Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Suvankar Ghorai | -- | 1707 | 2023-03-29 05:28:24 | | | |
| 2 | Conner Chen | Meta information modification | 1707 | 2023-03-31 04:32:44 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Dutta, R.; Rajendran, K.; Jana, S.K.; Saleena, L.M.; Ghorai, S. Extensive Use of Graphene in Detection of Viruses. Encyclopedia. Available online: https://encyclopedia.pub/entry/42600 (accessed on 07 February 2026).
Dutta R, Rajendran K, Jana SK, Saleena LM, Ghorai S. Extensive Use of Graphene in Detection of Viruses. Encyclopedia. Available at: https://encyclopedia.pub/entry/42600. Accessed February 07, 2026.
Dutta, Reshmi, Kokilavani Rajendran, Saikat Kumar Jana, Lilly M. Saleena, Suvankar Ghorai. "Extensive Use of Graphene in Detection of Viruses" Encyclopedia, https://encyclopedia.pub/entry/42600 (accessed February 07, 2026).
Dutta, R., Rajendran, K., Jana, S.K., Saleena, L.M., & Ghorai, S. (2023, March 29). Extensive Use of Graphene in Detection of Viruses. In Encyclopedia. https://encyclopedia.pub/entry/42600
Dutta, Reshmi, et al. "Extensive Use of Graphene in Detection of Viruses." Encyclopedia. Web. 29 March, 2023.
Copy Citation
Graphene and its derivatives offer considerable merits in biosensing applications for pathogenic virus detection.
graphene
diagnostics
graphene oxide
1. Introduction
Dengue virus (DENV), a member of the family Flaviviridae, is a single-stranded positive-sense RNA virus with a genome of 11 kb [1] and is transmitted by the female Aedes aegyptii mosquito vector [2]. DENV consists of three structural proteins, namely the membrane-associated protein M, core protein C, and envelope protein E, which are responsible for shaping the structure of the virus. In addition, it contains seven non-structural proteins, NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5, which play a role in the replication of the virus and many more cellular functions. The different serotypes of dengue are DENV-1, DENV-2, DENV-3, and DENV-4. Infection caused due to any of these serotypes is usually asymptomatic or flu-like symptoms develop, which might lead to severe dengue hemorrhagic fever or dengue shock syndrome [1]. A global study showed that about 390 million people get infected by dengue annually, of which 96 million exhibit clinical manifestations [2]. As of 23 September 2022, 110,473 dengue cases have been reported in India [3].
Conventional methods for dengue detection are laborious, time-consuming, costly and require skilled professionals. Hence, nanomaterial-based sensors can be a good option for dengue detection as they surpass all the issues mentioned above that conventional methods face. Nanomaterials have exceptional qualities, making them an ideal platform for usage in biosensors. They can be used for certain purposes by regulating physical and chemical properties, namely size, morphology, surface charge and solubility [4]. Such properties make nanomaterials ideal for their utility in biosensors to enhance target-specific reactions which respond to biochemical reactions such as temperature, pH, and the existence of enzymes [5]. Nanomaterials might boost the sensitivity of present diagnostic techniques due to features like high reactivity, adsorption capacity, particle size, and physicochemical bonding capacity. Utilizing nanoparticles might lead to enhancements in technology regarding time, specificity, mobility and convenience [4].
Graphene is one such material which is being used extensively in the development of sensors. Graphene is a premium material formed by a single-thick layer of sp2 hybridized carbon atoms arranged in a honeycomb lattice [6]. It is an extremely thin material and occurs as a few layers of graphite [7]. It displays outstanding electrochemical properties such as high thermal conductivity (above 3000 W m K−1) and low redox potential (−0.5 V–1.2) [8]. Optical absorption in the infrared region, a high surface area (2630 m2/g), completely impermeable to gas [9], and mechanical strength (about 1100 GPa) [10], are some of the properties for which graphene is used for diagnostic purposes [9][10]. The different forms of graphene include graphene oxide (GO), reduced graphene oxide (rGO), graphene sheets [7], and layered graphenes such as a few layered graphenes and multilayered graphene [7][11]. Some of the widely studied derivatives of graphene are GO and rGO (Figure 1). They display properties similar to graphene, such as flexibility, transparency, and low cytotoxicity. The hydrophilic nature and presence of reactive functional groups make them an ideal material to be used in a biosensor [12]. The hydrophilic feature plays a key role in assembling the biosensor as it permits film preparation by drop casting, spin coating, ink-jet printing, and electrode material processing. Research on these materials has successfully led to their use in different fields such as electronics, optics, sensors, and filtration [8]. The synthesis of GO involves vigorous oxidation of graphite–graphene solutions and hence leads to the production of a complex material having a variety of functional groups whose properties and quantity rely on the method of preparation. There is a tendency for functional groups of GO to get modified on the surface due to the attachment of redox species such as enzymes, peptides, or DNA and RNA [13]. In an acidic medium, graphite is oxidized to GO using Hummers’ and Offemans’ methods [14]. GO’s disrupted sp2 hybridized bonding is responsible for its electrically insulating nature, however, GO can also be a good semiconductor when oxidized extensively [15].
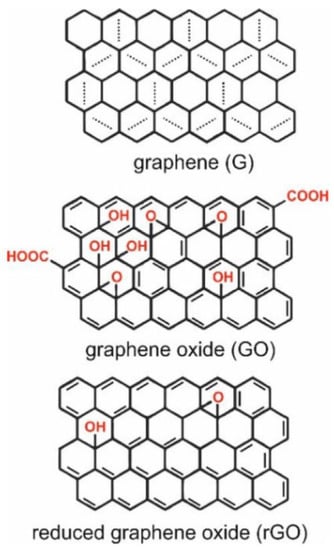
When graphene is converted to GO, it increases the hydrophilicity of the surface and results in the formation of big functional groups [18]. The presence of these oxygenated groups (hydroxyl and epoxy) thus aids in the formation of a stable dispersion in aqueous media and other polar solvents [14] and further enables biochemical and bioconjugation reactions to occur with ease [14][19]. GO is a remarkable adsorbant of proteins and antibodies, making it a useful biomaterial [20]. GO, on reduction, produces rGO by removing the oxidized functional groups of GO [21]. The reduction procedure establishes considerable changes involving surface properties in rGO [20]. The absence of most of the oxygen-containing functional groups from GO partly restores the sp2 structure [22] and improves the rGO conductivity [22][23]. Some unique properties of rGO involve conductivity, fluorescence quenching, peroxide-like enzyme activity, intrinsic Raman activity, oxidation-reduction capacity, and the ability to anchor different nanoparticles, enzymes, proteins, and nucleic acids [13]. While GO has inferior electrical properties, it is a better conductor and can retain its ability to disperse in water [24]. GO and rGO can be utilized in the preparation of a wide range of graphene-based nanocomposites. Some materials incorporated with graphene derivatives are nanoparticles, quantum dots, nanoclusters, polymers, and a variety of biomolecules [25]. Graphene and its derivatives undergo non-covalent interactions such as London forces, polarization, hydrophobic effects, and electrostatic force of attraction with adsorbates [26]. Such interactions confirm the adsorbate’s contact with a surface, leading to alterations in electronic characteristics that could be used in sensing. GO possesses a high loading ratio for biomolecules which can go up to 200% compared to other nanocarriers. This could be a useful feature for the development of sensitive electrochemical immunosensors [27]. A noteworthy point could be that 2D functionalized graphene derivatives and composites might undergo restacking. Restacking happens when multilayer Van der Waals materials are formed due to the powerful interaction of non-covalent interlayers. Developing a firm 3D network is crucial for yielding new and stable graphene nanocomposites that do not undergo restacking and possess beneficial features such as high surface area, tunable electronic features, improved conductivity and electrocatalytic characteristics, available inner space, and improved mechanical stability. Such features are suitable for systematic and selective biosensing [28].
Graphene and its derivatives offer considerable merits in biosensing applications for pathogenic virus detection. These materials are more conductive electrically and thermally, flexible, not heavy, biocompatible, and have a large surface area which is a gold standard for electrochemical platforms. Graphene, GO, and rGO successfully quench photoluminescence. Incorporating befitting nanomaterials into graphene and its derivatives can improve the quality of the known detection methods, e.g., Au–Ag nanoparticles transmit a notable surface-enhanced Raman scattering (SERS) signal. The sensor’s sensitivity is related to the transforming electronic features of graphene when the virus adsorbates are present. The large surface area of graphene-based materials helps the adsorption of analytes from the environment. When antibodies, aptamers, or nucleic acids are incorporated into graphene and its derivatives, they can bind to counterparts specific to a particular virus. This enhances the selectivity of a biosensor [13]. Therefore, biosensors utilizing graphene and its derivatives can be used efficiently for the detection of not only the dengue virus but also other upcoming pathogenic viruses.
2. Extensive Use of Graphene in the Detection of Viruses
The light, chemically stable, and conductive nature of graphene makes it a success in its use in the detection of viruses. The incorporation of functional groups into the hybrid structure leads to swift virus detection [13]. Graphene and its derivatives are often used in the detection of viruses due to their high specificity and less time expenditure. Polymerase chain reaction (PCR) and loop-mediated isothermal amplification (LAMP) are thought to be sensitive techniques in the detection of the foot and mouth disease virus (FMDV) [29]. PCR has certain limitations, which lead to the generation of false positive results. A nano-PCR system, which uses graphene oxide–gold nanoparticles nanocomposites (GO-AuNPs), exhibits improved PCR efficiency [30].
In recent years rGO has found wide application in electrochemical biosensing for the detection of the H1N1 (haemagglutinin type 1 and neuraminidase Type 1) strain of the influenza virus. Label-free detection of the H1N1 virus was done using an electrochemical immunosensor coated with rGO and incorporated with a microfluidic platform [31]. The rGO obtained from shellac, used in label-free electrochemical biosensors, proved to be a cost-effective method for the detection of the influenza virus H1N1 [32].
Low-cost ultrasensitive biosensors using graphene and its derivatives exhibit excellent and promising results in the detection of human papilloma virus (HPV16) DNA in patients suffering from HPV16-positive head and neck cancer [33]. Reduced graphene oxide field effect transistors (rGO-FETs) are used for highly sensitive and selective detection of HPV16 E7 protein. To acquire selective sensing, specific probes are used on the conducting rGO channel of the transistor [34].
Sulfonated magnetic nanoparticles functionalized with reduced graphene oxide (SMRGO), having antiviral properties, were used to eliminate herpes simplex virus type 1 (HSV-1). Graphene has remarkable photothermal properties that inactivate the viruses captured with near-infrared (NIR) irradiation [35]. Graphene-mediated surface-enhanced Raman scattering (G-SERS) is often used to identify norovirus (NoV) in a sample of human faeces. In this unique biosensing system, magnetic derivative molybdenum trioxide nanocubes functioned as the SERS nanotag, and single-layer graphene oxide (SLGO) served as the signal reporter. With this technology, rapid signal amplification for the detection of NoV could be achieved [36].
Detection of the hepatitis C virus (HCV) could be made using a highly sensitive assay that involves the use of reduced graphene oxide nanosheets (rGONS) and a hybridization chain reaction (HCR) amplification technique. This method uses the selective adsorption capacity of rGONS to fluorophore probes with different conformations [37]. The enhanced fluorescence quenching capacity of rGO leads to inverse upregulation of the assay sensitivity by a reduction in the background signal of the probes [38][39][40]. The methods of detecting different viruses using graphene are listed in Table 1.
Table 1. Graphene-based detection for different viruses. (H1N1—haemagglutinin type 1 and neuraminidase type 1 influenza virus, HPV—human papilloma virus, HSV1—herpes simplex virus 1, NoV—norovirus, HCV—hepatitis C virus).
| Virus Name | Electrode Material | Method of Detection | Reference |
|---|---|---|---|
| H1N1 | Graphene oxide | Electrochemical | [31] |
| H1N1 | Reduced graphene oxide | Electrochemical | [32] |
| HPV | Reduced graphene oxide | Electrochemical | [33] |
| HPV | Reduced graphene oxide | Field effect transistor | [34] |
| HSV1 | Reduced graphene oxide | Optical | [35] |
| NoV | Graphene oxide | Optical | [36] |
| HCV | Reduced graphene oxide | Optical | [37] |
References
- Idrees, S.; Ashfaq, U.A. A Brief Review on Dengue Molecular Virology, Diagnosis, Treatment and Prevalence in Pakistan. Genet. Vaccines Ther. 2012, 10, 6.
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. T he Global Distribution and Burden of Dengue. Nature 2013, 496, 504–507.
- Dengue Worldwide Overview. Available online: https://www.ecdc.europa.eu/en/dengue-monthly (accessed on 30 December 2022).
- Lim, J.-W.; Ahn, Y.-R.; Park, G.; Kim, H.-O.; Haam, S. Application of Nanomaterials as an Advanced Strategy for the Diagnosis, Prevention, and Treatment of Viral Diseases. Pharmaceutics 2021, 13, 1570.
- Huang, H.; Lovell, J.F. Advanced Functional Nanomaterials for Theranostics. Adv. Funct. Mater. 2017, 27, 1603524.
- He, J.; Fang, L. Controllable Synthesis of Reduced Graphene Oxide. Curr. Appl. Phys. 2016, 16, 1152–1158.
- Priyadarsini, S.; Mohanty, S.; Mukherjee, S.; Basu, S.; Mishra, M. Graphene and Graphene Oxide as Nanomaterials for Medicine and Biology Application. J. Nanostruct. Chem. 2018, 8, 123–137.
- Sharma, D.; Kanchi, S.; Sabela, M.I.; Bisetty, K. Insight into the Biosensing of Graphene Oxide: Present and Future Prospects. Arab. J. Chem. 2016, 9, 238–261.
- Bai, H.; Li, C.; Shi, G. Functional Composite Materials Based on Chemically Converted Graphene. Adv. Mater. 2011, 23, 1089–1115.
- Chen, D.; Feng, H.; Li, J. Graphene Oxide: Preparation, Functionalization, and Electrochemical Applications. Chem. Rev. 2012, 112, 6027–6053.
- Acik, M.; Mattevi, C.; Gong, C.; Lee, G.; Cho, K.; Chhowalla, M.; Chabal, Y.J. The Role of Intercalated Water in Multilayered Graphene Oxide. ACS Nano 2010, 4, 5861–5868.
- Chung, C.; Kim, Y.-K.; Shin, D.; Ryoo, S.-R.; Hong, B.H.; Min, D.-H. Biomedical Applications of Graphene and Graphene Oxide. Acc. Chem. Res. 2013, 46, 2211–2224.
- Vermisoglou, E.; Panáček, D.; Jayaramulu, K.; Pykal, M.; Frébort, I.; Kolář, M.; Hajdúch, M.; Zbořil, R.; Otyepka, M. Human Virus Detection with Graphene-Based Materials. Biosens. Bioelectron. 2020, 166, 112436.
- Konkena, B.; Vasudevan, S. Understanding Aqueous Dispersibility of Graphene Oxide and Reduced Graphene Oxide through p K a Measurements. J. Phys. Chem. Lett. 2012, 3, 867–872.
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The Chemistry of Graphene Oxide. Chem. Soc. Rev. 2010, 39, 228–240.
- Tadyszak, K.; Wychowaniec, J.K.; Litowczenko, J. Biomedical Applications of Graphene-Based Structures. Nanomaterials 2018, 8, 944.
- Popov, I.A.; Bozhenko, K.V.; Boldyrev, A.I. Is Graphene Aromatic? Nano Res. 2012, 5, 117–123.
- Mohan, V.B.; Lau, K.; Hui, D.; Bhattacharyya, D. Graphene-Based Materials and Their Composites: A Review on Production, Applications and Product Limitations. Compos. Part B 2018, 142, 200–220.
- Park, S.; Ruoff, R.S. Chemical Methods for the Production of Graphenes. Nat. Nanotechnol. 2009, 4, 217–224.
- Raslan, A.; Saenz del Burgo, L.; Ciriza, J.; Pedraz, J.L. Graphene Oxide and Reduced Graphene Oxide-Based Scaffolds in Regenerative Medicine. Int. J. Pharm. 2020, 580, 119226.
- Omar, N.A.S.; Fen, Y.W.; Abdullah, J.; Sadrolhosseini, A.R.; Mustapha Kamil, Y.; Fauzi, N.‘I.M.; Hashim, H.S.; Mahdi, M.A. Quantitative and Selective Surface Plasmon Resonance Response Based on a Reduced Graphene Oxide–Polyamidoamine Nanocomposite for Detection of Dengue Virus E-Proteins. Nanomaterials 2020, 10, 569.
- Zhang, Q.; Liu, X.; Meng, H.; Liu, S.; Zhang, C. Reduction Pathway-Dependent Cytotoxicity of Reduced Graphene Oxide. Environ. Sci. Nano 2018, 5, 1361–1371.
- Zhang, P.; Li, Z.; Zhang, S.; Shao, G. Recent Advances in Effective Reduction of Graphene Oxide for Highly Improved Performance Toward Electrochemical Energy Storage. Energy Environ. Mater. 2018, 1, 5–12.
- Lu, C.; Huang, P.-J.J.; Liu, B.; Ying, Y.; Liu, J. Comparison of Graphene Oxide and Reduced Graphene Oxide for DNA Adsorption and Sensing. Langmuir 2016, 32, 10776–10783.
- Krishnan, S.K.; Singh, E.; Singh, P.; Meyyappan, M.; Nalwa, H.S. A Review on Graphene-Based Nanocomposites for Electrochemical and Fluorescent Biosensors. RSC Adv. 2019, 9, 8778–8881.
- Georgakilas, V.; Tiwari, J.N.; Kemp, K.C.; Perman, J.A.; Bourlinos, A.B.; Kim, K.S.; Zboril, R. Noncovalent Functionalization of Graphene and Graphene Oxide for Energy Materials, Biosensing, Catalytic, and Biomedical Applications. Chem. Rev. 2016, 116, 5464–5519.
- Liu, Z.; Robinson, J.T.; Sun, X.; Dai, H. PEGylated Nanographene Oxide for Delivery of Water-Insoluble Cancer Drugs. J. Am. Chem. Soc. 2008, 130, 10876–10877.
- Vermisoglou, E.C.; Jakubec, P.; Malina, O.; Kupka, V.; Schneemann, A.; Fischer, R.A.; Zbořil, R.; Jayaramulu, K.; Otyepka, M. Hierarchical Porous Graphene–Iron Carbide Hybrid Derived From Functionalized Graphene-Based Metal–Organic Gel as Efficient Electrochemical Dopamine Sensor. Front. Chem. 2020, 8, 544.
- Bath, C.; Scott, M.; Sharma, P.M.; Gurung, R.B.; Phuentshok, Y.; Pefanis, S.; Colling, A.; Singanallur Balasubramanian, N.; Firestone, S.M.; Ungvanijban, S.; et al. Further Development of a Reverse-Transcription Loop-Mediated Isothermal Amplification (RT-LAMP) Assay for the Detection of Foot-and-Mouth Disease Virus and Validation in the Field with Use of an Internal Positive Control. Transbound. Emerg. Dis. 2020, 67, 2494–2506.
- Kim, J.-W.; Kim, M.; Lee, K.K.; Chung, K.H.; Lee, C.-S. Effects of Graphene Oxide-Gold Nanoparticles Nanocomposite on Highly Sensitive Foot-and-Mouth Disease Virus Detection. Nanomaterials 2020, 10, 1921.
- Singh, R.; Hong, S.; Jang, J. Label-Free Detection of Influenza Viruses Using a Reduced Graphene Oxide-Based Electrochemical Immunosensor Integrated with a Microfluidic Platform. Sci. Rep. 2017, 7, 42771.
- Joshi, S.R.; Sharma, A.; Kim, G.-H.; Jang, J. Low Cost Synthesis of Reduced Graphene Oxide Using Biopolymer for Influenza Virus Sensor. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 108, 110465.
- Farzin, L.; Sadjadi, S.; Shamsipur, M.; Sheibani, S. Electrochemical Genosensor Based on Carbon Nanotube/Amine-Ionic Liquid Functionalized Reduced Graphene Oxide Nanoplatform for Detection of Human Papillomavirus (HPV16)-Related Head and Neck Cancer. J. Pharm. Biomed. Anal. 2020, 179, 112989.
- Aspermair, P.; Mishyn, V.; Bintinger, J.; Happy, H.; Bagga, K.; Subramanian, P.; Knoll, W.; Boukherroub, R.; Szunerits, S. Reduced Graphene Oxide-Based Field Effect Transistors for the Detection of E7 Protein of Human Papillomavirus in Saliva. Anal. Bioanal. Chem. 2020, 413, 779–787.
- Deokar, A.R.; Nagvenkar, A.P.; Kalt, I.; Shani, L.; Yeshurun, Y.; Gedanken, A.; Sarid, R. Graphene-Based “Hot Plate” for the Capture and Destruction of the Herpes Simplex Virus Type 1. Bioconjug. Chem. 2017, 28, 1115–1122.
- Achadu, O.J.; Abe, F.; Suzuki, T.; Park, E.Y. Molybdenum Trioxide Nanocubes Aligned on a Graphene Oxide Substrate for the Detection of Norovirus by Surface-Enhanced Raman Scattering. ACS Appl. Mater. Interfaces 2020, 12, 43522–43534.
- Fan, J.; Yuan, L.; Liu, Q.; Tong, C.; Wang, W.; Xiao, F.; Liu, B.; Liu, X. An Ultrasensitive and Simple Assay for the Hepatitis C Virus Using a Reduced Graphene Oxide-Assisted Hybridization Chain Reaction. Analyst 2019, 144, 3972–3979.
- Liu, M.; Song, J.; Shuang, S.; Dong, C.; Brennan, J.D.; Li, Y. A Graphene-Based Biosensing Platform Based on the Release of DNA Probes and Rolling Circle Amplification. ACS Nano 2014, 8, 5564–5573.
- Abdelhamid, H.N.; Hussein, K.H. Graphene Oxide as a Carrier for Drug Delivery of Methotrexate. Biointerface Res. Appl. Chem. 2021, 11, 14726–14735.
- Kim, M.-G.; Shon, Y.; Lee, J.; Byun, Y.; Choi, B.-S.; Kim, Y.B.; Oh, Y.-K. Double Stranded Aptamer-Anchored Reduced Graphene Oxide as Target-Specific Nano Detector. Biomaterials 2014, 35, 2999–3004.
More
Information
Subjects:
Nanoscience & Nanotechnology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
592
Revisions:
2 times
(View History)
Update Date:
31 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




