Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Andrzej Łachacz | -- | 3246 | 2023-03-24 12:26:28 | | | |
| 2 | Catherine Yang | Meta information modification | 3246 | 2023-03-27 04:13:15 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Łachacz, A.; Kalisz, B.; Sowiński, P.; Smreczak, B.; Niedźwiecki, J. Transformation of Organic Soils Due to Artificial Drainage. Encyclopedia. Available online: https://encyclopedia.pub/entry/42517 (accessed on 07 February 2026).
Łachacz A, Kalisz B, Sowiński P, Smreczak B, Niedźwiecki J. Transformation of Organic Soils Due to Artificial Drainage. Encyclopedia. Available at: https://encyclopedia.pub/entry/42517. Accessed February 07, 2026.
Łachacz, Andrzej, Barbara Kalisz, Paweł Sowiński, Bożena Smreczak, Jacek Niedźwiecki. "Transformation of Organic Soils Due to Artificial Drainage" Encyclopedia, https://encyclopedia.pub/entry/42517 (accessed February 07, 2026).
Łachacz, A., Kalisz, B., Sowiński, P., Smreczak, B., & Niedźwiecki, J. (2023, March 24). Transformation of Organic Soils Due to Artificial Drainage. In Encyclopedia. https://encyclopedia.pub/entry/42517
Łachacz, Andrzej, et al. "Transformation of Organic Soils Due to Artificial Drainage." Encyclopedia. Web. 24 March, 2023.
Copy Citation
Organic soils that had been drained in order to obtain fertile agricultural land underwent changes leading to the formation of mursh (also known as moorsh). The mursh-forming process is a generic soil process that occurs in drained (artificially or naturally) organic soils, and leads to the changes in soil morphology, soil physical properties (including water retention capability), physicochemical properties, and chemical and biological properties.
Histosols
meadow soils
moorsh
mursh
peat
peatland degradation
1. Transformations of Organic Matter after Drainage
The effects of the transformation of organic matter during the mursh-forming process have been studied since the second half of the 20th century [1][2][3][4][5][6][7][8][9][10][11][12][13][14][15][16]. After drainage of the organic soil, oxidation of the SOM occurs, the quantity of soil carbon decreases, and the ash content increases. Organic matter undergoes microbial decomposition leading to CO2 and N2O emission to the atmosphere, as well as the production and increased availability of DOC and DON (dissolved organic carbon and nitrogen) in waters. Consequently, the loss of organic matter occurs, and the peatland subsides.
Peat soils in fens contain large amounts of nitrogen. Characteristic is the increase of total nitrogen in murshes (usually by 0.5–1.0%), and the decrease of organic carbon, which reduces the C:N ratio. This ratio is 16.3–17.8 in peats, and lower in murshes—12.1–12.5 (Table 1). Therefore, the C:N ratio can be treated as a simple indicator of the progress of the mursh-forming process [17][18]. The accumulation of nitrogen in murshes may be higher by 30–35% than in the peats from which they develop [1]. The mursh-forming process decomposes peat and delivers higher nitrogen availability. The transformation of nitrogen compounds depends on peat type and habitat conditions (mainly the water levels), and the type of vegetation [19]. In peats, nitrogen occurs in organic compounds in plant remnants, whereas in murshes it is mostly from fulvic acids and hydrolyzing compounds (hemicellulose and cellulose) [4]. As a result of mineralization, organic nitrogen compounds are transformed into mineral forms (N-NH4, N-NO3) and may be uptaken by plants. Due to nitrification processes, nitrous oxide is emitted to the atmosphere. Nitrogen compounds are accumulated in surface layers and bound by humus substances [1][20][21].
Okruszko et al. [22] found that the peat-mursh soils used for agriculture as grasslands (with groundwater levels during the growing season of 70–90 cm) released 574.1 kg of nitrogen per ha annually; 204.9 kg from this amount was uptaken by grassland vegetation and collected hay, and the remaining 369.2 kg could theoretically remain in soil organic matter. However, the authors stated that 63.4 kg of nitrogen were lost annually (washed into groundwater or emitted as N2O). In Poland, the amount of nitrogen released annually as a result of mineralization of organic matter of drained organic soils varies widely (60–400 kg ha−1). However, frequently, especially in deeply drained alder peats (rich in nitrogen), the amount of released nitrogen reaches 800–1000 kg ha−1, which is significantly higher than the amount that can be taken up by intensively managed grassland (300–400 kg ha−1) [1]. Since not all of the remaining nitrogen is fixed in humic compounds, it causes a threat to the environment (groundwater eutrophication, greenhouse effect).
Table 1. General characteristics of soil materials.
| Property, Unit | Sedge-Reed Peat 25–30 cm | Humous Mursh 5–10 cm | Alder-Wood Peat 55–60 cm | Proper Mursh 15–20 cm | Calcareous Mursh 0–32 cm |
Ferruginous Mursh 10–20 cm |
Mursh Of Mud Origin | Sandy Mursh | Semimurshic Material | Postmurshic Material |
|---|---|---|---|---|---|---|---|---|---|---|
| Source | [23] | [24] | [25] | [26] supplemented | [27] supplemented | |||||
| LOI, % | 89.9 | 85.3 | 79.3 | 72.0 | 43.9 | 32.1 | 51.4 | 39.6 | 13.9 | 5.8 |
| BD, Mg m−3 | 0.167 | 0.249 | 0.145 | 0.367 | 0.580 | n.d. | 0.757 | 0.540 | 0.951 | 1.211 |
| TP, % | 89.3 | 84.5 | 91.3 | 79.1 | 72.9 | n.d. | 68.0 | 74.8 | 60.6 | 51.3 |
| OC, % | 55.98 | 54.12 | 63.72 | 58.07 | 21.83 | 7.42 | 24.03 | 21.37 | 6.67 | 2.73 |
| TN, % | 3.15 | 4.47 | 3.91 | 4.64 | 1.58 | 0.47 | 2.00 | 1.43 | 0.53 | 0.25 |
| C:N | 17.8 | 12.1 | 16.3 | 12.5 | 13.8 | 15.8 | 12.0 | 15.1 | 12.6 | 11.0 |
| pHH2O | 6.1 | 5.4 | 6.0 | 5.4 | 7.6 | 6.0 | 6.0 | 6.4 | 6.2 | 5.8 |
| pHKCl | 5.5 | 4.9 | 5.3 | 5.0 | 7.3 | 5.0 | 5.3 | 5.7 | 5.3 | 4.9 |
| CEC, cmol(+) kg−1 | 174.5 | 190.7 | 146.1 | 153.9 | n.d. | 72.4 | 99.7 | 105.0 | 48.4 | 19.1 |
| BS, % | 68.3 | 42.2 | 77.7 | 63.8 | n.d. | 68.3 | 65.4 | 64.7 | 54.6 | 55.7 |
| CaCO3, % | 0.0 | 0.0 | 0.0 | 0.0 | 30.5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Catot, g kg−1 | 33.3 | 32.0 | 22.4 | 30.3 | 238.9 | 17.7 | 42.6 | 10.9 | 3.2 | 1.6 |
| CaHCl, g kg−1 | n.d. | n.d. | n.d. | n.d. | n.d. | 16.80 | 15.36 | 9.50 | 2.80 | 1.40 |
| Fetot, g kg−1 | 20.1 | 38.7 | 11.5 | 44.5 | 17.8 | 346.2 | 40.5 | 32.7 | 12.1 | 9.8 |
| FeHCl, g kg−1 | 16.5 | 32.0 | 4.5 | 14.3 | n.d. | 56.1 | 23.3 | 17.1 | 4.7 | 3.8 |
| Ptot, g kg−1 | 0.5 | 1.0 | 0.5 | 2.4 | 1.3 | 0.88 | 1.41 | 1.20 | 0.8 | 0.4 |
| PHCl, g kg−1 | n.d. | n.d. | n.d. | n.d. | n.d. | 0.26 | 0.36 | 0.30 | 0.15 | 0.09 |
| Mgtot, g kg−1 | 0.3 | 0.2 | 1.4 | 1.3 | 3.4 | 0.4 | 0.60 | 0.30 | 0.09 | 0.05 |
| MgHCl, g kg−1 | n.d. | n.d. | n.d. | n.d. | n.d. | 0.32 | 0.39 | 0.26 | 0.06 | 0.04 |
| Ktot, g kg−1 | 0.2 | 0.3 | 0.8 | 0.4 | 0.5 | 0.05 | 1.20 | 0.20 | 0.10 | 0.08 |
| KHCl, g kg−1 | n.d. | n.d. | n.d. | n.d. | n.d. | 0.02 | 0.30 | 0.13 | 0.06 | 0.05 |
Explanation: LOI—loss-on-ignition, BD—bulk density of dry soil, TP—total porosity, CEC—cation exchange capacity, BS—base saturation, CaCO3—calcium carbonate, determined according to the Scheibler’s method, tot—total content of elements, HCl—content of forms soluble in 0.5 M HCl extract.
The transformation of organic matter during the mursh-forming process is dynamic and depends on site conditions [28]. During the first phase after drainage, lasting several (or even dozens) years, intensive humification of SOM occurs, and some organic substances (cellulose, lignin, bitumen) are transformed to humus substances (first to fulvic and then to humic acids), and these new substances are enriched in oxygen functional groups, e.g., carboxylic, alcoholic, phenolic [29]. As a result, surface mursh horizons contain more humic compounds, which are traditionally divided into fulvic or humic acids. These compounds have different molecular weights, carbon and nitrogen contents, and stabilities, i.e., resistance to decomposition (humic acids have a resistance to decomposition than fulvic acids). In the first stages of plant decay, fulvic acids are formed and prevail [4], and in subsequent stages they are transformed (during condensation and polymerization) into other, more complex compounds (humic acids). However, in degraded murshes, the opposite process may occur, i.e., disintegration of humic acids to simple organic compounds. Therefore, it was assumed that the ratio of humic to fulvic acids may be a fairly specific index of the direction of changes taking place in drained organic soils [1]. In eutrophic sites, more fulvic acids are formed, whereas in the sites with lower trophism, polymerization and condensation of humic acids occurs [10]. Wójciak [10] also reported that in murshes formed from moss and sedge peats, humic acids prevailed and the ratio of humic acids to fulvic acids in some soils exceeded 3:1. In rush and alder peats, which are typical for eutrophic sites, the ratio of humic acids to fulvic acids was approximately 1:1. Kalisz et al. [12][13] stated that ratio of humic acids to fulvic acids is higher in the first stage of organic matter transformation in mursh soils and lower when the process is advanced.
The mursh-forming process also contributed to the decrease of humins and the increase of hot-water extractable carbon (HWC), which is a labile fraction of carbon compounds corresponding with the biomass of microorganisms [13][30]. Therefore, hot-water-extractable organic matter (HWOM) may be a good indicator of changes occurring in drained organic soils [31]. Becher [32] reported that after drainage, organic soils contain more humic acids and labile carbon fractions. Moreover, in murshes, humic acids contain more hydrogen, nitrogen, and phosphorus, and less carbon; the particles of humic acids are less oxidized; and humic acids contain more hydrophobic factions, as compared to peats. Water soluble fulvic acids represent the most mobile humus fraction and, according to Leinweber et al. [33], their molecular chemical composition and thermal properties may be objective ecological indicators of land use and peat degradation.
Peatlands in their natural state store carbon and are the sinks for carbon dioxide (CO2), but are also sources of methane (CH4) [34]. Drainage of peat soil reduces CH4 emissions, but changes the peatland into a source of CO2 due to aerobic peat mineralization [35]. It is estimated that about 1 Pg year−1 of CO2 is emitted from drained peatlands globally, which is equivalent to 10% of the CO2 emissions from the entire agriculture, forestry and land use sectors [36]. The most important factors regulating CO2 emissions from drained organic soils are organic matter and air in the topsoil. The rate of peat mineralization and CO2 emissions depends on the peat type, groundwater level, and temperature [37][38]. The emission rate is the highest for peatlands developed from alder peats and the lowest developed from Sphagnum peat. The release of CO2 changes seasonally in line with temperature changes. Therefore, the highest emissions are recorded between June and September. Oleszczuk et al. [38] also stated that peat mineralization and CO2 emission are most intense when the water level is 90 cm below the soil surface. Further lowering of the water results in drying of the upper peat layers, impeding peat mineralization, and reducing CO2 emission [39]. Rewetting (reswamping) of formerly drained peatlands may reduce CO2 emissions and restore the carbon sink function in peatlands [40].
2. Mineral Matter in Organic Soils after Drainage
In the evolution of drained organic soils, in terms of the influx and transformation of mineral substances (Figure 1), the influx of nutrients with atmospheric water is more or less constant over time and relatively insignificant compared to other sources. Also, the inflow of aeolian deposits is of less influence [41]. On the other hand, in undrained, wet organic soils, the influx of mineral matter together with the water supply (surface water inflow, spring groundwater inflow) is important. The content of mineral components in the waters flowing into the fens or transitional mires are very diverse, which is reflected in the vegetation and the nature of the accumulated peat. In general, the chemical composition of the mineral substrate as a result of biogeochemical cycles affects the nutrient content in the surface layers [42]. This relationship is stronger the shallower the peat deposit is. Drainage eliminates surface inflow, and a new source of mineral matter appears in the form of capillary infiltration of groundwater. Mineral compounds are released during the mineralization of organic matter. Changing the air and water conditions initiates the transformation of mineral compounds towards oxygenated forms. In case of agricultural use, there may also be an influx of minerals along with fertilizers and pesticides. Intensive mixing of soil components takes place and the share of mineral components increases, and organic matter is reduced as a result of mineralization. In case of shallow organic soils, mechanical tillage and mixing of soil surface layers with mineral subsoil is important. Plowing significantly increases the aeration of soil, and accelerates decomposition of organic matter [43]. Further intensive drainage eliminates the inflow of nutrients by capillary rise, because the groundwater level is too deep. This is particularly important in organic soils developed on a sandy subsoil, which is frequent in Poland. Mineral compounds can be leached out of the root zone, and the uptake by cultivated plants leads to soil impoverishment of nutrients and soil acidification. Further depletion of SOM leads to a change in the position of soils in the soil classification systems (from organic soil types to mineral ones).
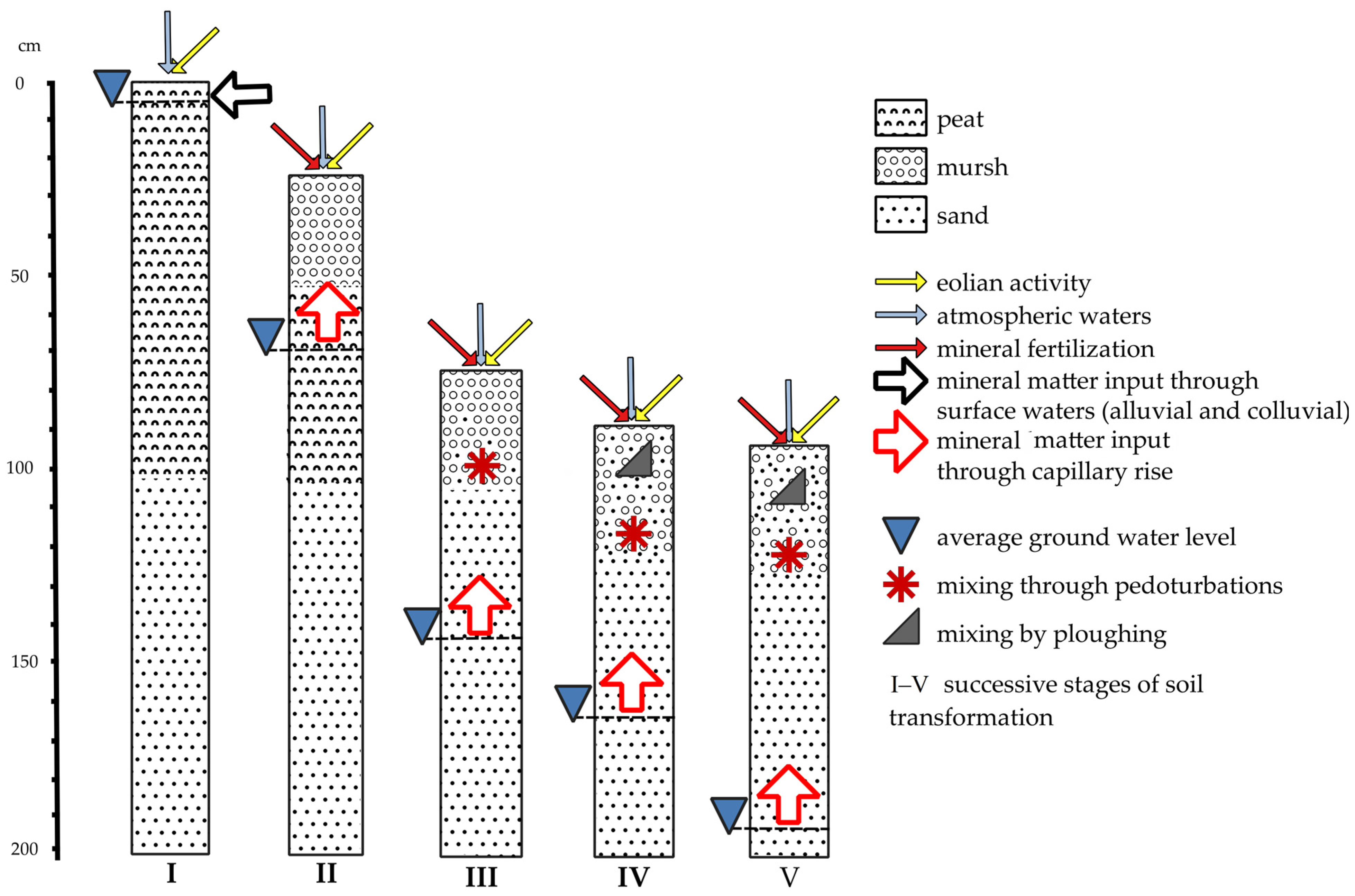
Figure 1. Successive stages of organic soil transformation showing the inflow of mineral matter to soil; (I)—PSC (2019): Hemic peat soil (Gleba torfowa hemowa), WRB (2022): Rheic Hemic Histosol; (II)—PSC (2019): Hemic murshic soil (Gleba murszowa hemowa), WRB (2022): Murshic Hemic Histosol; (III)—PSC (2019): Thin murshic soil (Gleba murszowa płytka), WRB (2022): Histic Gleysol (Arenic, Drainic, Mulmic); (IV)—PSC (2019): Typical semimurshic soil (Gleba murszowata typowa), WRB (2022): Mollic Gleysol (Arenic, Drainic, Mulmic); (V)—PSC (2019): Postmurshic soil (Gleba murszasta), WRB (2022): Umbric Gleysol (Arenic, Drainic, Humic, Nechic).
The assessment of plant-available nutrients in organic and mineral-organic soils (3–20% SOM) used as meadows, is possible in an extract of 0.5 M HCl [44][45], which turned out to be particularly useful for determining phosphorus fertilization needs at meadows located on drained peatlands [46]. The 0.5 M HCl enables extraction of mainly freshly formed (precipitated) mineral compounds, and to a lesser extent elements associated with humus. According to various authors [23][41][47][48], 74–89% of calcium, 25–71% of magnesium, 30–96% of iron, 29–75% of aluminum, 35–67% of potassium, and 13–38% phosphorus are extracted using 0.5 M HCl. The studies of Okruszko [1][49][50] revealed that the solubility, and thus the availability of chemical compounds for plants, decreases with the progress of the mursh-forming process, which is related to their aging, i.e., the transition from amorphous (colloidal) to crystalline forms.
The most important change concerns the loss of SOM, as it is 5–7% lower in murshes than in the peats lying below them. This is associated with an increase in bulk density, which in murshes is greater than 0.2 Mg m−3, and a decrease in total porosity (Table 1). The decomposition (mineralization) of SOM is associated with, among others, secretion of large amounts of H+, which changes soil reaction (decreasing the pH), as well as base saturation. This leads to changes in the sorption complex. In the first stage after drainage, the value of cation exchange capacity (CEC) increases, because humus acids formed in large quantities show greater cation sorbing capacity than the plant tissues present in peat. However, during the mursh-forming process, the CEC value decreases, which is associated to an increased share of hydrogen and transformations of the organic sorption complex. For example, the CEC in peat amounts to 157.8 cmol (+) kg−1, whereas in murshes developed from them it is merely 137.1 cmol (+) kg−1. The average CEC of mursh in the soil profile was only 86.9% of the capacity of the underlying peats [51]. High CEC in organic soils may lead to strong binding of cations and, for example, deficiency of copper to plants [52][53].
The chemical composition of peat, including additions of mineral alluvial and colluvial sediments, as well as content of Fe, Ca, Mg, and P, determines the formation of humus-mineral and humus-metallic bondings [1][5][10][12][23]. In murshes, iron hydroxide forms complex bondings with organic compounds [7][9][23]. Mineral-organic bondings may be formed during various stages of organic matter transformation [3]. In Poland, as early as the 1950s, studies of mineral-humus connections in drained organic soils were undertaken using physical fractionation in heavy fluids [7][54][55]. The developed method [55] included the separation of four fractions with a specific density (g cm−3): >2.88, 2.88–1.94, 1.94–1.59, and <1.59. Higher content of the heavy fraction indicates that the evolution of soils after drainage will move towards black earths (with the mollic horizon), in which organic matter is stable and humus is permanently connected with the mineral fraction. The research carried out by Kalisz et al. [12][56] and Długosz et al. [57] indicate that the addition of a fine-grained mineral fraction (silt, clay) has a positive effect on the stability of organic matter in drained peatlands.
Of all the metallic elements, organic soils contain the largest amounts of calcium and iron (Table 1). The amounts of calcium in murshes are related to Ca release during peat decomposition. However, Ca is displaced by hydrogen in the sorption complex during mursh formation, and also leached down the soil profile, therefore it is rarely accumulated in the topsoil (murshes). The Ca content in murshes correlates with the content in peats and depends on local geochemical conditions and water flow. In the first period after drainage, the Ca content may be even higher in murshes than in peats. However, with the progress of the mursh-forming process, Ca decreases, which is referred to as their decalcitation. The annual Ca leaching from drained organic soils may reach 260–840 kg ha−1 [1][23]. During mursh-formation, iron accumulates (primary it comes from the decomposition of peat plant remains), providing 433 kg Fe2O3 ha−1 annually [6]. However, the main source of iron comes from water capillary rise, which provides Fe2+, later oxidized to Fe3+ in the aeration zone. Murshes contain mainly “free” (Fe in easily soluble or colloidal compounds) and “bound” (Fe bound humus) iron forms [23].
In some soils a particularly high accumulation of calcium or iron in murshes occurs, which justifies the need to distinguish carbonate and ferruginous murshes. Liwski et al. [58], on the basis of total content of calcium and iron distinguished, calcareous murshes (over 4.3% Ca) and ferruginous murshes (over 4.2% Fe). The iron content in ferruginous murshes is usually 7–10.5% (up to 14–17.5%) [1]. In carbonate murshes, the accumulation of calcium carbonate had taken place before peat drainage, and in peats it originated from groundwater or surface water. Such murshes were developed from peats lying on limestone in river valleys at loess areas [59], or from carbonate spring formations, or from shallow peats lying on carbonate lake sediments [24]. Carbonate murshes have higher content of magnesium, and their sorption properties result from the predominance of calcium and magnesium cations. Other chemical characteristics of these soil formations are primarily determined by the properties of the peats from which they originated, and the SOM content. Both calcium and iron can also precipitate above the groundwater level in drained organic soils. Under specific conditions, a meadow lime horizon and/or a layer of iron ore deposited directly below the shallow organic horizon may be formed. Such accumulation is related to the discontinuity of water capillary rise between the mineral subsoil (usually sandy) and organic topsoil, and can also impair the physical properties of soils. In the ferruginous concretions that build iron ores, the content of elemental iron can reach up to 28% [55]. An increased contents of calcium carbonate or iron compounds are associated with a lower SOM content, which modifies soil properties. Ferruginous murshes may have a higher content of phosphorus, while other properties remain similar to murshes with similar SOM content. If the mursh contains vivianite, the peat-mursh soil does not require P fertilization. Unlike accumulation of iron compounds, vivianite precipitation occurs only during peat formation. The content of phosphorus extracted with 0.5 M HCl in murshes with vivianite is in the range of 870–1300 mg P kg−1, but may reach 2200 mg P kg−1 [1].
Generally murshes, apart from ferruginous and calcareous murshes (Table 1), contain similar amounts of iron and calcium. However, during long term drainage, iron prevails, and calcium is leached out. The presence of large amounts of chemically active (reactive) calcium compounds (also magnesium to some extent) and iron (also aluminum to some extent) in drained organic soils affect, among others, the solubility and availability of phosphorus for plants [47][49][50][60][61]. Considering the above, mursh soils can be classified as either of the lime type (pedocal) or the sesquioxide type (pedalfer), but most often they belong to the mixed type (calalfer) [49].
Phosphorus accumulates as a result of the mursh-forming process. Therefore, its content is usually higher in murshes than in peats. The mursh-forming process leads to the release of phosphorus from peat and increases its availability to plants [49][50]. The share of phosphorus in mineral forms in its total content increases in murshes (33–35%) in relation to peat (26–28%) [48]. In murshes, phosphorus is present in different proportions and various combinations: with humus (approx. 60–90% of total P), iron, and calcium. The complexation of iron and aluminum with humus compounds reduces the P sorption capacity, which means that phosphorus in murshes can occur in forms available to plants [23]. However, as a result of the long-term drainage at agricultural areas, phosphorus-iron compounds increasingly gain in solidity and their availability for plants diminishes [1].
Typical for peats and the murshes is the scarcity of potassium [62]. The process of mursh formation does not significantly affect the distribution of potassium in the soil profile [1]. The content of magnesium in organic soils is several to dozens times lower than that of calcium and behaves similarly to calcium. Aluminum, on the other hand, behaves similarly to iron and its content increases in murshes, which is caused by its sorption by colloidal organic substances [1]. Piaścik [23] indicated that the total aluminum content in mursh soils is 3–15 times lower than that of iron, and mursh horizons contain 1.5–3 times more Fe than the underlying peat.
The calculated coefficients of enrichment (for unfertilized soils) in mineral elements in the surface (mursh) layer (0–5 cm) in relations to underlying peat (45–50 cm) proves the above statements [63]: 4 for aluminum and zinc, 3–4 for potassium, 2–3 for manganese and cobalt, 1–2 for magnesium, iron, chromium, and copper. However, for calcium it is only 0.6, which suggests leaching of Ca in drained organic soils.
The properties of murshes can also be modified by the addition of a mineral clastic fraction and, consequently, mud-derived murshes and sandy murshes usually found in river valleys can be distinguished [26][57] (Table 1). A significant addition of mineral particles decreases SOM content, and modifies soil physical (bulk density increases, total porosity decreases) and chemical properties. Generally, murshes developed from organic muds contain more potassium, which results from the presence of K in clay minerals. Chemical properties of sandy murshes are influenced by the decreasing SOM and significant addition of sand and silt. These murshes are a transition to semimurshic and postmurshic soil materials.
References
- Okruszko, H. Transformation of fen-peat soils under the impact of draining. Zesz. Probl. Post. Nauk Rol. 1993, 406, 3–73.
- Okruszko, H. Phenomenon of peat soil degradation in the light of experiments. Acta Agroph. 2000, 26, 7–15.
- Okruszko, H. Muck soils of valley peatlands, and their chemical and physical properties. Rocz. Nauk. Rol. Ser. F 1960, 74, 5–89.
- Kozakiewicz, A. Characteristics of the organic matter of peat soils and the peats of low-moors. Rocz. Glebozn.–Soil Sci. Annu. 1962, 11, 73–100, (In Polish with English Summary).
- Marcinek, J. Studies of chemical composition of organic substance of peat soils. Zesz. Probl. Post. Nauk Roln. 1962, 34, 129–137, (In Polish with English Summary).
- Okruszko, H.; Kozakiewicz, A. Humification and mineralization as mucking process elements in peat soils. Zesz. Probl. Post. Nauk Roln. 1973, 146, 63–76, (In Polish with English Summary).
- Walczyna, J. Organic-mineral compounds in drained hydrogenic soils and evolution trends of these soils. Rocz. Glebozn.–Soil Sci. Annu. 1974, 25, 179–200, (In Polish with English Summary).
- Drozd, J. Changes of nitrogen content in humic compounds of muck soils formed under different hydrological conditions. Rocz. Glebozn.–Soil Sci. Annu. 1986, 37, 195–203, (In Polish with English Summary).
- Sapek, A.; Sapek, B. The use of 0.5 M sodium hydroxide extract for characterizing humic substances from organic formations. Rocz. Glebozn.–Soil Sci. Annu. 1986, 37, 139–147, (In Polish with English Summary).
- Wójciak, H. Properties of the organic matter in drained peat soils. Diss. Monogr. UWM Olszt. 2004, 97, 1–71, (In Polish with English Summary).
- Matyka-Sarzyńska, D.; Sokołowska, Z. Usefulness of humification number in studies of the state of secondary transformation as comparison to water holding capacity of mucks. Acta Agroph. 2004, 106, 553–564, (In Polish with English Summary).
- Kalisz, B.; Lachacz, A.; Glazewski, R. Transformation of some organic matter components in organic soils exposed to drainage. Turk. J. Agric. For. 2010, 34, 245–256.
- Kalisz, B.; Lachacz, A.; Glazewski, R. Effects of peat drainage on labile organic carbon and water repellency in NE Poland. Turk. J. Agric. For. 2015, 39, 20–27.
- Fortuniak, K.; Pawlak, W.; Bednorz, L.; Grygoruk, M.; Siedlecki, M.; Zieliński, M. Methane and carbon dioxide fluxes of a temperate mire in Central Europe. Agric. For. Meteorol. 2017, 232, 306–318.
- Becher, M.; Kalembasa, D.; Kalembasa, S.; Symanowicz, B.; Jaremko, D.; Matyszczak, A. A New Method for Sequential Fractionation of Nitrogen in Drained Organic (Peat) Soils. Int. J. Environ. Res. Public Health 2023, 20, 2367.
- Smolczynski, S.; Kalisz, B.; Urbanowicz, P.; Orzechowski, M. Effect of Peatland Siltation on Total and Labile C, N, P and K. Sustainability 2021, 13, 8240.
- Krüger, J.P.; Leifeld, J.; Glatzel, S.; Szidat, S.; Alewell, C. Biogeochemical indicators of peatland degradation—A case study of temperate bog in northern Germany. Biogeosciences 2015, 12, 2861–2871.
- Lasota, J.; Błońska, E. C:N:P stoichiometry as an indicator of Histosol drainage in lowland and mountain forest ecosystems. For. Ecosyst. 2021, 8, 39.
- Maciak, F.; Liwski, S. Carbon and nitrogen mineralization in peat soils depending on their decomposition degree and addition of plant residues (grass roots). In Proceedings of the 8th International Peat Congress, Leningrad, Russia, 15–19 August 1988; Section 4. pp. 265–274.
- Ilnicki, P. Peatlands and Peat; (Torfowiska i torf); Wydawnictwo Akademii Rolniczej: Poznań, Poland, 2002; p. 606. (In Polish)
- Truba, M.; Oleszczuk, R. An analysis of some basic chemical and physical properties of drained fen peat and moorsh soil layers. Ann. Wars. Univ. Life Sci.–SGGW Land Reclam. 2014, 46, 69–78.
- Okruszko, H.; Sapek, B.; Szuniewicz, J. Balance of mineral components in peat soil utilized for a grassland over 24 years. In Proceedings of the 8th International Peat Congress, Leningrad, Russia, 15–19 August 1988; Section 3. pp. 226–233.
- Piaścik, H. Changes in peat-muck soils of the Mazurian Lakeland with particular regard to changes in the content of calcium, iron and aluminium. Zesz. Nauk. ART Olszt. Rol. 1977, 23, 3–60, (In Polish with English Summary).
- Meller, E. Shallow Organogenic-Calcareous Soils on Lacustrine Chalk and Their Transformation Resulted from Cultivation; Wydawnictwo Akademii Rolniczej w Szczecinie: Szczecin, Poland, 2006; 115p, (In Polish with English Summary).
- Meller, E.; Malinowski, R. Chemical properties of post-bog soils with bog iron ore of “Stare Bagno” located in the Karpina river valley. Rocz. Glebozn.–Soil Sci. Annu. 2010, 61, 159–163, (In Polish with English Summary).
- Kalisz, B.; Łachacz, A. Content of nutrients, heavy metals and exchangeable cations in riverine organic soils. Pol. J. Soil Sci. 2009, 42, 43–52.
- Łachacz, A. Origin and Properties of Shallow Organogenic Soils of the Mazury and Kurpie Plain; Dissertations and Monographs of University of Warmia and Mazury in Olsztyn: Olsztyn, Poland, 2001; 119p, (In Polish with English Summary).
- Liwski, S.; Maciak, F.; Okruszko, H.; Churski, T.; Gotkiewicz, J.; Oświt, J.; Pacowski, R.; Szuniewicz, J.; Żurek, S. Characteristics of soil formation and soils and their evolution due to draining and different utilization. Pol. Ecol. Stud. 1984, 10, 313–346.
- Boguta, P.; Sokołowska, Z. Statistical relationship between selected physicochemical properties of peaty-muck soils and their fraction of humic acids. Int. Agroph. 2014, 28, 269–278.
- Smreczak, B.; Ukalska-Jaruga, A. Dissolved organic matter in agricultural soils. Soil Sci. Annu. 2021, 72, 132234.
- Heller, C.; Weiß, K. Approaching a standardized method for the hot-water extraction of peat material to determine labile SOM in organic soils. Commun. Soil Sci. Plant Anal. 2015, 46, 1044–1060.
- Becher, M. Organic Matter Transformation Degree in the Soils of the Upper Liwiec River; Rozprawa Naukowa Uniwersytetu Przyrodniczo-Humanistycznego w Siedlcach: Siedlce, Poland, 2013; (In Polish with English Summary).
- Leinweber, P.; Schulten, H.-R.; Kalbitz, K.; Meissen, R.; Jancke, H. Fulvic acid composition in degraded fenlands. J. Plant Nutr. Soil Sci. 2001, 164, 371–379.
- Frolking, S.; Talbot, J.; Jones, M.C.; Treat, C.C.; Kauffman, J.B.; Tuittila, E.-S.; Roulet, N. Peatlands in the Earth’s 21st century climate system. Environ. Rev. 2011, 19, 371–396.
- Maljanen, M.; Sigurdsson, B.D.; Guðmundsson, J.; Oskarsson, H.; Huttunen, J.T.; Martikainen, P.J. Greenhouse gas balances of managed peatlands in the Nordic countries-present knowledge and gaps. Biogeosciences 2010, 7, 2711–2738.
- Edenhofer, O.; Pichs-Madruga, R.; Sokona, Y.; Farahani, E.; Kadner, S.; Seyboth, K.; Adler, A.; Baum, I.; Brunner, S.; Eickemeier, P.; et al. (Eds.) Climate Change 2014: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014.
- Oleszczuk, R.; Łachacz, A.; Kalisz, B. Measurements versus estimates of soil subsidence and mineralization rates at peatland over 50 years (1966–2016). Sustainability 2022, 14, 16459.
- Oleszczuk, R.; Regina, K.; Szajdak, L.; Höper, H.; Maryganova, V. Impact of agricultural utilization of peat soils on the greenhouse gas balance. In Peatlands and Climate Change; Strack, M., Ed.; International Peat Society: Jyväskylä, Finland, 2008; pp. 70–97.
- Renger, M.; Wessolek, G.; Schwaerzel, K.; Sauerbrey, R.; Siewert, C. Aspects of peat conservation and water management. J. Plant Nutr. Soil Sci. 2002, 165, 487–493.
- Joosten, H.; Tapio-Bistrom, M.-L.; Tol, S. Peatlands-Guidance for Climate Change Mitigation through Conservation, Rehabilitation and Sustainable Use; Food and Agriculture Organisation of the United Nations and Wetlands International: Rome, Italy, 2012.
- Bieniek, B. Changes of the chemical composition of peat soils occurring at a deep drainage under conditions of the Masurian Lake District. Rocz. Nauk. Rol. Ser. F 1988, 80, 203–225, (In Polish with English Summary).
- Urvas, L. The influence of different subsoil types on the Ca, K and P status, and pH of the corresponding mould layer. Ann. Agric. Fenn. 1983, 22, 186–192.
- Kowaliński, S. Moorsh soils and their metamorphoses effected by plough tillage. (Gleby murszowe i ich przeobrażenia pod wpływem uprawy płużnej). Pr. Wrocławskiego Tow. Nauk. Ser. B 1964, 124, 1–139, (In Polish with English Summary).
- Sapek, A.; Sapek, B. Testing of grassland soils in Poland. Comm. Soil Sci. Plant Anal. 1992, 23, 2165–2171.
- Sapek, A.; Sapek, B. Methods of Chemical Analyses of Organic Soils. (Metody Analizy Chemicznej Gleb Organicznych); Materiały Instruktażowe IMUZ Falenty 115; Instytut Melioracji i Użytków Zielonych: Falenty, Poland, 1997; 80p. (In Polish)
- Okruszko, H.; Walczyna, J. Determination of abundance in phosphorus of meadow organic soils using the extraction in 0.5 n HCl. Rocz. Nauk. Rol. Ser. F 1970, 77, 437–453, (In Polish with English Summary).
- Przesmycka, W. Occurrence of aluminium, iron and calcium phosphates in peat-muck soil profiles. Wiadomości Inst. Melior. Użytków Ziel. 1974, 12, 117–138, (In Polish with English Summary).
- Przesmycka, W. Content of mineral compounds of phosphorus in reclaimed grassland soils of the Wizna Peatland. Rocz. Nauk. Rol. Ser. F 1976, 79, 93–107, (In Polish with English Summary).
- Okruszko, H. Changes in phosphorus content of organic hydromorphic soils due to drainage. Rocz. Glebozn.–Soil Sci. Annu. 1964, 14, 183–195.
- Okruszko, H. Determination of phosphorus fertilization needs of peat soils on example of the Kuwasy peatland. Wiadomości Inst. Melior. Użytków Ziel. 1964, 4, 9–69, (In Polish with English Summary).
- Piaścik, H.; Łachacz, A. The effects of the muck-forming process on the sorptive properties of peat soils. Pol. J. Soil Sci. 2001, 34, 69–76.
- Sapek, B.; Sapek, A. Changes in the properties of humus substances and the sorption complex in reclaimed peat soils. Int. Peat J. 1987, 2, 99–117.
- Sapek, B.; Sapek, A.; Stawiński, J. The effect of moorsh forming process on the specific surface area and sorption properties of peat-moorsh soils. In Proceedings of the 8th International Peat Congress, Leningrad, Russia, 15–19 August 1988; Section 4. pp. 288–294.
- Rząsa, S. Genesis and evolution of mineral muck soils in the terrain being drained. Rocz. Wyższej Szkoły Rol. Pozn. 1963, 18, 151–223, (In Polish with English Summary).
- Rząsa, S. Physical fractioning of humus bonds with help of heavy liquids. Rocz. Glebozn.–Soil Sci. Annu. 1958, 7, 217–220, (In Polish with English Summary).
- Kalisz, B.; Urbanowicz, P.; Smólczyński, S.; Orzechowski, M. Impact of siltation on the stability of organic matter in drained peatlands. Ecol. Indic. 2021, 130, 108149.
- Długosz, J.; Kalisz, B.; Łachacz, A. Mineral matter composition of drained floodplain soils in north-eastern Poland. Soil Sci. Annu. 2018, 69, 184–193.
- Liwski, S.; Okruszko, H.; Kalińska, D. Differentiation and content of chemical compounds in organogenic soils of Biebrza Mires. Zesz. Nauk. Akad. Rol. Wrocławiu 1981, 134, Rolnictwo 38, 97–109, (In Polish with English Summary).
- Zawadzki, S. Investigations on the origin and evolution of bog soils rich in calcium carbonate in the Lublin District. Ann. Univ. Maria Curie-Skłodowska Sect. E 1958, 12, 1–86, (In Polish with English Summary).
- Ziółek, M. Phosphorus forms in organic soils with a varying degree of transformation (on the example of the Lublin Polesie region. Pol. J. Soil Sci. 2007, 49, 179–194.
- Debicka, M.; Bogacz, A.; Kowalczyk, K. Phosphorus behaviour and its basic indices under organic matter transformation in variable moisture conditions: A case study of fen organic soils in the Odra River Valley, Poland. Agronomy 2021, 11, 1997.
- Lucas, R.E. Organic Soils (Histosols). Formation, Distribution, Physical and Chemical Properties and Management for Crop Production; Research Report 435 (Farm Science); Michigan State University: East Lansing, MI, USA, 1982; p. 77.
- Sapek, A.; Gotkiewicz, J. Utilization and fertilization effect on the content of mineral elements in profiles of peat soil from the Kuwasy bog. Rocz. Nauk. Rol. Ser. F 1977, 79, 113–134, (In Polish with English Summary).
More
Information
Subjects:
Soil Science
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
701
Revisions:
2 times
(View History)
Update Date:
27 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




