
- Bian, Y.; Zhang, C.; Wang, H.; Cao, Q. Degradable Nanofiber for Eco-Friendly Air Filtration: Progress and Perspectives. Sep. Purif. Technol. 2023, 306, 122642.
- Thomas, S.; Seufert, B.; Serrano-Garcia, W.; Devisetty, M.; Khan, R.; Puttananjegowda, K.; Alcantar, N. Eco-Friendly, Biodegradable, and Biocompatible Electrospun Nanofiber Membranes and Applications. In Sustainable Nanotechnology: Strategies, Products, and Applications; Pathak, Y.V., Parayil, G., Patel, J.K., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2022; pp. 173–199.
- Niu, H.; Lin, T. Fiber Generators in Needleless Electrospinning. J. Nanomater. 2012, 2012, 725950.
- Pathak, Y.V.; Parayil, G.; Patel, J.K. (Eds.) Sustainable Nanotechnology: Strategies, Products, and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2022.
More
Information
Subjects:
Electrochemistry; Materials Science, Ceramics
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
660
Revisions:
2 times
(View History)
Update Date:
04 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




