Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hisashi Kato-Noguchi | -- | 2294 | 2023-03-30 04:01:01 | | | |
| 2 | Sirius Huang | Meta information modification | 2294 | 2023-03-30 05:00:36 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kato-Noguchi, H. Function of Defensive Molecules Momilactones A and B. Encyclopedia. Available online: https://encyclopedia.pub/entry/42628 (accessed on 07 February 2026).
Kato-Noguchi H. Function of Defensive Molecules Momilactones A and B. Encyclopedia. Available at: https://encyclopedia.pub/entry/42628. Accessed February 07, 2026.
Kato-Noguchi, Hisashi. "Function of Defensive Molecules Momilactones A and B" Encyclopedia, https://encyclopedia.pub/entry/42628 (accessed February 07, 2026).
Kato-Noguchi, H. (2023, March 30). Function of Defensive Molecules Momilactones A and B. In Encyclopedia. https://encyclopedia.pub/entry/42628
Kato-Noguchi, Hisashi. "Function of Defensive Molecules Momilactones A and B." Encyclopedia. Web. 30 March, 2023.
Copy Citation
Labdane-related diterpenoids, momilactones A and B were isolated and identified in rice husks in 1973 and later found in rice leaves, straws, roots, root exudate, other several Poaceae species and the moss species Calohypnum plumiforme. Momilactones in rice plants suppressed the growth of fungal pathogens, indicating the defense function against pathogen attacks. Rice plants also inhibited the growth of adjacent competitive plants through the root secretion of momilactones into their rhizosphere due to the potent growth-inhibitory activity of momilactones, indicating a function in allelopathy.
allelopathy
biosynthesis
diterpenoid
Echinochloa crus-galli
elicitation
momilactone
Oryza sativa
pathogen
rice blast
1. Introduction
Labdane-related diterpenoids, momilactones A and B (Figure 1) were first isolated and identified in rice husks as potent germination and growth-inhibitory substances in 1973 [1]. Momilactones were later isolated from rice leaves as phytoalexins against fungal pathogens such as the rice blast fungus Magnaporthe oryzae [2][3]. The concentrations of momilactones increased 2 days after infection with Magnaporthe oryzae, and momilactones suppressed the further growth of the fungus [4][5]. The fungal elicitors chitosan and cholic acid also induced the accumulation of momilactone A in rice leaves and suspension-cultured rice cells [6][7].
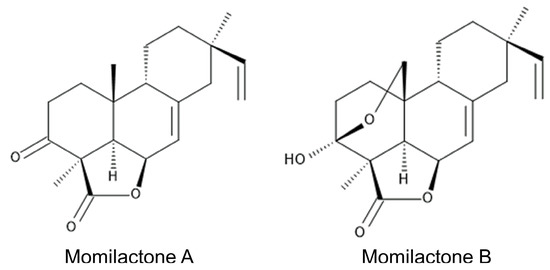
Figure 1. Momilactones.
The function of momilactones, especially momilactone A, as phytoalexins has been extensively studied, and the evidence suggests that momilactones may play a role in the rice defense function against fungal pathogens [8][9][10].
The first finding of rice allelopathy was made in field examinations in Arkansas, U.S.A., where 191 of over 5000 rice accessions suppressed the growth of the aquatic weed Heteranthera limosa [11]. Allelopathy is defined as the chemical interactions among various plant species [12]. Certain plants release some secondary metabolites, termed allelochemicals, into their immediate environment, and these allelochemicals affect the growth and development of other plant species nearby [13][14][15][16][17]. The observation of rice allelopathy led to large field screening programs. Among over 16,000 rice germplasm collections of the USDA-ARS from 99 countries, 412 rice accessions suppressed the growth of Heteranthera limosa, and 145 rice accessions suppressed the growth of Ammannia coccinea [18][19]. More than 40 rice cultivars among 1000 rice collections inhibited the growth of Echinochloa crus-galli and Cyperus difformis [20]. Screening programs in the field and/or laboratories have also been carried out in several other countries, and it was found that certain rice cultivars released allelochemicals from their root systems into their immediate environments, such as rhizosphere soil, cultural solutions and other incubation media [21][22][23][24]. Thereafter, momilactones A and B were again isolated and identified in rice root exudates as rice allelochemicals [25][26]. It was also found that rice plants released momilactones throughout their life cycles with sufficient amounts of momilactones for allelopathy [27][28].
Momilactones are synthesized in rice plants from geranylgeranyl diphosphate, which is also a precursor of other phytoalexins and a plant hormone, gibberellic acid [29]. Momilactones are synthesized and accumulated in rice leaves as phytoalexins and secreted into their root zones as allelochemicals [30][31]. A gene cluster related to momilactone synthesis was found on chromosome 4 of the rice genome. Momilactones were later found in some other Gramineae plant species and the moss species Calohypnum plumiforme (syn. Hypnum plumaeform) as allelochemicals [32][33][34][35].
2. Defense Function against Pathogens, Microbes and Insects
2.1. Rice Blast Fungal Pathogen
Infection with the rice blast pathogen Magnaporthe oryzae (syn. Pyricularia oryzae; renamed from Magnaporthe grisea) induced momilactone A accumulation in rice leaves. The accumulation was abundant at the edges of necrotic lesions, which are symptoms of the infection of leaves [36]. Blast fungus susceptibility diffed among rice cultivars, and tolerance to the fungus correlated positively with momilactone A accumulation in rice leaves [37]. Blast-fungus-resistant rice mutants accumulated momilactone A 2 days after fungus inoculation, and the concentration of momilactone A was 100–400-fold greater than that in wild-type rice and suppressed the further growth of the fungus [4][5]. Exogenously applied momilactone A also suppressed the growth of the fungus on agar media [5]. In addition, the susceptibility of momilactone-deficient rice mutants to the blast fungus was high compared to wild-type rice [38]. These observations suggest that momilactone A may prevent the subsequent spread of the fungus infection through the increased production of momilactone A after pathogen infection.
2.2. Other Fungal Pathogens
Momilactones A and B inhibited the growth of the pathogenic fungi Rhizoctonia solani, Blumeria graminis, Fusarium oxysporum, Fusarium solani, Botrytis cinereal and the Colletrichum gloesporides complex [39][40]. Infection with Xanthomonas oryzae pv. oryzae, which causes bacterial blight, increased jasmonic acid and momilactone A concentrations in rice leaves [41]. Jasmonic acid is a plant defense signaling hormone and induces several defense responses for protection [42][43][44].
2.3. Anti-Microbe Activity
Momilactone A inhibited the mycelia growth of the mushroom Coprinus cinereus [45] and the cyanobacteria Microcystis aeruginosa [46]. Momilactones A and B inhibited the growth of the bacteria Escherichia coli, Pseudomonas putida (former name, Pseudomonas ovalis), Bacillus cereus and Bacillus pumilus [39].
2.4. Insect Attack
An insect attack by the white-back planthopper (Sogatella furcifera) induced the accumulation of momilactone A in rice leaves through a jasmonic acid-mediated pathway [47]. The digestive waste of the rice brown planthopper (Nilaparvata lugens) induced momilactone A and B accumulation in rice leaves. Filtration and heat treatments of digestive wastes reduced their accumulation. A symbiont of the insect, Serratia marcescens, in the digestive waste also induced the accumulation of momilactones A and B [48]. The function of momilactones A and B against insect attacks is not clear.
3. Function in Allelopathy
A considerable number of rice accessions or cultivars have been found to suppress the growth of several other plant species, including weed species, when these rice and other plants were grown together under field and/or laboratory conditions [11][21][22][23][24][49]. These observations suggest that rice is allelopathic and contains allelochemicals. A compound causing the growth-inhibitory effect of rice was later isolated from its root exudates and identified as momilactone B [25]. Momilactone A was also identified in rice secretory fluid [26]. These investigations suggest that momilactones A and B may function as rice allelochemicals.
3.1. Activities of Momilactones A and B as Allelochemicals
Momilactones A and B inhibited the growth of several plant species, including weed species such as Echinochloa crus-galli and Echinochloa colonum. Both Echinochloa species are known as the most noxious weeds in rice fields because of their potential to significantly disturb rice production [50][51]. Momilactones A and B inhibited the root and shoot growth of Echinochloa crus-galli at concentrations greater than 3 μM and 1 μM, respectively, and the root and shoot growth of Echinochloa colonum at concentrations greater than 10 μM and 1 μM, respectively [52]. Table 1 shows the concentrations of momilactones A and B required for 50% growth inhibition (defined as IC50) of target plant species. Smaller values of IC50 indicate the higher susceptibly of the target plants to momilactones. On the basis of IC50 values, monocotyledonous weed plant species (Echinochloa crus-galli, Echinochloa colonum, Phleum pretense, Digitaria sanguinalis and Lolium multiflorum) showed higher susceptibly compared to dicotyledonous plant species (Arabidopsis thaliana, Lepidium sativum, Lactuca sativa and Medicago sativa) [52][53][54][55]. In addition, momilactone B showed much higher growth-inhibitory activity than momilactone A, which has also been confirmed by other bioassay systems [55][56][57][58][59].
Table 1. The concentrations (μM) required for 50% growth inhibition (IC50) of various plant species.
| Momilactone A | Momilactone B | Reference | |||
|---|---|---|---|---|---|
| Target Plant Species | Roots | Shoots | Roots | Shoots | |
| Echinochola crus-gall | 28.7 | 46.4 | 6.1 | 6.3 | [53] |
| Echinochloa colonum | 65.4 | 240 | 5.04 | 12.5 | [52] |
| Phleum pratense | 76.5 | 157 | 5.6 | 7.9 | [55] |
| Digitaria sanguinalis | 98.5 | 275 | 9.5 | 12.4 | [55] |
| Lolium multiflorum | 91.9 | 138 | 6.9 | 6.5 | [55] |
| Arabidopsis thiliana | 203 | 84.4 | 12 | 6.5 | [54] |
| Lepidium sativum | 425 | 285 | 6.3 | 4.6 | [35] |
| Lactuca sativa | 472 | 395 | 54.3 | 77.9 | [55] |
| Medicago sativa | 379 | 315 | 67.8 | 82.4 | [55] |
On the other hand, momilactones A and B showed relatively weak inhibitory activity on rice growth compared to Echinochloa crus-galli. The rice roots and shoots were suppressed by momilactones A and B at concentrations greater than 300 μM and 100 μM, respectively [52][53]. Thus, the effect of momilactones on rice was only 1% of that on Echinochloa crus-galli, which was inhibited at concentrations greater than 3 μM and 1 μM for roots and shoots, respectively, as described above [52][53]. In addition, momilactones A and B did not cause any visible damage to rice plants at concentrations that were phytotoxic to other plant species [52][53][54][55]. These observations suggest that the toxicity of momilactones A and B to rice plants is much less than that to other plant species. The resistance mechanism of rice to momilactones is unknown. This tolerance may possibly involve either rapid secretion, the insensitivity of the molecular target and/or the degradation of momilactones.
3.2. Concentration and Secretion of Momilactones
The endogenous concentrations of momilactones A and B, respectively, in rice were 4.5 μg/g and 3.0 μg/g of rice straw [60] and 4.9 μg/g and 2.9 μg/g of rice husks [61]. Momilactone B was found in rice seedlings 7 days after germination, and the concentrations of momilactones A and B increased until day 80 after germination, which is when flowering is initiated [52][62][63][64]. The 80-day-old rice plants contained momilactones A and B at 140 μg/g and 95 μg/g in rice plants, respectively [52][64]. Considering their reported concentrations, the ratio of momilactone A to momilactone B is 1.5–1.6.
The secretion of momilactone B from rice roots was observed 3 days after germination [62]. The levels of momilactone A and B secretion increased up to day 80 after germination and decreased thereafter [52][63]. The secretion levels of momilactones A and B at day 80 were 1.1 and 2.3 μg per plant per day, respectively [52][63], which indicates that the secretion ratio of momilactone B to momilactone A is 2.1. The observation suggests that rice secretes momilactones A and B into its rhizosphere throughout its entire life cycle, and the secretion increases until flowering initiation. Thus, it may be possible that rice allelopathy increases over this time frame. In addition, momilactone B was secreted at a higher rate than momilactone A, even though the concentration of momilactone A is higher than that of momilactone B in rice plants, which suggests that momilactone B may be preferentially secreted into the rhizosphere over momilactone A. Plants are reported to secrete a wide range of compounds from their roots through their cell membranes, for example, by proton-pumping mechanisms, plasmalemma-derived exudation and endoplasmic-derived exudation [65][66][67]. However, the mechanism of the exudation of momilactones from rice roots is unknown.
3.3. Contribution of Momilactones to Rice Allelopathy
When eight cultivars of rice seedlings (7 days old) were incubated for four days with Echinochloa crus-galli seedlings (4 days old) in a buffered bioassay medium, all rice cultivars suppressed the growth of Echinochloa crus-galli with different suppression levels. All rice cultivars produced and secreted momilactones A and B into the media, and the concentrations of momilactones A and B in the media were 0.21–1.45 μM and 0.66–3.84 μM, respectively [53]. Based on the growth-inhibitory activity and secreted amounts of momilactones A and B in the media, momilactone A may only account for 1.0–4.9% of the observed growth inhibition of Echinochloa crus-galli by the respective rice cultivars. By contrast, momilactone B may account for 58.8–81.9% of the observed growth inhibition. In addition, the momilactone B concentration in the media was significantly (p < 0.01) correlated with the extent of the growth suppression of Echinochloa crus-galli by these eight rice cultivars [53]. A similar correlation was also found between the level of momilactone B secretion and the extent of the growth suppression of Lactuca sativa by these rice cultivars [68][69]. The observations suggest that momilactone B may be a major contributor to the allelopathic activity of rice, and the secretion levels of momilactone B reflect the variation in allelopathic activity observed rice cultivars. The leaf, straw and husk extracts of 41 rice cultivars differed in their growth-inhibitory activity against Alisma plantago-aquatica. The concentration of momilactone B in the extracts was also correlated with the inhibitory activity of the extracts [70].
3.4. Genetic Evidence for Momilactones in Rice Allelopathy
Momilactone-biosynthesis-deficient mutants (cps4 and ksl4) were obtained through insertion gene knockouts for OsCPS4 and OsKSL4 [71][72]. Allelopathic activity after removing all syn-copalyl diphosphate-derived labdane-related diterpenoids (cps4 mutant) or, more selectively, only momilactones (ksl4 mutant) was compared to the respective wild-type rice. The wild types showed allelopathic activity, whereas both mutants lost this activity [73]. The investigation suggests that the loss of allelopathic activity may be attributed to the specific loss of momilactones, which verifies the involvement of momilactones in rice allelopathy.
3.5. Inhibitory Mechanism
Molecular targets of momilactone B were investigated through SDS-PAGE and two-dimensional gel electrophoresis with MALDI-TOF-MS. Momilactone B suppressed the germination of Arabidopsis thaliana and inhibited the breakdown of the storage proteins cruciferina, cruciferin 2 and cruciferin 3 during germination [74]. The breakdown of these proteins is essential to construct cell structures for germination and seedling growth [75][76][77]. The application of momilactone B to Arabidopsis thaliana seedlings inhibited the accumulation of amyrin synthase LUP2, subtilisin-like serine protease, β-glucosidase and malate synthase [78]. Those proteins are involved in the production of intermediates and metabolic turnover for cell structures [79][80][81][82]. On the contrary, momilactone B induced the accumulation of translationally controlled tumor protein, 1-cysteine peroxiredoxin 1 and glutathione-S-transferase [75]. These proteins elevate the tolerance to drought and oxidative stress conditions [83][84][85]. In addition, glutathione-S-transferase showed herbicide detoxification activity [86], and 1-cysteine peroxiredoxin 1 showed germination-inhibitory activity under unfavorable conditions [87]. These observations suggest that momilactone B may cause growth inhibition through the suppression of metabolic turnover and the production of intermediates and induce tolerance to stress conditions.
3.6. Induction of Rice Allelopathy and Momilactone
The allelopathic activity of rice was increased by nutrient deficiency, which is often caused by competition with neighboring plants [88][89][90]. The nutrient-deficient condition also increased the production and secretion of momilactone B from rice [91]. In addition, the allelopathic activity of rice was also elevated by either nearby Echinochloa crus-galli plants or their root exudates [91][92][93][94]. This elevation was not only owing to nutrient competition between rice and Echinochloa crus-galli [95][96]. The momilactone B concentration in rice and its secretion level from rice were also increased by either Echinochloa crus-galli or its root exudates. Rice may recognize certain components of the root exudation of Echinochloa crus-galli, and the compounds trigger the increased production and secretion of momilactone B [91][95][96]. Other weed species, namely, Eclipta prostate and Leptochola chinensis, also increased the secretion of momilactone B [97].
Rice allelopathic activity was also elevated by jasmonic acid [98]. The application of jasmonic acid and cantharidin with UV irradiation also increased the concentration of momilactone B in rice and the secretion levels of momilactones from rice roots into its rhizosphere [99]. As momilactones, especially momilactone B, have strong allelopathic activity, as described previously, such increasing secretion levels of momilactones may provide a competitive advantage for rice through the suppression of the growth of nearby competing plant species.
References
- Kato, T.; Kabuto, C.; Sasaki, N.; Tsunagawa, M.; Aizawa, H.; Fujita, K.; Kato, Y.; Takahashi, N. Momilactones, growth inhibitors from rice, Oryza sativa L. Tetrahedron Lett. 1973, 39, 3861–3864.
- Cartwright, D.; Langcake, P.; Pryce, R.J.; Leworthy, D.P.; Ride, J.P. Chemical activation of host defense mechanisms as a basis for crop protection. Nature 1977, 267, 511–513.
- Cartwright, D.W.; Langcake, P.; Pryce, R.J.; Leworthy, D.P.; Ride, J.P. Isolation and characterization of two phytoalexins from rice as momilactones A and B. Phytochemistry 1981, 20, 535–537.
- Takahashi, A.; Kawasaki, T.; Henmi, K.; Shii, K.; Kodama, O.; Satoh, H.; Shimamoto, K. Lesion mimic mutants of rice with alterations in early signaling events of defense. Plant J. 1999, 17, 535–545.
- Hasegawa, M.; Mitsuhara, I.; Seo, S.; Imai, T.; Koga, J.; Okada, K.; Yamane, H.; Ohashi, Y. Phytoalexin accumulation in the interaction between rice and the blast fungus. Mol. Plant-Micro. Intrac. 2010, 23, 1000–1011.
- Agrawal, G.K.; Rakwal, R.; Tamogami, S.; Yonekura, M.; Kubo, A.; Saji, H. Chitosan activates defense/stress response(s) in the leaves of Oryza sativa seedlings. Plant Physiol. Biochem. 2002, 40, 1061–1069.
- Shimizu, T.; Jikumaru, Y.; Okada, A.; Okada, K.; Koga, J.; Umemura, K.; Minami, E.; Shibuya, N.; Hasegawa, M.; Kodama, O.; et al. Effects of a bile acid elicitor, cholic acid, on the biosynthesis of diterpenoid phytoalexins in suspension-cultured rice cells. Phytochemistry 2008, 69, 973–981.
- Tamogami, S.; Kodama, O. Coronatine elicits phytoalexin production in rice leaves (Oryza sativa L.) in the same manner as jasmonic acid. Phytochemistry 2000, 54, 689–694.
- Jung, Y.H.; Lee, J.H.; Agrawal, G.K.; Rakwal, R.; Kim, J.A.; Shim, J.K.; Lee, S.K.; Jeon, J.S.; Koh, H.J.; Lee, Y.H.; et al. The rice (Oryza sativa) blast lesion mimic mutant, blm, may confer resistance to blast pathogens by triggering multiple defense-associated signaling pathways. Plant Physiol. Biochem. 2005, 43, 397–406.
- Okada, A.; Shimizu, T.; Okada, K.; Kuzuyama, T.; Koga, J.; Shibuya, N.; Nojiri, H.; Yamane, H. Elicitor induced activation of the methylerythritol phosphate pathway toward phytoalexins biosynthesis in rice. Plant Mol. Biol. 2007, 65, 177–187.
- Dilday, R.H.; Nastasi, P.; Smith, R.J., Jr. Allelopathic observations in rice (Oryza sativa L.) to ducksalad (Heteranthera limosa). Proc. Arkansas. Acad. Sci. 1989, 43, 21–22.
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: Orlando, FL, USA, 1984; pp. 1–422.
- Putnam, A.R.; Tang, C.S. Allelopathy: State of the science. In The Science of Allelopathy; Putnam, A.R., Tang, C.S., Eds.; John Wiley and Sons: Ithaca, NY, USA, 1986; pp. 1–19.
- Inderjit. Plant phenolics in allelopathy. Bot. Rev. 1996, 62, 186–202.
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266.
- Bonanomi, G.; Sicurezza, M.G.; Caporaso, S.; Esposito, A.; Mazzoleni, S. Phytotoxicity dynamics of decaying plant materials. New Phytol. 2006, 169, 571–578.
- Belz, R.G. Allelopathy in crop/weed interactions—An update. Pest Manag. Sci. 2007, 63, 308–326.
- Dilday, R.H.; Lin, J.; Yan, W. Identification of allelopathy in the USDA-ARS rice germplasm collection. Aust. J. Exp. Agric. 1994, 34, 907–910.
- Dilday, R.H.; Yan, W.G.; Moldenhauer, K.A.K.; Gravois, K.A. Allelopathic activity in rice for controlling major aquatic weeds. In Allelopathy in Rice; Olofsdotter, M., Ed.; International Rice Research Institute: Manila, Philippines, 1998; pp. 7–26.
- Hassan, S.M.; Aidy, I.R.; Bastawisi, A.O.; Draz, A.E. Weed management using allelopathic rice varieties in Egypt. In Allelopathy in Rice; Olofsdotter, M., Ed.; International Rice Research Institute: Manila, Philippines, 1998; pp. 27–37.
- Kim, K.U.; Shin, D.H. Rice allelopathy research in Korea. In Allelopathy in Rice; Olofsdotter, M., Ed.; International Rice Research Institute: Manila, Philippines, 1998; pp. 39–43.
- Olofsdotter, M.; Navarez, D.; Rebulanan, M.; Streibig, J.C. Weed-suppressing rice cultivars: Does allelopathy play a role? Weed Res. 1999, 39, 441–454.
- Pheng, S.; Adkins, S.; Olofsdotter, M.; Jahn, G. Allelopathic effects of rice (Oryza sativa L.) on the growth of awnless barnyardgrass (Echinochloa colona (L.) Link): A new form for weed management. Cambodian J. Agri. 1999, 2, 42–49.
- Kato-Noguchi, H.; Ino, T. Assessment of allelopathic potential of root exudate of rice seedlings. Biol. Plant. 2001, 44, 635–638.
- Kato-Noguchi, H.; Ino, T.; Sata, N.; Yamamura, S. Isolation and identification of a potent allelopathic substance in rice root exudates. Physiol. Plant. 2002, 115, 401–405.
- Kato-Noguchi, H.; Ino, T.; Ota, K. Secretion of momilactone A from rice roots to the rhizosphere. J. Plant Physiol. 2008, 165, 691–696.
- Kato-Noguchi, H. Allelopathic substance in rice root exudates: Rediscovery of momilactone B as an allelochemical. J. Plant Physiol. 2004, 161, 271–276.
- Kato-Noguchi, H.; Peters, R.J. The role of momilactones in rice allelopathy. J. Chem. Ecol. 2013, 39, 175–185.
- Schmelz, E.A.; Huffaker, A.; Sims, J.W.; Christensen, S.A.; Lu, X.; Okada, K.; Peters, R.J. Biosynthesis, elicitation and roles of monocot terpenoid phytoalexins. Plant J. 2014, 79, 659–678.
- Kato-Noguchi, H. Convergent or parallel molecular evolution of momilactone A and B: Potent allelochemicals, momilactones have been found only in rice and the moss Hypnum plumaeforme. J. Plant Physiol. 2011, 168, 1511–1516.
- Serra, N.S.; Shanmuganathan, R.; Becker, C. Allelopathy in rice: A story of momilactones, kin recognition, and weed management. J. Exp. Bot. 2021, 72, 4022–4037.
- Zhang, J.; Peters, R.J. Why are momilactones always associated with biosynthetic gene clusters in plants? Proc. Natl. Acad. Sci. USA 2020, 117, 13867–13869.
- Kobayashi, K.; Shigemori, H.; Kato-Noguch, H. Allelopathic potential of Hypnum plumaeforme L. and its allelopathic substances. In Proceedings of the 4th Asia-Pacific Conference on Chemical Ecology, from Biomolecules to Ecosystems an Interactive Chemical Message for our Future, Tsukuba, Japan, 10–14 September 2007; p. 77.
- Nozaki, H.; Hayashi, K.I.; Nishimura, N.; Kawaide, H.; Matsuo, A.; Takaoka, D. Momilactone A and B as allelochemicals from moss Hypnum plumaeforme: First occurrence in bryophytes. Biosci. Biotech. Biochem. 2007, 71, 3127–3130.
- Kato-Noguchi, H.; Kobayashi, K.; Shigemori, H. Allelopathy of the moss Hypnum plumaeforme by the production of momilactone A and B. Weed Res. 2009, 49, 621–627.
- Umemura, K.; Ogawa, N.; Shimura, M.; Koga, J.; Usami, H.; Kono, T. Possible role of phytocassane, rice phytoalexin, in disease resistance of rice against the blast fungus Magnaporthe grisea. Biosci. Biotech. Biochem. 2003, 67, 899–902.
- Dillon, V.M.; Overton, J.; Grayer, R.J.; Harborne, J.B. Differences in phytoalexin response among rice cultivars of different resistance to blast. Phytochemistry 1997, 44, 599–603.
- Toyomasu, T.; Usui, M.; Sugawara, C.; Otomo, K.; Hirose, Y.; Miyao, A.; Hirochik, H.; Okad, K.; Shimizu, T.; Koga, J.; et al. Reverse-genetic approach to verify physiological roles of rice phytoalexins: Characterization of a knockdown mutant of OsCPS4 phytoalexin biosynthetic gene in rice. Physiol. Plant. 2014, 150, 55–62.
- Fukuta, M.; Xuan, T.D.; Deba, F.; Tawata, S.; Dang Khanh, T.; Chung, M.I. Comparative efficacies in vitro of antibacterial, fungicidal, antioxidant, and herbicidal activities of momilatones A and B. J. Plant Interac. 2007, 2, 245–251.
- Gu, C.Z.; Xia, X.M.; Lv, J.; Tan, J.W.; Baerson, S.R.; Pan, Z.Q.; Song, Y.Y.; Zeng, R.S. Diterpenoids with herbicidal and antifungal activities from hulls of rice (Oryza sativa). Fitoterapia 2019, 136, 104183.
- Liu, H.; Li, X.; Xiao, J.; Wang, S. A convenient method for simultaneous quantification of multiple phytohormones and metabolites: Application in study of rice-bacterium interaction. Plant Methods 2012, 8, 1–12.
- Howe, G.A.; Major, I.T.; Koo, A.J. Modularity in jasmonate signaling for multistress resilience. Ann. Rev. Plant Biol. 2018, 69, 387–415.
- Mostafa, S.; Wang, Y.; Zeng, W.; Jin, B. Plant responses to herbivory, wounding, and infection. Int. J. Mol. Sci. 2022, 23, 7031.
- Nguyen, T.H.; Goossens, A.; Lacchini, E. Jasmonate: A hormone of primary importance for plant metabolism. Curr. Opin. Plant Biol. 2022, 67, 102197.
- Hanai, H.; Ishida, S.; Saito, C.; Maita, T.; Kusano, M.; Tamogami, S.; Noma, M. Stimulation of mycelia growth in several mushroom species by rice husks. Biosci. Biotech. Biochem. 2005, 69, 123–127.
- Chung, I.M.; Ali, M.; Ahmad, A.; Chun, S.C.; Kim, J.T.; Sultana, S.; Kim, J.S.; Seo, B.R. Steroidal constituents of rice (Oryza sativa) hulls with Algicidal and Herbicidal activity against blue-green algae and duckweed. Phytochem. Anal. 2007, 18, 133–145.
- Kanno, H.; Hasegawa, M.; Kodama, O. Accumulation of salicylic acid, jasmonic acid and phytoalexins in rice, Oryza sativa, infested by the white-backed planthopper, Sogatella furcifera (Hemiptera: Delphacidae). Appl. Entomol. Zool. 2012, 47, 27–34.
- Wari, D.; Alamgir, K.M.; Mujiono, K.; Hojo, Y.; Tani, A.; Shinya, T.; Nakatania, H.; Galis, I. Brown planthopper honeydew-associated symbiotic microbes elicit momilactones in rice. Plant Signal. Behav. 2019, 14, 1655335.
- Azmi, M.; Abdullah, M.Z.; Fujii, Y. Exploratory study on allelopathic effect of selected Malaysian rice varieties and rice field weed species. J. Trop. Agric. Food Sci. 2000, 28, 39–54.
- Rao, A.N.; Johnson, D.E.; Sivaprasad, B.; Ladaha, J.K.; Mortimer, A.M. Weed management in direct-seeded rice. Adv. Agron. 2007, 93, 153–255.
- Kong, C.H. Rice allelopathy. Allelopathy J. 2008, 22, 261–278.
- Kato-Noguchi, H.; Ota, K.; Ino, T. Release of momilactone A and B from rice plants into the rhizosphere and its bioactivities. Allelopathy J. 2008, 22, 321–328.
- Kato-Noguchi, H.; Hasegawa, M.; Ino, T.; Ota, K.; Kujime, H. Contribution of momilactone A and B to rice allelopathy. J. Plant Physiol. 2010, 167, 787–791.
- Kato-Noguchi, H.; Ota, K.; Kujime, H. Absorption of momilactone A and B by Arabidopsis thaliana L. and the growth inhibitory effects. J. Plant Physiol. 2012, 169, 1471–1476.
- Kato-Noguchi, H.; Ota, K. Biological activities of rice allelochemicals momilactone A and B. Rice Res. 2013, 1, 2.
- Takahashi, N.; Kato, T.; Tsunagawa, M.; Sasaki, N.; Kitahara, Y. Mechanisms of dormancy in rice seeds. II. New growth inhibitors, momilactone-A and -B isolated from the hulls of rice seeds. Jpn. J. Breed. 1976, 26, 91–98.
- Kato, T.; Tsunakawa, M.; Sasaki, N.; Aizawa, H.; Fujita, K.; Kitahara, Y.; Takahashi, N. Growth and germination inhibitors in rice husks. Phytochemistry 1977, 16, 45–48.
- Chung, I.M.; Hahh, S.J.; Ahmad, A. Confirmation of potential herbicidal agents in hulls of rice, Oryza sativa. J. Chem. Ecol. 2005, 31, 1339–1352.
- Toyomasu, T.; Kagahara, T.; Okada, K.; Koga, J.; Hasegawa, M.; Mitsuhashi, W.; Sassa, T.; Yamane, H. Diterpene phytoalexins are biosynthesized in and exuded from the roots of rice seedlings. Biosci. Biotechnol. Biochem. 2008, 72, 562–567.
- Lee, C.W.; Yoneyama, K.; Takeuchi, Y.; Konnai, M.; Tamogami, S.; Kodama, O. Momilactones A and B in rice straw harvested at different growth stages. Biosci. Biotechnol. Biochem. 1999, 63, 1318–1320.
- Chung, I.-M.; Kim, T.K.; Kim, S.H. Evaluation of allelopathic potential and quantification of momilactone A, B from rice hull extracts and assessment of inhibitory bioactivity on paddy field weeds. J. Agric. Food. Chem. 2006, 54, 2527–2536.
- Kato-Noguchi, H.; Ino, T. Rice seedlings release momilactone B into the environment. Phytochemistry 2003, 63, 551–554.
- Kato-Noguchi, H.; Ino, T.; Ichii, M. Changes in release level of momilactone B into the environment from rice throughout its life cycle. Func. Plant Biol. 2003, 30, 995–997.
- Kato-Noguchi, H.; Ino, T. Possible involvement of momilactone B in rice allelopathy. J. Plant Physiol. 2005, 162, 718–721.
- Hawes, M.C.; Gunawardena, U.; Miyasaka, S.; Zhao, X. The role of root border cells in plant defense. Trends Plant Sci. 2000, 5, 128–133.
- Bais, H.P.; Park, S.W.; Weir, T.L.; Callaway, R.M.; Vivanco, J.M. How plants communicate using the underground information superhighway. Trends Plant Sci. 2004, 9, 26–32.
- Badri, D.V.; Vivanco, J.M. Regulation and function of root exudates. Plant Cell Environ. 2009, 32, 666–681.
- Kato-Noguchi, H.; Ino, T. Concentration and release level of momilactone B in the seedlings of eight rice cultivars. J. Plant Physiol. 2005, 162, 965–969.
- Kato-Noguchi, H.; Ino, T.; Kujime, H. The relation between growth inhibition and secretion level of momilactone B from rice root. J. Plant Interact. 2010, 5, 87–90.
- Mennan, H.; Ngouajio, M.; Sahin, M.; Isik, D.; Altop, E.K. Quantification of momilactone B in rice hulls and the phytotoxic potential of rice extracts on the seed germination of Alisma plantago-aquatica. Weed Biol. Manag. 2012, 12, 29–39.
- Zhang, J.; Li, C.; Wu, C.; Xiong, L.; Chen, G.; Zhang, Q.; Wang, S. RMD: A rice mutant database for functional analysis of the rice genome. Nucleic Acids Res. 2006, 34, D745–D748.
- Jeon, J.S.; Lee, S.; Jung, K.H.; Jun, S.H.; Jeong, D.H.; Lee, J.; Kim, C.; Jang, S.; Yang, K.; Nam, J.; et al. T-DNA insertional mutagenesis for functional genomics in rice. Plant J. 2000, 22, 561–570.
- Xu, M.; Galhano, R.; Wiemann, P.; Bueno, E.; Tiernan, M.; Wu, W.; Chung, I.M.; Gershenzon, J.; Sesma, A.T.; Peters, R.J. Genetic evidence for natural product-mediated plant-plant allelopathy in rice (Oryza sativa). New Phytol. 2012, 193, 570–575.
- Kato-Noguchi, H.; Ota, K.; Kujime, H.; Ogawa, M. Effects of momilactone on the protein expression in Arabidopsis germination: Arabidopsis and momilactone. Weed Biol. Manag. 2013, 13, 19–23.
- Finkelstein, R.R.; Tenbarge, K.M.; Shumway, J.E.; Crouch, M.L. Role of ABA in maturation of rapeseed embryos. Plant Physiol. 1985, 78, 630–636.
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.M.; Nonogaki, H. Seeds: Physiology of Development, Germination and Dormancy, 3rd ed; Springer: New York, NY, USA, 2012; pp. 1–408.
- Job, C.; Rajjou, L.; Lovigny, Y.; Belghazi, M.; Job, D. Patterns of protein oxidation in Arabidopsis seeds and during germination. Plant Physiol. 2005, 138, 790–802.
- Kato-Noguchi, H.; Kitajima, S. Momilactone sensitive proteins in Arabidopsis thaliana. Nat. Prod. Commun. 2015, 10, 729–732.
- Ohyama, K.; Suzuki, M.; Kikuchi, J.; Saito, K.; Muranaka, T. Dual biosynthetic pathways to phytosterol via cycloartenol and lanosterol in Arabidopsis. Proc. Natl. Acad. Sci. USA 2009, 106, 725–730.
- Kuroha, T.; Okuda, A.; Arai, M.; Komatsu, Y.; Sato, S.; Kato, T.; Tabata, S.; Satoh, S. Identification of Arabidopsis subtilisin-like serine protease specifically expressed in root stele by gene trapping. Physiol. Plant. 2009, 137, 281–288.
- Gallardo, K.; Job, C.; Groot, S.P.C.; Puype, M.; Demol, H.; Vandekerckhove, J.; Job, D. Proteomics of Arabidopsis seed germination. A comparative study of wild-type and gibberellin-deficient seeds. Plant Physiol. 2002, 129, 823–837.
- Eastmond, P.J.; Graham, I.A. Re-examining the role of the glyoxylate cycle in oilseeds. Trends Plant Sci. 2001, 6, 72–77.
- Stacy, R.A.P.; Nordeng, T.W.; Culianez-Macia, F.A.; Reidunn, B.; Aalen, R.B. The dormancy-related peroxiredoxin anti-oxidant, PER1, is localized to the nucleus of barley embryo and aleurone cells. Plant J. 1999, 19, 1–8.
- Kim, S.Y.; Paeng, S.K.; Nawkar, G.M.; Maibam, P.; Lee, E.S.; Kim, K.S.; Lee, D.H.; Park, D.J.; Kang, S.B.; Kim, M.R.; et al. The 1-cys peroxiredoxin, a regulator of seed dormancy, functions as a molecular chaperone under oxidative stress conditions. Plant Sci. 2011, 181, 119–124.
- Fanucchi, F.; Alpi, E.; Olivieri, S.; Cannistraci, C.V.; Bachi, A.; Alpi, A.; Alessio, M. Acclimation increases freezing stress response of Arabidopsis thaliana at proteome level. Biochim. Biophys. Acta 2012, 1824, 813–825.
- Neuefeind, T.; Reinemer, P.; Bieseler, B. Plant glutathione S-transferases and herbicide detoxification (Review). Biol. Chem. 1997, 378, 199–205.
- Haslekås, C.; Viken, M.K.; Grini, P.E.; Nygaard, V.; Nordgard, S.H.; Meza, T.J.; Aalen, R.B. Seed 1-cysteine peroxiredoxin antioxidants are not involved in dormancy, but contribute to inhibition of germination during stress. Plant Physiol. 2003, 133, 1148–1157.
- Kim, K.U.; Shin, D.H.; Lee, I.J.; Kim, H.Y.; Kim, K.U.; Shin, D.H. Rice allelopathy in Korea. In Rice Allelopathy; Kim, K.U., Shin, D.H., Eds.; Kyungpook National University: Taegu, Korea, 2000; pp. 57–82.
- Song, B.; Xiong, J.; Fang, C.; Qiu, L.; Lin, R.; Liang, Y.; Lin, W. Allelopathic enhancement and differential gene expression in rice under low nitrogen treatment. J. Chem. Ecol. 2008, 34, 688–695.
- Shen, L.; Lin, W. Effects of phosphorus levels on allelopathic potential of rice co-cultured with barnyardgrass. Allelopathy J. 2007, 19, 393–402.
- Kato-Noguchi, H. Barnyard grass-induced rice allelopathy and momilactone B. J. Plant Physiol. 2011, 168, 1016–1020.
- Zhao, H.; Li, H.; Kong, C.; Xu, X.; Liang, W. Chemical response of allelopathic rice seedlings under varying environmental conditions. Allelopathy J. 2005, 15, 105–110.
- Kong, C.H.; Li, H.B.; Hu, F.; Xu, X.H.; Wang, P. Allelochemicals released by rice roots and residues in soil. Plant Soil 2006, 288, 47–56.
- Li, L.L.; Zhao, H.H.; Kong, C.H. (–)-Loliolide, the most ubiquitous lactone, is involved in barnyardgrass-induced rice allelopathy. J. Exp. Bot. 2020, 71, 1540–1550.
- Kato-Noguchi, H. The chemical cross talk between rice and barnyardgrass. Plant Signal. Behav. 2011, 6, 1207–1209.
- Kato-Noguchi, H.; Ino, T. The chemical-mediated allelopathic interaction between rice and barnyard grass. Plant Soil 2013, 370, 267–275.
- Yang, X.F.; Kong, C.H. Interference of allelopathic rice with paddy weeds at the root level. Plant Biol. 2017, 19, 584–591.
- Bi, H.H.; Zeng, R.Z.; Su, L.M.; An, M.; Luo, S.H. Rice allelopathy induced by methyl jasmonate and methyl salicylate. J. Chem. Ecol. 2007, 33, 1089–1103.
- Kato-Noguchi, H.; Kujime, H.; Ino, T. UV-induced momilactone B accumulation in rice rhizosphere. J. Plant Physiol. 2007, 164, 1548–1551.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
888
Revisions:
2 times
(View History)
Update Date:
30 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




