
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Thomas Miedaner | -- | 2815 | 2023-03-27 16:50:31 | | | |
| 2 | Thomas Miedaner | Meta information modification | 2815 | 2023-03-27 17:06:42 | | | | |
| 3 | Catherine Yang | Meta information modification | 2815 | 2023-03-28 02:42:28 | | |
Video Upload Options
Toxigenic Fusarium species are among the most important plant pathogens in agriculture. They may infect nearly every plant species and comprise about 300 phylospecies. The worldwide most important toxigenic species is F. graminearum producing the trichothecenes deoxynivalenol or nivalenol and the estrogenic zearalenone. Among the economically most important diseases are Fusarium head blight (FHB) of cereals, Fusarium crown rot of wheat and barley, Gibberella/Fusarium ear and stalk rot of maize. Mycotoxins are harmful to humans and animals and a great worldwide threat. The global economic losses caused by toxigenic Fusarium diseases are immense. They can only be controlled by a combination of measures including agronomic practices and resistant varieties. With genomic techniques new insights into the Fusarium-host pathosystem will be possible.
1. Introduction and Taxonomy
Fusarium is a large genus of fungi found in natural and agricultural environments on all continents (except Antarctica)[1]. The genus was first described in 1809 by the German naturalist Johann Heinrich Friedrich Link for species with "canoe-shaped conidia ... characterised by a foot-shaped or notched base to the basal cell"[2]. Fusarium refers to the asexual ('anamorphic') haploid stage of growth in which macroconidia and/or microconidia are produced, depending on the species. The sexual stage ('teleomorph') of most Fusarium species belongs to Gibberella or Nectria leading to production of ascospores. For many species no sexual stage has been observed, yet. Another spore form are chlamydospores, thick-walled spores for long-term survival in the soil. According to the International Code of Nomenclature for Algae, Fungi and Plants (ICN) single names for fungal species should be used since 2011, and by most taxonomists “Fusarium” was chosen[3]. Taxonomy is a big challenge in this genus because of the high diversity among and within species. Orginally based only on morphological features, molecular approaches brought the term “species complex” where new species are defined based on their molecular phylogeny. The F. graminearum species complex (FGSC) currently comprises around 15 lineages given species rank (“phylospecies”) and differing by sequences of defined genes and geographical occurrence[4]. However, several of these lineages are interfertile, thus representing a single biological species. According to sequence data, the genus Fusarium now includes at least 300 phylospecies, 20 species complexes and nine lineages. The term "toxigenic Fusarium species" does not include F. oxysporum, because this species does not produce mycotoxins
2. Diseases and Mycotoxins
Toxigenic Fusarium species are plant pathogens infecting hundreds of species from apples to zucchini, however, some Fusarium spp. can also challenge animals and immunocompromised humans. In plants, two categories of symptoms are observed: blights and rots, like Fusarium head blight or Gibberella ear rot (Figure 1), or cankers and growth distortions, like bakanae and crazy top (Table 1).
|
|
|
|
(a) |
(b) |
Figure 1. The two main diseases of Fusarium spp. in Central Europe: (a) Fusarium head blight in wheat caused by Fusarium culmorum, (b) Gibberella ear rot in maize caused by F. graminearum, both from artificial infection.
Table 1. Examples of economically important diseases caused by toxigenic Fusarium species.
|
Disease |
Causing species |
Hosts |
Region1 |
|
Seedling blight |
F. graminearum, F. culmorum, F. poae, |
Small-grain cereals |
Temp |
|
Fusarium crown rot |
F. pseudograminearum |
Wheat, barley |
Temp |
|
Gibberella ear rot |
F. graminearum |
Maize |
Temp |
|
Fusarium ear rot |
F. verticillioides, F. proliferatum, |
Maize, Sorghum |
Temp, |
|
Stalk rot |
F. graminearum, F. equiseti, F. culmorum |
Maize |
Temp |
|
Fusarium (tuber) dry rot |
Fusarium sambucinum, (Fusarium solani) |
Potato |
Temp |
|
Pitch canker |
F. circinatum |
Pinus spp. |
Temp, |
|
Bakanae |
F. fujikuroi |
Rice |
Trop |
|
Crazy top |
F. sacchari |
Sugarcane |
Trop |
1 Temp=temperate regions, trop = subtropical/tropical regions.
Toxigenic Fusarium species can infect all plant parts in all growth stages (seedlings, roots and foots, crowns, leaves, heads, ears). They are generally unspecialized and some species can infect a wide array of host plants. F. culmorum, for example, may cause diseases in about 40 plant species including cereals, potato, apple, sugar beet, asparagus, carnation, leak, peas, turfgrasses. On the other hand, the same disease can be caused by an array of Fusarium spp (Table 1). They usually survive in plant debris or soil and infect by rain-splashed conidia or airborne ascospores when the weather is favorable. Different species have different temperature optima. F. culmorum and F. langsethiae prefer cool temperate climate, F. graminearum and F. verticillioides predominate in warm temperate climate. Others are preferring subtropical or tropical climate (F. longipes, F. scirpi). Fusarium species are considered as hemibiotrophs. After a short biotrophic phase during penetration they transition to necrotrophs as the infection progresses. They produce a wide variety of toxins, some of them are known to support the infection i.e., are true mycotoxins, such as deoxynivalenol (DON) (Table 2). One species can produce several mycotoxins and, conversely, the same mycotoxin can be produced by several species.
Table 2. Most important mycotoxins produced by Fusarium species.
|
Mycotoxin |
Producing species (examples) |
|
Trichothecene, type A |
F. poae, F. sporotrichioides, F. langsethiae |
|
Trichothecene, type B |
F. graminearum, F. culmorum, F. poae |
|
Zearalenone (ZON) |
F. graminearum, F. culmorum |
|
Fumonisins (FUM) |
F. verticillioides, F. proliferatum |
|
Moniliformin |
F. avenaceum, F. subglutinans, F. temperatum, F. fujikuroi |
|
Beauvericin |
F. avenaceum, F. subglutinans, F. temperatum, F. fujikuroi |
Mycotoxins have a great diversity, the trichothecenes, for example, are a family of about 200 substances[5]. Also within each mycotoxin different chemotypes might exist, for example within DON the precursors 3-Acetyl-DON or 15-Acetyl DON can be found, depending on the isolate. Isolates of F. graminearum and F. culmorum are either producing DON or NIV. Among the foods most frequently contaminated with Fusarium toxins in the European Union (EU), cereals ranked first, and of these, maize and wheat had the highest mycotoxin levels. These mycotoxins are of great concern because they endanger global food security and animal health[6]. Fusarium toxins have a very broad spectrum of action. Trichothecenes block protein and DNA synthesis, causing cell damage that can lead to nausea, vomiting, bloody diarrhea, and kidney damage. Immunosuppressive, embryotoxic and teratogenic effects have also been observed. Zearalenone causes fertility problems and even abortions in female livestock caused by its strong estrogenic activity. Fumonisins are considered highly carcinogenic and have been implicated in oesophageal and lung cancer in southern Africa and China.
The global economic losses caused by Fusarium diseases are immense. They do not only reduce grain yield, but also result in reduced baking, malting or feeding quality and mycotoxin contamination, that might downgrade the harvest. As the same Fusarium species can infect both wheat and maize (Table 1), a large proportion of the acreage in the temperate climate zone is affected. In a global survey of disease burden, Fusarium head blight was the second most important wheat disease with an estimated global yield loss of 2.85% and the highest yield loss in China (8.75%)[7]. Fusarium stalk rot ranked first among maize diseases, reducing yield by 4.58%, with the highest loss in the Indo-Gangetic Plain (5.84%). Fusarium ear rot in maize ranked fourth with a global loss of 2.38%, again with the highest loss in the Indo-Gangetic Plain (4.52%). In the (sub)tropical regions, maize is one of the most important staple crops for human nutrition, especially in Central and South America and Africa where daily 200 – 400 g maize are consumed per capita on a national level.
3. Genetics and Genomics of Fusarium Aggressiveness and Host Resistance
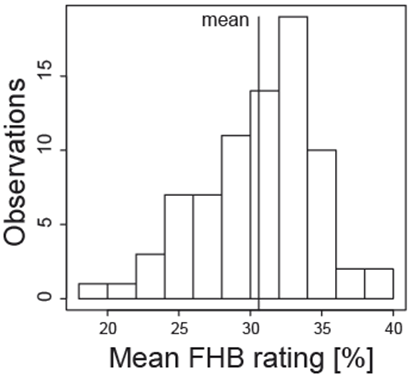
Only a few toxigenic Fusarium species are intensively examined for their within-species variation. From early on, the large variability of isolates have been observed for colony growth characteristics, mycelium color or spore production. This could be confirmed for phenotypic traits in field experiments with artificial FHB infection. Aggressiveness is “the quantitative ability of an isolate to cause disease on a susceptible host”[8]. Large genetic variation of aggressiveness measured by symptom development and DON production was detected among 77 isolates of F. graminearum in multi-environmental field trials (Figure2a, b).
Isolates of F. culmorum and F. graminearum are not very specific in their host range. They can infect all cereal species. A similar ranking for all cereal species and tested cultivars was found. The NIV-producing isolate was the least aggressive isolate compared to the DON-producing isolates. Despite large differences on the susceptibility of the host side, aggressiveness was a stable, highly heritable trait. Similarly, variation in aggressiveness can be found in other diseases.
|
|
|
|
Figure 2. (a) Aggressiveness and (b) deoxynivalenol (DON) production of 77 isolates of Fusarium graminearum tested on a susceptible winter wheat genotype across four location-year combinations;[9] (c) Resistance of 585 winter wheat genotypes tested by an aggressive Fusarium culmorum isolate across five location-year combinations
Aggressiveness is inherited quantitatively, i.e. by many genes. There is no race specifity of the toxigenic Fusarium species. All isolates react similarly to hosts with different resistance reactions, no significant isolate × genotype interaction occurs.
More than 19 complete Fusarium genomes have been published that are available in different data bases although not all are annotated[10]. Candidate-gene based association mapping of F. graminearum[9] and resequencing of 92 isolates of F. culmorum[11] showed that single genes have only smaller effects on aggressiveness. In the latter study, 20 and 27 single nucleotide polymorphisms (SNPs) were detected in wheat to control aggressiveness and DON content, respectively, of which 10 overlapped. Most SNPs had only a small effect, however, four SNPs showed a higher proportion of genetic variance ranging from 12 to 48%. The SNP with the highest effect was associated with a trichothecene-producing gene. In future, pathogenomics as a high resolution technique will lead to the identification of more genes and their regulators associated with pathogenicity and aggressiveness and primary and secondary metabolism.
Like aggressiveness, host resistance is inherited quantitatively (Fig. 2c). A large number of genes, called quantitative trait loci (QTL), underlie this variation and in addition the environment and genotype × environment interaction play a large role. Most QTLs are specific to the populations and environments in which they have been mapped and have only small effects on resistance. Exceptions are several genes/QTLs from Chinese wheat varieties with large, stable effects, e.g. Fhb1-Fhb5, which are now used worldwide to improve resistance to FHB in bread wheat, durum wheat, and triticale[12]. By refined mapping strategies, promising meta-QTLs and candidate genes can be detected for improving Fusarium resistances with reduced mycotoxin accumulation and integrated into genomics-assisted backcross breeding strategies.
FHB resistance of wheat to different Fusarium species is also unspecific. In contrast, the resistance of different growth stages within cereal species is usually not correlated, e.g. the correlation between foot rot and head blight severity or young-plant stage and either foot rot or head blight in wheat are not significant for F. culmorum and F. graminearum. Resistance mechanisms most likely differ for the different diseases although they are initiated by the same Fusarium species. In resistance breeding, therefore, each growth stage-dependent resistance has to be targeted separately.
4. Control and Management of Fusarium Diseases
Fusarium diseases have a complex etiology, only an integrated approach that considers several factors at the same time can control the risk of Fusarium infection. Most knowledge has been accumulated about management of FHB in wheat. The following tools have been promoted.
- Biocontrol by application of mycoparasitic fungi such as Trichoderma harzianum which has an antagonistic effect against Fusarium spp. causing foot rot, root rot, and crown rot or the bacterium Burkholderia cepacia reducing infections with F. solani.
- Fungicides have lower effects due to a very short application window.
- Crop rotation with non-cereals, e.g. rapeseed, alfalfa, potato, sugar beet.
- Residue and soil management with the aim of rapidly decomposing Fusarium-containing plant residues or burying them in the soil by ploughing.
- Measures to reduce mycotoxin levels during harvest, storage and processing
- Resistant cultivars reduce fungal growth, damage and mycotoxin contamination of the host.
Biocontrol agents („biopesticides“) have not yet found widespread commercial use in the field. Fungicides may control FHB, especially some azoles, like tebuconazole or metconazole, and can reduce DON in the crop by 50-80%. However, a fungicide application close to the infection from 2 days before to maximal 4 days after a rainfall event in the critical period from the end of ear emergence till end of flowering is necessary for maximal efficacy[13]. Fungicide resistance development of most of the Fusarium species has been observed. Crop rotation together with appropriate soil management is one of the most effective measures against Fusarium although it might not always be economical. A soil management that allows rapid decomposition of stubble residues and other organic material on the surface considerably reduce the risk of infection.
Five risk factors have been determined during a 4-years study in Germany to increase FHB infection in wheat[14]: (1) warm, wet weather at flowering, (2) maize as previous crop, (3) minimum tillage, (4) susceptible variety, (5) application of a strobilurin fungicide. The most important risk is the weather at flowering. When it is dry enough, the other risk factors do not apply. When the weather is appropriate for infection, combining several risk factors has a synergistic effect. A single risk factor might increase the risk of DON contamination three-fold whereas four risk factors together increase the risk for DON contamination 56-fold.
Resistant cultivars are the most economically viable and environmentally friendly measure available worldwide to reduce the impact of Fusarium on the crop and the levels of mycotoxins in the harvest. However, due to complex quantitative inheritance, dependence on morphological traits and environmental conditions, it is not easy to select for Fusarium resistance. In all cases, there are no disease-free cultivars available (Fig. 2c), only cultivars with fewer symptoms. Selecting these quantitatively resistant genotypes requires a reliable, quantitative phenotyping platform in the field with testing a large number of genotpyes across several locations and/or years, and statistical analysis. The main problem is to breed Fusarium-resistant genotypes that are commercially successful, because very often the most resistant lines are too tall, susceptible to other diseases or yield considerably less. The situation in Germany might be an example. Out of a total of 117 recommended varieties in 2022, 23 varieties (20%) have a good FHB resistance (Table 3). Of these, 19 varieties have a short to medium plant height, 14 varieties are tolerant to lodging and 6 varieties also have a high grain yield. One variety of these is, however, susceptible to yellow rust, while two others are highly susceptible to leaf rust.
Table 3. Number of recommended varieties fulfilling various breeding goals in Germany from a total of 117 varieties[15].
|
Breeding goal |
Criterium1 |
Number of cultivars |
|
Fusarium head blight |
1-4 |
23 |
|
+ Plant height |
1-5 |
19 |
|
+ Lodging |
1-4 |
14 |
|
+ Grain yield |
7-9 |
6 |
1 1=lowest susceptibility, very short, no lodging, very low grain yield.
9=highest susceptibility, very tall, full lodging, maximal grain yield.
Harvest should be carried out as soon as threshability is reached without delay[13]. Grain with a moisture content >15% should be airdried. Heavily infected early crops should be harvested, stored and processed separately. By increasing the speed of the wind, both the more heavily infested husks and some of the lighter Fusarium-infected grains can be removed during threshing or later during appropriate cleaning steps, thereby reducing mycotoxin contents. During storage, basic hygiene measures should be applied. DON is largely heat stable and cannot be destroyed by storage. In contrast, mycotoxin concentration may increase as a result of increased temperature and humidity favoring a prolonged fungal growth in storage. During further processing, the DON content can be reduced to some extent by appropriate hulling procedures, as the toxin concentration is usually higher in the outer layers of the grain.
5. Conclusions and Prospects
Toxigenic Fusarium species are a large group of rather unspecialized, ubiquitous plant pathogens that will keep us busy for a long time to come, as intensive agricultural production methods encourage their spread and harmful effects. While there has been a huge amount of research into FHB in small-grain cereals worldwide over the last few decades, other areas are still lagging behind. Genomic methods might allow in future to identify the causes of both pathogen aggressiveness and host resistance and may accelerate resistance selection. However, the challenge for plant breeders remains to combine Fusarium resistance with excellent agronomic traits in commercially competitive varieties. This is especially challenging in high-yielding environments. Therefore, all methods of disease management must be combined to limit human and animal exposure to Fusarium toxins. Particularly in (sub)tropical areas, where warm and humid climates provide an ideal environment for Fusarium species, this is a major challenge.
Global warming will initially lead to a northward spread of FHB in temperate climates, with implications for wheat and maize breeding. A temperature-induced change in the species composition that cause a certain disease complex can already be observed. For example, F. culmorum, which used to be important in northern Germany, is now mainly found in Scandinavia, while F. verticillioides is already regularly found on maize in Germany in warm summers. Thus, the cold-tolerant species will tend to migrate northwards, while the more thermophilic species will follow from the south. It is therefore to be expected that the mycotoxin profiles will shift in future. Increasing dryness during flowering could lead to reduced Fusarium incidence, but the fungi might also adapt to varying weather given their great environmental plasticity.
References
- Summerell, B.A.; Laurence, M.H.; Liew, E.C.; Leslie, J.F Biogeography and phylogeography of Fusarium: a review. Fungal Diversity 2010, 44, 3-13, https://doi.org/10.1007/s13225-010-0060-2.
- Burgess, L.W. McAlpine memorial lecture - a love affair with Fusarium. Australas. Plant Pathol. 2014, 43, 359-368, https://doi.org/10.1007/s13313-013-0261-8.
- Crous, P.W.; Lombard, L.; Sandoval-Denis, M.; Seifert, K.A.; Schroers, H.-J.; Chaverri, P.; Gené, J.; Guarro, J.; Hirooka, Y.; Bensch, K.; et al.et al. Fusarium: more than a node or a foot-shaped basal cell. . Stud. Mycol. 2021, 98, 100116, https://doi.org/10.1016/j.simyco.2021.100116.
- Starkey, D.E.; Ward, T.J.; Aoki, T.; Gale, L.R.; Kistler, H.C.; Geiser, D.M.; Suga, H.; Tóth, B.; Varga, J.; O’Donnell, K.; et al. Global molecular surveillance reveals novel Fusarium head blight species and trichothecene toxin diversity . Fungal Genet. Biol. 2007, 44, 1191-1204, https://doi.org/10.1016/j.fgb.2007.03.001.
- McCormick, S.P.; Stanley, A.M.; Stover, N.A.; Alexander, N.J. Trichothecenes: from simple to complex mycotoxins. Toxins 2011, 3, 802-814, https://doi.org/10.3390/toxins3070802.
- Ekwomadu, T.I.; Akinola, S.A.; Mwanza, M. Fusarium mycotoxins, their metabolites (free, emerging, and masked), food safety concerns, and health impacts. Int. J. Environ. Res. Public Health 2021, 18, 11741, https://doi.org/10.3390/ijerph182211741.
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430-439, https://doi.org/10.1038/s41559-018-0793-y.
- Van der Plank, J.E. . Plant diseases: epidemics and control; Academic Press: New York, USA, 1963; pp. 364.
- Talas, F.; Würschum, T.; Reif, J.C.; Parzies, H.K.; Miedaner, T. Association of single nucleotide polymorphic sites in candidate genes with aggressiveness and deoxynivalenol production in Fusarium graminearum causing wheat head blight. BMC Genet. 2012, 13, 14, https://doi.org/10.1186/1471-2156-13-14.
- Rampersad, S.N. Pathogenomics and management of Fusarium diseases in plants . Pathogens 2020, 9, 340, https://doi.org/10.3390/pathogens9050340.
- Miedaner, T.; Vasquez, A.; Castiblanco, V.; Castillo, H.E.; Foroud, N.; Würschum, T.; Leiser, W. Genome-wide association study for deoxynivalenol production and aggressiveness in wheat and rye head blight by resequencing 92 isolates of Fusarium culmorum. BMC Genomics 2021, 22, 630, https://doi.org/10.1186/s12864-021-07931-5.
- Buerstmayr, M.; Steiner, B.; Buerstmayr, H. Breeding for Fusarium head blight resistance in wheat—progress and challenges. Plant Breed. 2020, 139, 429-454, https://doi.org/10.1111/pbr.12797.
- Ährenfusariosen in Weizen [Fusarium head blight in wheat, in German . https://www.lfl.bayern.de/ips/getreide/072198/index.php. Retrieved 2023-3-27
- Obst, A.; Lepschy, J.; Beck, R.; Bauer, G.; Bechtel, A. The risk of toxins by Fusarium graminearum in wheat—interactions between weather and agronomic factors. Mycotoxin Res. 2000, 16 (Suppl.1), 16-20, https://doi.org/10.1007/BF02942972.
- Bundessortenamt. Beschreibende Sortenliste für Getreide, Mais, Öl- und Faserpflanzen, Leguminosen, Rüben, Zwischenfrüchte [German descriptive list of varieties for cereals, maize, oil and fibre plants, pulse crops, beets, catch crops; in German]; Bundessortenamt: Hannnover, 2022; pp. 368pp.




 (a)
(a) (b)
(b)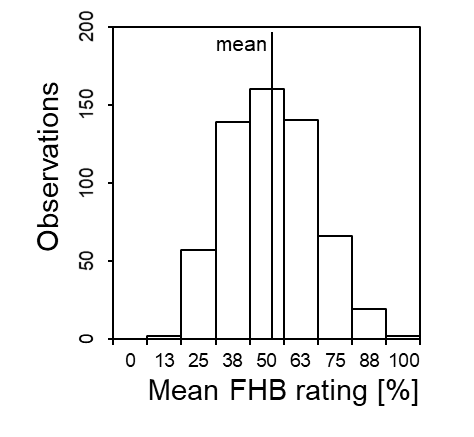 (c)
(c)

