1. Protein Structure of Ubiquitin-Specific Peptidase 16
1.1. Domain Architecture and Conservation
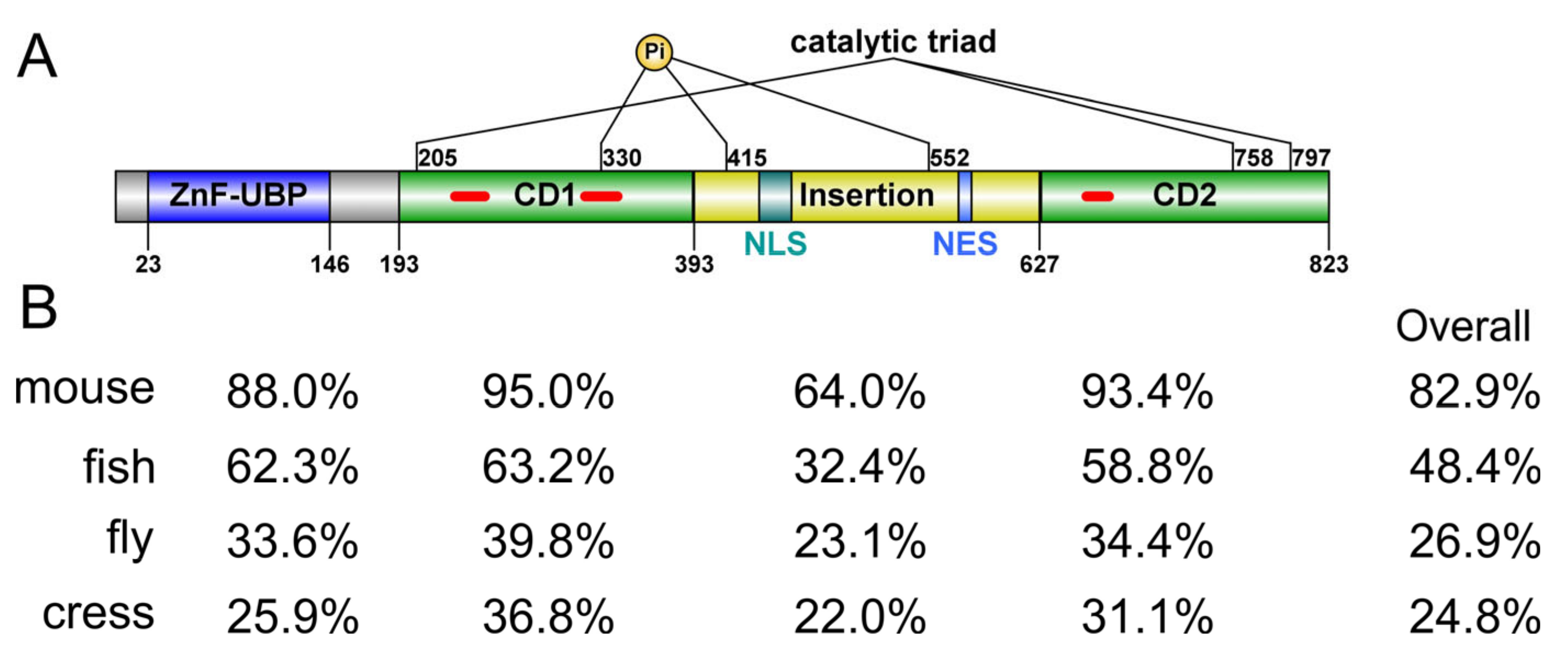
Human ubiquitin-specific peptidase 16 (USP16) is an active DUB of 823 a.a. It features a UBP-type zinc-finger domain (ZnF-UBP) at the
N-terminus (a.a. 23–146) followed by a catalytic domain (CD, a.a. 193–823) that is bifurcated by a long, disordered insertion (a.a. 393–627) of about 230 residues (
Figure 1A). The closest paralog of human USP16 is USP45, which has 38% sequence identity and the same domain architecture. USP45 has been found to play a role in DNA damage repair
[1], but it has not been linked to histone deubiquitination. To understand the relationship between USP16 and histone H2A deubiquitination, researchers searched for putative USP16 orthologs from representative species of invertebrates, vertebrates, and plants. Notably, budding and fission yeasts (
Saccharomyces cerevisiae and
Schizosaccharomyces pombe) do not have an apparent ortholog for USP16. Orthologs of both USP16 and USP45 can be found in vertebrate genomes. Still, there is only one USP16 ortholog from
Drosophila melanogaster, annotated as Usp16-45, which is highly divergent from orthologs from other species (
Figure 1B and
Figure 2), with a 26.9% sequence identity to human USP16. However, USP16 orthologs in the
Arabidopsis thaliana genome are less obvious. It has been previously shown that
Arabidopsis thaliana does contain H2Aub and Polycomb Group (PcG) protein homologs
[2][3]. One reference protein (NP_567705, UBP16), despite being annotated as plant Usp16, contains a much shorter MYND-type ZnF domain instead of a UBP-type ZnF domain. Using the human USP16 ZnF-UBP domain as the query sequence, researchers identified two plant gene products as the closest putative homologs, UBP1 (NP_565753) and UBP2 (NP_563719), with 24.0% and 25.9% sequence identity, respectively. In comparison, plant UBP16 has only 17.1% sequence identity. Researchers thus designate UBP1 and UBP2 as the
Arabidopsis thaliana orthologs of human USP16. Such designation is purely based on bioinformatic analysis and requires future experimental evidence for support. The fact that fly has only one ancestor Usp16-45 gene but higher organisms, including plants, have two genes, USP1, and USP45, suggests a gene duplication event during evolution involving USP16, rendering USP16 fine-tuned for its functional roles. Indeed, it has been suggested USP16 and USP45 may arise from a novel whole genome duplication event
[4]. Comparing the sequence identity of different parts of USP16, the core CD and ZnF-UBP domain are more conserved than the rest of the protein (
Figure 1B), suggesting it likely employs similar mechanisms for substrate recognition but may have different modes of regulation involving the disordered regions. Except for mouse and human USP16, the function of orthologs in other organisms is yet to be investigated.
Figure 1. Domain architecture and conservation of USP16. (A) Sequence feature of human USP16. ZnF-UBP, UBP-type zinc finger domain; CD1/CD2, bifurcated catalytic domain; NLS, nuclear localization signal; NES, nuclear export signal; Pi, phosphorylation site. Short insertions in the catalytic domain are indicated with red lines. Domain boundaries are defined based on the predicted or experimental structures. (B) Sequence identity of the individual domains of USP16 and the full-length protein from different species (mouse: NP_077220; fly: NP_572220; zebrafish: NP_001139569; cress: NP_563719) compared to those of human USP16 (NP_006438). Pairwise sequence alignment was carried out using Smith-Waterman local alignment algorithm.
Figure 2. Sequence alignment of USP16 from five species. Sequences of human, mouse, zebrafish, fly, and thale cress USP16 homologs were aligned MAFFT algorithm. The sequences are colored according to percentage identity. Sequence fragments that exist only in one of the five homologs are removed from the alignment for clarity and indicated with a marker. The image was prepared using Jalview.
1.2. Overall Structure
The previous study demonstrated that USP16 forms a tetramer in solution
[5]. The size of the protein and the long predicted disordered insertion within the CD render it recalcitrant for structure determination by NMR or X-ray crystallography. Only an NMR structure of the ZnF-UBP domain has been experimentally determined
[6]. Researchers resort to AlphaFold
[7] for the structure of full-length USP16. AlphaFold-multimer
[8] predicts a tightly packed tetrameric structure where the Ub-binding pocket of the CD is occupied by other subunits, which is not physiologically meaningful. Therefore, without further experimental data, researchers limit discussion to the monomeric structure of USP16 predicted by AlphaFold (
Figure 3). As expected, the predicted structure forms two well-defined domains (ZnF-UBP and USP) of relatively high confidence, along with long disordered insertions. A lysine-rich region (a.a. 437–464) in the middle of the insertion is predicted to form an α-helix with relatively high confidence (
Figure 3A).
Figure 3. Structure of USP16. (
A) AlphaFold-predicted structure of monomeric, full-length USP16 is colored according to the confidence of prediction (pLDDT value 0–100). Blue indicates high confidence. Zinc ions chelated to the ZnF-UBP domain are shown as orange spheres. Known phosphorylated residues, Ser330, 415, and 552, are presented as green spheres. (
B) Superposition of the catalytic domains of USP16 (AlphaFold2 predicted) and USP7 (PDB: 1NB8) was carried out using PyMOL
[9]. The fingers, thumb, and palm domain of USP16 are colored orange, green and blue, respectively. The USP7 catalytic domain is colored grey. While the core domains of USP7 and USP16 align well, it is evident that additional structural elements arise from the short insertions in USP16. (
C) The catalytic triad of USP16. Residues C205, H758, and S797 form a hydrogen-bonded catalytic triad. D798, despite being close to the catalytic triad, is not arranged in the necessary conformation and, thus, is not a catalytic residue.
1.3. ZnF-UBP Domain
The experimentally determined ZnF-UBP structure matches the AlphaFold-predicted structure very well. It adopts a two-layer sandwich containing four α-helices surrounding a five-stranded β-sheet. The ZnF-UBP chelates three zinc ions in a cross-braced fashion. The ZnF-UBP domain has been shown to bind the free C-terminal tail of Ub with micromolar affinity
[6]. Furthermore, an enzymatic assay
[10] using ubiquitin C-terminal 7-amido-4-methylcoumarin (Ub-AMC) as a substrate reveals that full-length USP16 has a 3.5-fold smaller Michaelis-Menten specificity constant (
kcat/
KM) than the CD, suggesting the ZnF-UBP domain plays a regulatory role, although the molecular details of such remain elusive.
1.4. Catalytic Domain
In the AlphaFold-predicted structure of full-length USP16, disordered residues (a.a. 571–604) in a segment of low-confidence prediction occupy the Ub binding pocket loosely (Figure 3A). Without experimental evidence, researchers have no reason to suggest this is the actual case under physiological conditions. Researchers limit the discussion of the CD structure to the core domain without the disordered regions. The CD of USP16 adopts a typical hand-like USP fold with palm, thumb, and fingers subdomains. Compared to the prototypic structure of the CD from USP7 (PDB: 1NB8, Figure 3B), the CD of USP16 contains extra structural elements in the thumb subdomain: a β-hairpin (a.a. 231–251) insertion and an extended helix-turn-helix motif insertion in the thumb subdomain. USP16 also contains an extra short α-helix at the tip of the fingers subdomain formed by a 17 residue insertion (Figure 3B).
USPs are cysteine proteases that feature a catalytic Cys-His-Asn/Asp triad. However, USP16 is one of three USPs that utilizes a Ser instead of an Asn/Asp (Cys-His-Ser), the other two being USP30 and USP45, the closest USP16 paralog
[11]. The AlphaFold predicted USP16 structure confirms that residues Cys205, His758, and Ser797 form a catalytic triad (
Figure 3C) and are poised in a productive conformation, meaning a properly arranged hydrogen-bonded network is present to enable the deprotonation of the cysteine thiol group for nucleophilic attack. Note that a neighboring Asp798 is not utilized as a catalytic residue (
Figure 3C).
1.5. Disordered Region
The disordered region of a protein is often involved in post-translational modifications and protein-peptide interactions. Recently, a conserved CRM1-dependent nuclear export signal (NES, a.a. 572–581) and a conserved non-canonical nuclear localization signal (NLS, a.a. 437–459) were identified within the long insertion of the CD
[12]. The NLS motif binds to the RPS27a subunit of the pre-40S ribosome to promote the maturation of the 40S ribosomal subunit. It is interesting to observe that the predicted structure of this motif is an α-helix
[13]. Hence, although the long insertion within the CD is not required for its catalytic activity, it most likely plays important regulatory roles in the subcellular localization and substrate recognition of USP16.
Although USP16 contains multiple predicted CDK phosphorylation sites (a.a. 97, 146, 189, 217, 277, 552, and 600), researchers experimentally identified phosphorylated Ser330, Ser415, and Ser552
[14]. Both Ser415 and Ser552 are located on the disordered insertion, while Ser330 is located in the thumb subdomain (
Figure 3A) and distant from the Ub binding pocket. Phosphorylation of these Ser residues is unlikely to affect substrate Ub recognition. Researchers further demonstrated that Ser552 is the major phosphorylation site, and its phosphorylation allows the nuclear import of USP16, which correlates with H2A deubiquitination at the onset of mitosis
[14]. Thus, USP16 pSer552 is specifically linked to mitotic progression. Notably, Ser552 is not conserved in mice, zebrafish, or fruit flies (
Figure 2). Thus, these organisms may employ distinct mechanisms for the global H2A deubiquitination during the M phase. The enzymes responsible for Ser330 and Ser415 phosphorylation have not yet been defined. The linker between the ZnF-UBP and USP domains of USP16 (a.a. 137–196) are mainly disordered based on AlphaFold prediction (
Figure 3A). Researchers previously found the region interacts with a HECT-type E3 Ub ligase HERC2
[15]. The formation of a DUB/E3 complex is a common theme found throughout the Ub proteasome system. The effect of USP16/HERC2 interaction on the activity of USP16 remains to be elucidated.
2. Mechanisms Regulating the mRNA and Protein Levels of Ubiquitin-Specific Peptidase 16
2.1. Gene Copy Number
In the human genome,
USP16 is located on chromosome 21. Triplication of part or the entirety of chromosome 21 causes Down syndrome
[16]. Furthermore, triplication of
USP16 increases the mRNA and protein levels by 0.5-fold
[17]. This relatively minor increase has been implicated in the defects of multiple somatic cell lineage and progenitor cells in Down syndrome
[17]. RNA interference, deletion, or inactivation of USP16 in one of the triplicated copies can mitigate these stem cell defects and may represent alternate approaches to ameliorate these disorders
[17][18][19].
2.2. NF-κB (Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells)
Three NF-κB binding sites were identified in the promoter region of USP16
[20]. Overexpression of p65 (also called RelA), one of the NF-κB family of transcription factors, significantly increases USP16 transcript levels. Strong activators of the NF-κB pathway, such as lipopolysaccharide (LPS) and tumor necrosis factor-alpha (TNF-α), also positively regulate USP16 transcription. Therefore, the transcription of USP16 is under the control of the NF-κB pathway
[20]. Interestingly, USP16 also regulates the NF-κB pathway (
Figure 4A) in a feedback loop. Mass spectrometry analyses identified USP16 as an interacting protein of IKKβ (inhibitor of nuclear factor kappa-B kinase subunit beta), the key kinase of the NF-κB transcription factor p105
[21]. IKKβ is ubiquitinated at Lys238, and USP16 reverses this modification. USP16 DUB activity enhances IKKβ interaction with p105 and promotes its phosphorylation
[21]. Consequently, USP16 is critical for activating NF-κB-targeted genes, including USP16 itself (
Figure 4A). This positive feedback mechanism may allow USP16 and the NF-κB pathway to adapt the immune system to stimuli or stress more quickly.
Figure 4. Substrates and functions of USP16 in the cytoplasm and nucleus. (A) Transcription of USP16 is under the control of the NF-κB pathway, a pathway also regulated by USP16. (B) Ct-HBx inhibits the expression level of USP16 in hepatocellular carcinoma. (C) USP16 functions as a major deubiquitinase of H2A, and lncEPAT binds to USP16 and blocks its recruitment to chromatin. (D) The C-terminal HECT domain of HERC2 interacts with the coiled-coil domain of USP16 and possibly recruits USP16 to DNA damage foci. (E) USP16 deubiquitinates PLK1 for mitotic chromosome alignment. BubR1 is a core component of the mitotic checkpoint complex. (F) USP16 deubiquitinates c-Myc in prostate cancer. (G) USP16 deubiquitinates calcineurin for T cell maintenance. CNA, Calcineurin A; CNB, calcineurin B; CaM, calmodulin; AP1, activating protein 1, a transcription factor. (H) USP16 deubiquitinates IGF2BP3 in gallbladder cancer. MST, mammalian STE20-like kinase; LATS, large tumor suppressor kinase; YAP/TAZ, Yes-associated protein 1 (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ); TEAD4, TEA domain family member (TEAD) 4. (I) USP16 inhibits p38 activation and deubiquitinates JAK1 in K-RAS-driven lung tumorigenesis. (J) USP16 deubiquitinates the low-density lipoprotein receptor for coronary artery function. (K) USP16 deubiquitinates RPS27a for 40S ribosomal subunit biogenesis and maturation, and (L) USP16 deubiquitinates Tektin for male fertility.
2.3. Gene Fusion
Chronic myelomonocytic leukemia (CMML) is a heterogeneous hematopoietic disease exhibiting either myeloproliferative or myelodysplastic pathological properties
[22]. In a study involving 30 CMML samples from 29 patients, one contained an inversion of chromosomal 21q21-22
[23]. This chromosomal translocation resulted in the fusion of RUNX1 and USP16 genes, containing exon 1 of USP16 fused to exon 15 of RUNX1 and lacking a start codon. However, the fusion gene does contain multiple ATG codons in exons 5–7 in RUNX1
[23]. While this fusion disrupts both the USP16 and RUNX1 genes, the potential for generating truncated RUNX1 protein and its implication to CMML pathogenesis has not been explored.
2.4. HBV X (HBx) Protein
Hepatitis B virus (HBV) infection is a major cause of hepatocellular carcinoma (HCC). The major pathogenic protein of HBV is HBV X (HBx) protein, which has been shown to promote HCC growth and metastasis
[24]. A carboxyl-terminal truncated HBx (Ct-HBx) is often observed in HCC tumor tissues. The DUB expression profiles of cells overexpressing Ct-HBx showed decreased levels of USP16, suggesting that the expression of USP16 may be negatively regulated in a manner dependent on Ct-HBx
[25] (
Figure 4B). Down-regulation of USP16 in the liver tumor cell line promotes its colony formation and tumor growth, leading to stem-like features. USP16 overexpression abolishes the tumorigenic ability of the Ct-HBx. USP16 was found to be frequently downregulated in human HCCs and correlates with advanced tumor stages and disease progression
[25]. While this research identifies USP16 downregulation as a critical regulator in Ct-HBx-driven HCC growth, the targets of USP16 and its mechanism in this disease remain unclear.
3. Cytoplasmic and Nuclear Subcellular Localization of Ubiquitin-Specific Peptidase 16
As noted in previous studies, USP16 is predominately localized to the cytoplasm during interphase and is in a hypophosphorylated state
[14][26]. However, SDS-PAGE analysis of purified USP16 isolated from
Spodoptera frugiperda Sf9 cells was instrumental in identifying a slow-migrating proteoform that could be relinquished upon phosphatase treatment. Phosphorylation of Ser552 (pSer552) by cyclin-dependent kinase 1 (CDK1) was established to be the major modification
[14]. While pSer552 is not required for tetramer formation, DUB activity, the substrate specificity of USP16, or transcriptional regulation by USP16, it is essential for cell proliferation and cell cycle progression through the G2/M phase. The underlying mechanism is the disruption of the interaction between USP16 and the nuclear export protein CRM1 (chromosomal maintenance 1, also known as exportin 1), resulting in its nuclear localization. Thus, Ser552 functions as the phosphorylation site regulating the nuclear retention of USP16 when cells enter the M phase of the cell cycle
[14].
Furthermore, an NES between a.a. 572–581 was shown to contribute to the cytoplasmic localization of USP16
[12]. Whereby, following mitosis, USP16 is rapidly exported from the nucleus to the cytoplasm. As well, a non-canonical NLS sequence, a.a. 437–459, overlapping the predicted monopartite NLS signal, a.a. 439–456, was identified in USP16
[12]. Considering the predominant cytoplasmic localization of USP16, this NLS is weak. Enforced nuclear localization of USP16 abolishes DNA double-strand break (DSB) repair, possibly due to the unrestrained DUB activity
[12]. This may link to the reduced DNA damage repair ability in Down syndrome cells
[14]. Besides deubiquitinating H2Aub, recent studies also identified many cytoplasmic and nuclear substrates of USP16 (
Figure 4 and
Table 1). Below, researchers summarize these studies, highlighting the diverse regulation of USP16 in physiological and pathological processes.
Table 1. Summary of the substrates and functions of USP16.
| |
Substrates |
Functions |
References |
| Nucleus |
H2AK119ub |
Deubiquitinates H2AK119ub and activates gene expression |
[5][17][27][28][29][30][31][32] |
| H2AK15ub |
Deubiquitinates H2AK15ub in DNA damage response |
[15] |
| PLK1 |
Deubiquitinates PLK1 in chromosome alignment |
[33] |
| c-Myc |
Deubiquitinates and stabilizes c-Myc and promotes prostate cancer cell growth |
[34] |
| Cytoplasm |
Calcineurin A |
Deubiquitinates calcineurin A and regulates NFAT-targeted genes |
[35] |
| IGF2BP3 |
Deubiquitinates IGF2BP3 and promotes gallbladder cancer |
[36] |
| JAK1 |
Deubiquitinates JAK1 in lung tumorigenesis |
[37] |
| LDLR |
Deubiquitinates LDLR in atherosclerosis and coronary artery diseases |
[38] |
| RPS27a |
Deubiquitinates RPS27a for 40S subunit maturation |
[13] |
| Tektin |
Deubiquitinates Tektin for microtubule filament formation during spermiogenesis |
[39] |
| IKKβ |
Deubiquitinates IKKβ and activates NF-κB-targeted genes |
[21] |