Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rocío Díaz | -- | 2070 | 2023-03-20 14:05:06 | | | |
| 2 | Rita Xu | Meta information modification | 2070 | 2023-03-21 02:41:14 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Díaz-Puertas, R.; Álvarez-Martínez, F.J.; Falco, A.; Barrajón-Catalán, E.; Mallavia, R. Antibiotic-Resistant Bacteria. Encyclopedia. Available online: https://encyclopedia.pub/entry/42357 (accessed on 13 January 2026).
Díaz-Puertas R, Álvarez-Martínez FJ, Falco A, Barrajón-Catalán E, Mallavia R. Antibiotic-Resistant Bacteria. Encyclopedia. Available at: https://encyclopedia.pub/entry/42357. Accessed January 13, 2026.
Díaz-Puertas, Rocío, Francisco Javier Álvarez-Martínez, Alberto Falco, Enrique Barrajón-Catalán, Ricardo Mallavia. "Antibiotic-Resistant Bacteria" Encyclopedia, https://encyclopedia.pub/entry/42357 (accessed January 13, 2026).
Díaz-Puertas, R., Álvarez-Martínez, F.J., Falco, A., Barrajón-Catalán, E., & Mallavia, R. (2023, March 20). Antibiotic-Resistant Bacteria. In Encyclopedia. https://encyclopedia.pub/entry/42357
Díaz-Puertas, Rocío, et al. "Antibiotic-Resistant Bacteria." Encyclopedia. Web. 20 March, 2023.
Copy Citation
Antibiotic-resistant bacteria (ARB) is a growing global health threat, leading to the search for alternative strategies to combat bacterial infections. Phytochemicals, which are naturally occurring compounds found in plants, have shown potential as antimicrobial agents. The use of nanotechnology combined with antibacterial phytochemicals could help achieve greater antibacterial capacity against ARB by providing improved mechanical, physicochemical, biopharmaceutical, bioavailability, morphological or release properties.
nanotechnology
nanofibers
nanoparticles
green synthesis
electrospinning
1. Introduction
Bacterial infections pose a major threat to human health worldwide, especially when resistant to conventional antibiotics. In 2019, over 4.95 million fatalities worldwide were associated with antimicrobial resistance (AMR) illnesses, among which 1.27 million were directly linked by them, i.e., fatalities that could have been prevented if the infections had been susceptible to antibiotics, thereby becoming a leading cause of death worldwide in low-resource environments [1]. Due to the alarming appearance of antibiotic-resistant bacteria (ARB) on multiple antibiotics, their rapid spread, and the slow discovery of new antibiotics, conventional therapies are gradually losing effectiveness [2]. There are several factors contributing to the development of ARB, including: (i) the overuse and the misuse of antibiotics [3], often as a consequence of a lack of new ones [4], (ii) poor infection control measures [5], (iii) genetic factors, and (iv) environmental factors [6].
Alternative and complementary treatments to antibiotics have been steadily pursued in the last few decades to address this issue [7]. In this sense, it is predicted that natural sources still harbor a huge number of bioactive molecules that are yet to be discovered, particularly within plants (kingdom Plantae) [8]. Plant extracts can contain a wide variety of phytochemicals such as polyphenols, alkaloids, and terpenoids with proven antibacterial capacity, even against ARB [9][10]. Phytochemicals are usually less potent than traditional antibiotics, although often endowed with therapeutically interesting properties such as molecular promiscuity or AMR-modifying capacities. Not in vain, plant extracts have been used by human communities since ancient times, when scientific knowledge was practically nil and only reduced to trial-and-error screenings [11]. Nowadays, the development of modern technology can help to optimize the use of these phytochemicals and to enhance their benefits for human health, as is the case for nanotechnology.
In recent years, the use of nanotechnology in biomedical applications has been increasing rapidly, observing a pronounced upward trend in the number of scientific articles published in this regard. Nanomaterials such as nanofibers (NFs) and nanoparticles (NPs) can tackle limitations related to traditional approaches [12] and provide beneficial morphologies and surface features to fight bacteria [13]. In addition, the characteristics of these nanomaterials, i.e., size, shape, constituents, and surface, can adjust their mechanical, biological, and physicochemical properties to match the required needs [14]. The polymeric matrices of nanomaterials can result in desirable characteristics, such as small size and high surface-to-volume ratio. These properties can enhance the permeability and solubility of drugs encapsulated within them, making them ideal for drug delivery. Phytochemicals could potentially utilize these features to exert their antimicrobial effects [15]. These features can also improve the biopharmaceutical properties of the final products, with a special interest on low bioavailable compounds [16].
2. Antimicrobial Capacity of Phytochemicals
As briefly mentioned above, poultices and infusions have been prepared from local plants for medicinal purposes since ancient times, including curing bacterial infections [11]. Since the discovery and implementation of antibiotics in the middle of the 20th century, the use of plants as antimicrobials has been drastically reduced. However, the rise of ARB has pushed researchers to search for new antimicrobial compounds from various sources, thus revisiting the plant world as it represents a large reservoir of bioactive molecules with therapeutic potential yet to be explored in depth [17].
One of the main advantages of the use of phytochemicals for antimicrobial purposes is their multifactorial capacity or molecular promiscuity. While traditional antibiotics usually act on a specific bacterial molecular target with great efficacy, phytochemicals can bind to several, but generally with less affinity than that of antibiotics. Such promiscuity or multitarget affinity potentially hinders the generation of possible resistance mechanisms in the bacteria [18]. As it is schematized in Figure 1, the different bacterial molecules targeted by phytochemicals include cell wall [19] and the cell membrane components [20] as well as proteins with diverse locations and functions [21], and thus with the ability of even interfering in the nutrient metabolism and motility [22]. In addition to these pharmacological direct activities, it has been shown that there are phytochemicals, such as certain polyphenols, that are capable of sensitizing ARB by reversing their resistance mechanisms and making them more susceptible to traditional drugs [23][24].
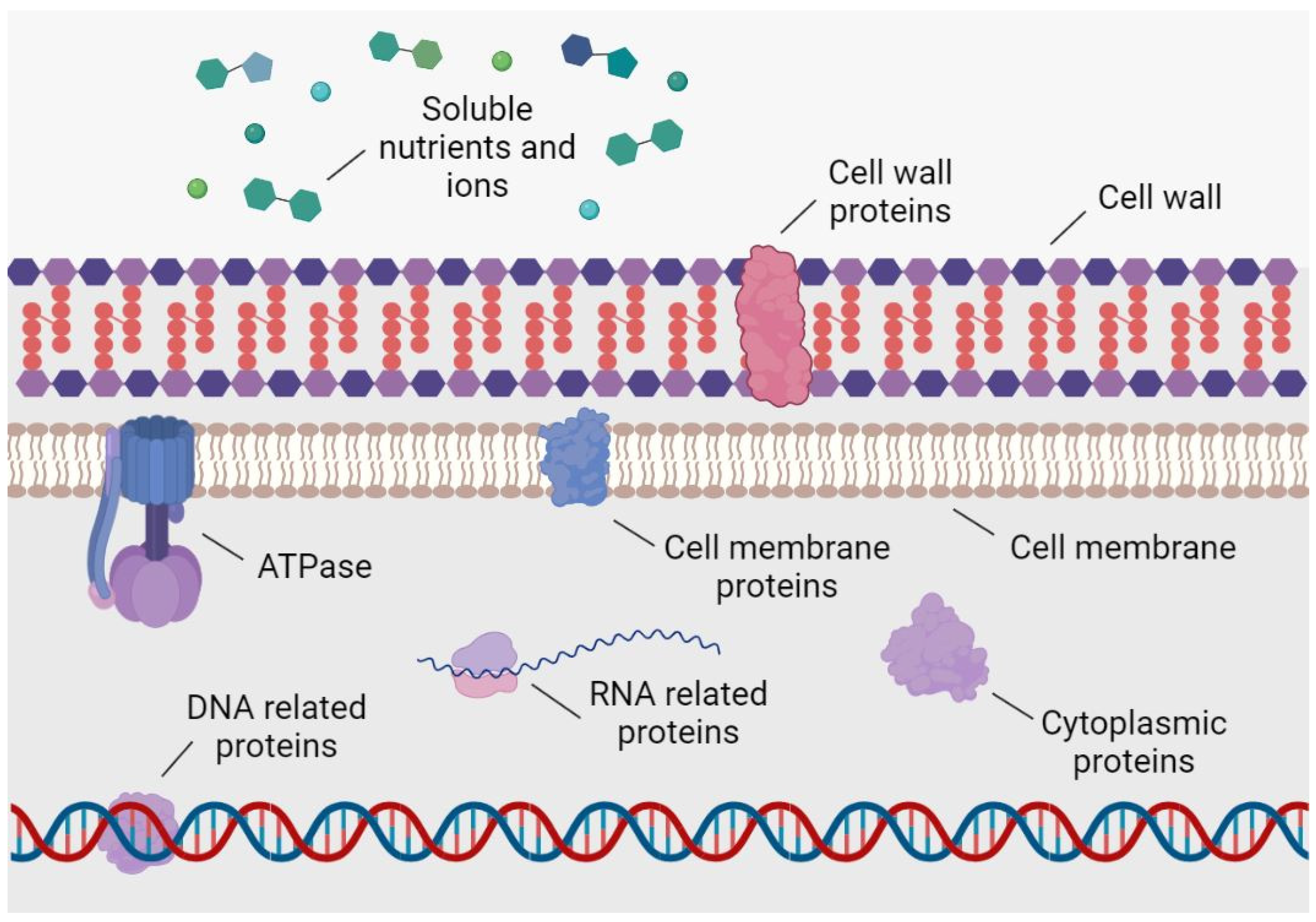
Figure 1. Main bacterial molecular targets of phytochemicals with antibacterial activity.
Phytochemicals may also act in synergy with antibiotics, such as antibiotic adjuvants [25], enhancing their antimicrobial activity and potentially reducing the amount of antibiotic needed to treat an infection [26]. This combination may help to slow the development of AMR, as well as to minimize their adverse effects and environmental impact [27]. Additionally, phytochemicals may help to boost the immune system and improve the overall health of an individual, conferring protection to infection [28].
To date, the literature on AMR to phytochemicals is limited. One example is a study linking genetic changes in Lysteria monocytogenes (deletion of the sigB gene) with increased resistance to carvacrol, thereby showing that it is feasible for bacteria to develop resistance to specific polyphenols with unique or few molecular targets [29]. Another example of AMR to botanicals is the presence of “tannin-resistant” Gram-positive bacteria (Streptococcus sp.) at sites of high exposure to these polyphenols, such as goat, sheep, and deer rumens [30]. This type of bacteria is thought to protect ruminants from possible tannin anti-nutritional dietary influences [31]. Mechanisms by which bacteria overcome the inhibitory effects of tannins on growth include substrate modification, dissociation of tannin-substrate complexes, formation of extracellular polysaccharides, cell membrane modification, and metal ion chelation [32]. Importantly, bacteria prevalent in ruminant gastrointestinal tannin media may not themselves be resistant. This resistance may be more related to improve the nutrient accessibility of bacteria in the special microenvironment of the ruminant stomach [33]. However, drug resistance seems unlikely to develop when complex mixtures of polyphenols affecting multiple molecular targets on bacterial cells are used [34][35], and so it occurs for plant extracts that contain an amalgam of phytochemicals [36].
One of the main limitations for the use of phytochemicals as antibacterial agents is the low availability and poor pharmacokinetic properties. Widely studied phytochemicals such as quercetin [37] and curcumin [38] present these limitations. The use of drug delivery systems, such as nanomaterials, could help to overcome these limitations [39]. In the following sections, the combinations of phytochemicals and different types of nanomaterials will be described.
3. Nanofibers
NFs are one-dimensional nanomaterials whose properties make them suitable for a wide range of applications, including drug delivery [40][41], tissue engineering [42], water/air filtration [43], energy storage [44], protective clothing [45], sensors or photocatalytics [46], among others [47][48]. One of the key features of NFs is their large surface area to volume ratio, which allows them to interact with their surroundings in ways that are not possible with larger fibers [49]. This can make them more effective at adsorbing or filtering small molecules or particles [50].
NFs can be prepared from natural or synthetic polymers, metals, ceramics, semiconducting, composite, and carbon-based materials [51]. Synthetic and natural polymers are particularly used in the synthesis of NFs for biomedical applications due to their biocompatibility, biodegradability, and processability [13][52]. Synthetic polymers include polyethilene glycol (PEG), a water-soluble biocompatible polymer with good drug-carrying capacity [53]; polyvinyl alcohol (PVA), a water-soluble biocompatible polymer [54]; polyvinylpyrrolidone (PVP), a water-soluble polymer with high biocompatibility [55]; polycaprolactone (PCL), a biocompatible and biodegradable polymer [56]; polylactic acid (PLA), a biocompatible, biodegradable polymer that is often used in drug delivery applications [57]; or polyethyleneimine (PEI), a cationic polymer with good drug-carrying capacity and ability to evade the immune system [58]. The most widely used natural polymers for the synthesis of NFs in drug delivery applications are chitosan (CS), a biocompatible, biodegradable polymer derived from chitin [59]; gelatin, a protein derived from collagen, which is a natural polymer found in connective tissue [60]; alginate, a natural polymer derived from brown seaweed, brown algae (Ochrophyta, Phaeophyceae) and bacteria (Azotobacter vinelandii and Pseudomonas species) [61]; hyaluronic acid, a natural polymer found in connective tissue [62]; or dextran, a natural polymer derived from glucose [63]. Natural polymers are biocompatible and have good drug-carrying capacity, making them useful in drug delivery and tissue engineering applications [64]. Overall, the choice of polymer for NF synthesis for drug delivery applications depends on the specific requirements of the application, including the desired drug-carrying capacity, biocompatibility, and other factors. As for other nanomaterials, it is also important to consider the intended route of administration and the stability of the drug in the polymer matrix [65].
3.1. Synthesis of Polymeric NFs
Polymeric NFs can be synthesized using different technologies, such as electrospinning, self-assembly, template-based synthesis, polymerization or sonochemical synthesis [51]. Among the different methods that exist to produce them, electrospinning is the most used because it is simple, cheap, versatile, reproducible, and scalable [66]. Recently, the term “green electrospinning” has emerged as a method for synthesizing NFs using environmentally-friendly and sustainable materials and processes. It involves the use of biodegradable, biocompatible, and renewable materials, as well as energy-efficient and low-waste production methods. It is based on the use of natural or biosynthetic biodegradable polymers and the use of non-toxic solvents [67].
Electrospinning allows NFs to be created by loading and expelling a polymer solution through a needle subjected to a high-voltage electric field. The solution with the desired polymer is drawn into a syringe attached to a needle and pumped at low speeds until a drop forms at the tip of the needle. Subsequently, the solution is subjected to a high electrical charge produced by a high voltage source. As the voltage increases, the drop at the tip of the needle begins to deform until it begins to exert a magnitude of force, such as the surface tension of the solution itself. At this time, a cone shape with convex sides and a rounded tip, known as Taylor cone, begins to form [68]. When a certain voltage threshold is reached, a jet of liquid begins to be emitted. During the movement of the jet between the needle and the collector, the solvent evaporates and a solid polymer fiber is collected. The collector is also connected to the high voltage source and is usually made of conductive metal [69].
Drug encapsulation in NFs can be performed by electrospinning using methods such as blend electrospinning, coaxial electrospinning, and emulsion electrospinning (Figure 2). In blend electrospinning, the drug is mixed with the polymeric solution before the electrospinning process. Therefore, the drug is expected to be dispersed in the polymeric matrix and uniformly distributed in the NFs [70]. Coaxial electrospinning is based on the co-spinning of two solutions using two needles located coaxially, one with the polymeric solution and the other with the therapeutic solution. Core-shell fibers are obtained, where normally the polymeric matrix is found in the outer core and the therapeutic agent is incorporated in the inner core [13]. Emulsion electrospinning solutions are based on two or more immiscible liquid phases that will be electrospun together using the same set up as blend electrospinning [70]. The distribution of the compounds in the NFs depends on their molecular weight. It has been observed that high molecular weight compounds tend to form core-shell structures, while low molecular weight compounds are distributed throughout the NFs [71].
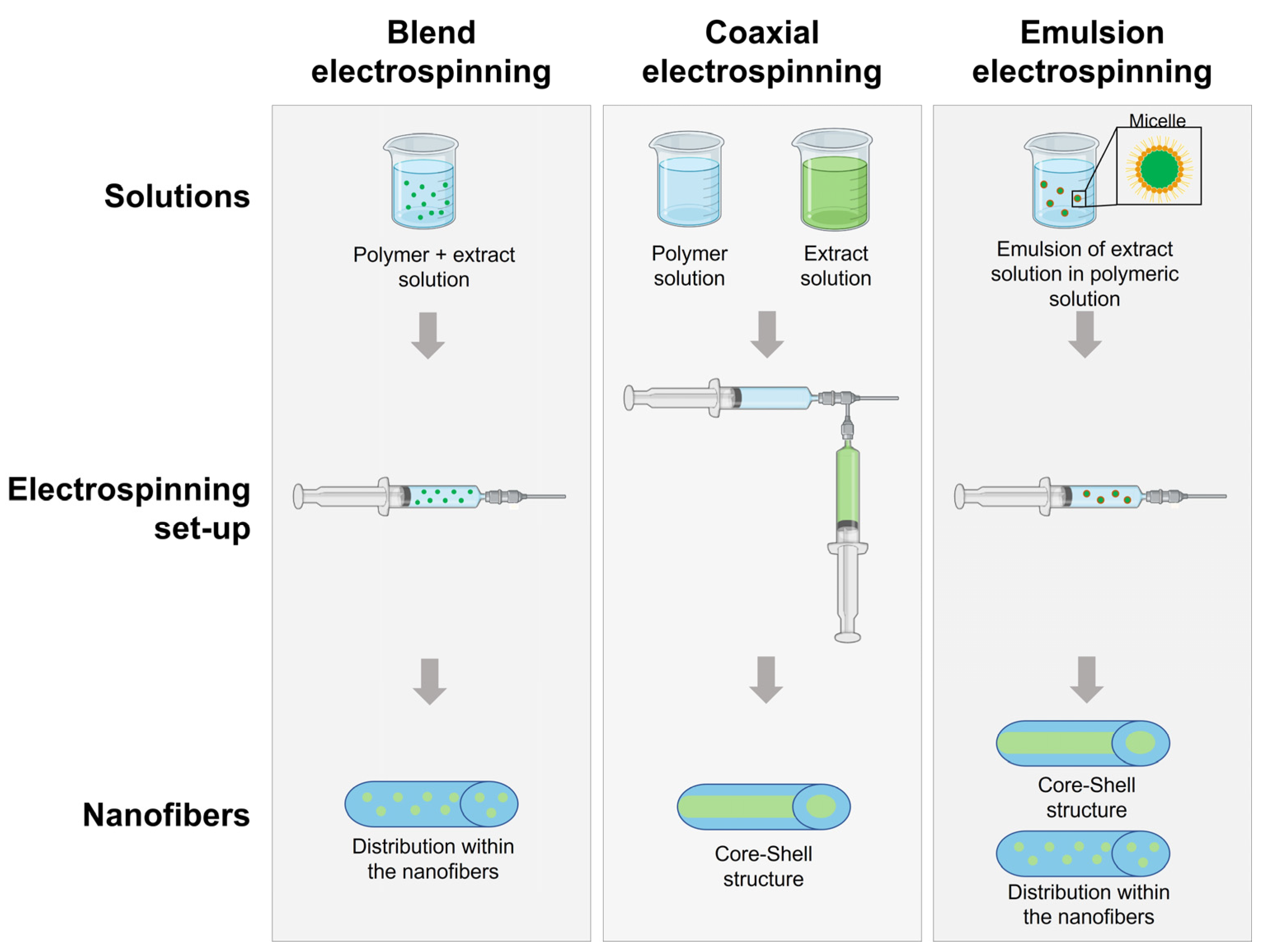
Figure 2. Diagram of electrospinning process for the manufacturing of NFs loaded with plant extracts.
3.2. Antibacterial Properties of Polymeric NFs
NFs can exert their antibacterial activity per se through a variety of mechanisms depending on the specific properties of the NFs, polymers used, and the type of bacteria being targeted. NFs with a small pore size or high surface area can physically entrap bacteria, preventing them from growing or spreading [72]. The surface chemistry of NFs can also affect their ability to interact with bacteria. For example, NFs with a positive charge may be able to attract and kill negatively charged bacteria, while those with a hydrophobic surface may be able to inhibit the growth of hydrophilic bacteria [13]. NFs can be designed to release antimicrobial agents, which can kill or inhibit the growth of bacteria [73]. NFs can also stimulate the immune system, helping to fight off bacterial infections [74]. In addition, by utilizing a polymer with antimicrobial capabilities, such as CS, NFs can exhibit their antibacterial activity. The antibacterial activity of CS can be attributed to its adsorptive characteristics to bacterial cells due to electrostatic interactions between the polycationic structure of CS and the anionic groups found on the bacterial cell surface [75]. This causes permeabilization of the cell membrane and the loss of essential constituents as enzymes, nucleotides, ions, and death of the bacterial cell.
References
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655.
- Huemer, M.; Mairpady Shambat, S.; Brugger, S.D.; Zinkernagel, A.S. Antibiotic resistance and persistence—Implications for human health and treatment perspectives. EMBO Rep. 2020, 21, e51034.
- Malik, B.; Bhattacharyya, S. Antibiotic drug-resistance as a complex system driven by socio-economic growth and antibiotic misuse. Sci. Rep. 2019, 9, 9788.
- Hays, J.P.; Ruiz-Alvarez, M.J.; Roson-Calero, N.; Amin, R.; Murugaiyan, J.; van Dongen, M.B.M.; Global, A.M.R.I.A.N. Perspectives on the Ethics of Antibiotic Overuse and on the Implementation of (New) Antibiotics. Infect. Dis. Ther. 2022, 11, 1315–1326.
- Avershina, E.; Shapovalova, V.; Shipulin, G. Fighting Antibiotic Resistance in Hospital-Acquired Infections: Current State and Emerging Technologies in Disease Prevention, Diagnostics and Therapy. Front. Microbiol. 2021, 12, 2044.
- Larsson, D.G.J.; Flach, C.-F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269.
- AlSheikh, H.M.; Sultan, I.; Kumar, V.; Rather, I.A.; Al-Sheikh, H.; Tasleem Jan, A.; Haq, Q.M. Plant-Based Phytochemicals as Possible Alternative to Antibiotics in Combating Bacterial Drug Resistance. Antibiotics 2020, 9, 480.
- Van vuuren, S.; Viljoen, A. Plant-Based Antimicrobial Studies-Methods and Approaches to Study the Interaction between Natural Products. Planta Med. 2011, 77, 1168–1182.
- Álvarez-Martínez, F.J.; Rodríguez, J.C.; Borrás-Rocher, F.; Barrajón-Catalán, E.; Micol, V. The antimicrobial capacity of Cistus salviifolius and Punica granatum plant extracts against clinical pathogens is related to their polyphenolic composition. Sci. Rep. 2021, 11, 588.
- Jubair, N.; Rajagopal, M.; Chinnappan, S.; Abdullah, N.B.; Fatima, A. Review on the Antibacterial Mechanism of Plant-Derived Compounds against Multidrug-Resistant Bacteria (MDR). Evid.-Based Complement. Altern. Med. 2021, 2021, 3663315.
- Tapsell, L.C.; Hemphill, I.; Cobiac, L.; Sullivan, D.R.; Fenech, M.; Patch, C.S.; Roodenrys, S.; Keogh, J.B.; Clifton, P.M.; Williams, P.G.; et al. Health benefits of herbs and spices: The past, the present, the future. Med. J. Aust. 2006, 185, S1–S24.
- Barani, M.; Zeeshan, M.; Kalantar-Neyestanaki, D.; Farooq, M.A.; Rahdar, A.; Jha, N.K.; Sargazi, S.; Gupta, P.K.; Thakur, V.K. Nanomaterials in the Management of Gram-Negative Bacterial Infections. Nanomaterials 2021, 11, 2535.
- Hamdan, N.; Yamin, A.; Hamid, S.A.; Khodir, W.K.; Guarino, V. Functionalized Antimicrobial Nanofibers: Design Criteria and Recent Advances. J. Funct. Biomater. 2021, 12, 59.
- Spizzirri, U.G.; Aiello, F.; Carullo, G.; Facente, A.; Restuccia, D. Nanotechnologies: An Innovative Tool to Release Natural Extracts with Antimicrobial Properties. Pharmaceutics 2021, 13, 230.
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731.
- Mira, A.; Rubio-Camacho, M.; Alarcón, D.; Rodríguez-Cañas, E.; Fernández-Carvajal, A.; Falco, A.; Mallavia, R. L-Menthol-Loadable Electrospun Fibers of PMVEMA Anhydride for Topical Administration. Pharmaceutics 2021, 13, 1845.
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards Advances in Medicinal Plant Antimicrobial Activity: A Review Study on Challenges and Future Perspectives. Microorganisms 2021, 9, 2041.
- Álvarez-Martínez, J.F.; Barrajón-Catalán, E.; Encinar, A.J.; Rodríguez-Díaz, C.J.; Micol, V. Antimicrobial Capacity of Plant Polyphenols against Gram-positive Bacteria: A Comprehensive Review. Curr. Med. Chem. 2020, 27, 2576–2606.
- Dimech, G.S.; Soares, L.A.L.; Ferreira, M.A.; de Oliveira, A.G.V.; Carvalho, M.d.C.; Ximenes, E.A. Phytochemical and Antibacterial Investigations of the Extracts and Fractions from the Stem Bark of Hymenaea stigonocarpa Mart. ex Hayne and Effect on Ultrastructure of <i>Staphylococcus aureus</i> Induced by Hydroalcoholic Extract. Sci. World J. 2013, 2013, 862763.
- Meng, X.; Li, D.; Zhou, D.; Wang, D.; Liu, Q.; Fan, S. Chemical composition, antibacterial activity and related mechanism of the essential oil from the leaves of Juniperus rigida Sieb. et Zucc against Klebsiella pneumoniae. J. Ethnopharmacol. 2016, 194, 698–705.
- Tiwari, V. Molecular insight into the therapeutic potential of phytoconstituents targeting protein conformation and their expression. Phytomedicine 2019, 52, 225–237.
- Kim, G.; Xu, Y.; Zhang, J.; Sui, Z.; Corke, H. Antibacterial Activity and Multi-Targeting Mechanism of Dehydrocorydaline From Corydalis turtschaninovii Bess. Against Listeria monocytogenes. Front. Microbiol. 2022, 12, 3957.
- Hatano, T.; Kusuda, M.; Inada, K.; Ogawa, T.-o.; Shiota, S.; Tsuchiya, T.; Yoshida, T. Effects of tannins and related polyphenols on methicillin-resistant Staphylococcus aureus. Phytochemistry 2005, 66, 2047–2055.
- Sousa, V.; Luís, Â.; Oleastro, M.; Domingues, F.; Ferreira, S. Polyphenols as resistance modulators in Arcobacter butzleri. Folia Microbiol. 2019, 64, 547–554.
- Wright, G.D. Antibiotic Adjuvants: Rescuing Antibiotics from Resistance. Trends Microbiol. 2016, 24, 862–871.
- Khameneh, B.; Eskin, N.A.M.; Iranshahy, M.; Fazly Bazzaz, B.S. Phytochemicals: A Promising Weapon in the Arsenal against Antibiotic-Resistant Bacteria. Antibiotics 2021, 10, 1044.
- Kumar, M.; Jaiswal, S.; Sodhi, K.K.; Shree, P.; Singh, D.K.; Agrawal, P.K.; Shukla, P. Antibiotics bioremediation: Perspectives on its ecotoxicity and resistance. Environ. Int. 2019, 124, 448–461.
- Bhattacharya, S.; Paul, S.M.N. Efficacy of phytochemicals as immunomodulators in managing COVID-19: A comprehensive view. VirusDisease 2021, 32, 435–445.
- Ait-Ouazzou, A.; Espina, L.; Gelaw, T.K.; de Lamo-Castellví, S.; Pagán, R.; García-Gonzalo, D. New insights in mechanisms of bacterial inactivation by carvacrol. J. Appl. Microbiol. 2013, 114, 173–185.
- Brooker, J.D.; O’Donovan, L.A.; Skene, I.; Clarke, K.; Blackall, L.; Muslera, P. Streptococcus caprinus sp.nov., a tannin-resistant ruminal bacterium from feral goats. Lett. Appl. Microbiol. 1994, 18, 313–318.
- Butler, L.G. Antinutritional Effects of Condensed and Hydrolyzable Tannins. In Plant Polyphenols: Synthesis, Properties, Significance; Hemingway, R.W., Laks, P.E., Eds.; Springer US: Boston, MA, USA, 1992; pp. 693–698.
- Smith, A.H.; Zoetendal, E.; Mackie, R.I. Bacterial Mechanisms to Overcome Inhibitory Effects of Dietary Tannins. Microb. Ecol. 2005, 50, 197–205.
- Monagas, M.; Urpi-Sarda, M.; Sánchez-Patán, F.; Llorach, R.; Garrido, I.; Gómez-Cordovés, C.; Andres-Lacueva, C.; Bartolomé, B. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct. 2010, 1, 233–253.
- Gupta, P.D.; Birdi, T.J. Development of botanicals to combat antibiotic resistance. J. Ayurveda Integr. Med. 2017, 8, 266–275.
- Warnke, P.H.; Becker, S.T.; Podschun, R.; Sivananthan, S.; Springer, I.N.; Russo, P.A.J.; Wiltfang, J.; Fickenscher, H.; Sherry, E. The battle against multi-resistant strains: Renaissance of antimicrobial essential oils as a promising force to fight hospital-acquired infections. J. Cranio-Maxillofac. Surg. 2009, 37, 392–397.
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Micol, V. Tackling Antibiotic Resistance with Compounds of Natural Origin: A Comprehensive Review. Biomedicines 2020, 8, 405.
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273.
- Hassanzadeh, K.; Buccarello, L.; Dragotto, J.; Mohammadi, A.; Corbo, M.; Feligioni, M. Obstacles against the Marketing of Curcumin as a Drug. Int. J. Mol. Sci. 2020, 21, 6619.
- Vinayak, M.; Maurya, A. Quercetin Loaded Nanoparticles in Targeting Cancer: Recent Development. Anti-Cancer Agents Med. Chem. 2019, 19, 1560–1576.
- Torres-Martinez, J.E.; Cornejo Bravo, M.J.; Serrano Medina, A.; Pérez González, L.G.; Villarreal Gómez, J.L. A Summary of Electrospun Nanofibers as Drug Delivery System: Drugs Loaded and Biopolymers Used as Matrices. Curr. Drug Deliv. 2018, 15, 1360–1374.
- Martínez-Ortega, L.; Mira, A.; Fernandez-Carvajal, A.; Mateo, C.R.; Mallavia, R.; Falco, A. Development of A New Delivery System Based on Drug-Loadable Electrospun Nanofibers for Psoriasis Treatment. Pharmaceutics 2019, 11, 14.
- Nemati, S.; Kim, S.-j.; Shin, Y.M.; Shin, H. Current progress in application of polymeric nanofibers to tissue engineering. Nano Converg. 2019, 6, 36.
- Sundarrajan, S.; Tan, K.L.; Lim, S.H.; Ramakrishna, S. Electrospun Nanofibers for Air Filtration Applications. Procedia Eng. 2014, 75, 159–163.
- Wei, J.; Geng, S.; Pitkänen, O.; Järvinen, T.; Kordas, K.; Oksman, K. Green Carbon Nanofiber Networks for Advanced Energy Storage. ACS Appl. Energy Mater. 2020, 3, 3530–3540.
- Baji, A.; Agarwal, K.; Oopath, S.V. Emerging Developments in the Use of Electrospun Fibers and Membranes for Protective Clothing Applications. Polymers 2020, 12, 492.
- Asgari, S.; Mohammadi Ziarani, G.; Badiei, A.; Ajalloueian, F.; Vasseghian, Y. Electrospun composite nanofibers as novel high-performance and visible-light photocatalysts for removal of environmental pollutants: A review. Environ. Res. 2022, 215, 114296.
- Lou, L.; Osemwegie, O.; Ramkumar, S.S. Functional Nanofibers and Their Applications. Ind. Eng. Chem. Res. 2020, 59, 5439–5455.
- Falco, A.; Mallavia, R. Electrospun Nanomaterials: Applications in Food, Environmental Remediation, and Bioengineering. Nanomaterials 2020, 10, 1714.
- Vimala Bharathi, S.K.; Murugesan, P.; Moses, J.A.; Anandharamakrishnan, C. 3.43-Recent Trends in Nanocomposite Packaging Materials. In Innovative Food Processing Technologies; Knoerzer, K., Muthukumarappan, K., Eds.; Elsevier: Oxford, UK, 2021; pp. 731–755.
- Choi, H.-J.; Kumita, M.; Hayashi, S.; Yuasa, H.; Kamiyama, M.; Seto, T.; Tsai, C.-J.; Otani, Y. Filtration Properties of Nanofiber/Microfiber Mixed Filter and Prediction of its Performance. Aerosol Air Qual. Res. 2017, 17, 1052–1062.
- Deepthi, S. New Perspective of Nano Fibers: Synthesis and Applications. In Nanofibers; Brajesh, K., Ed.; IntechOpen: Rijeka, Croatia, 2021; p. Ch. 1.
- Mira, A.; Mateo, C.R.; Mallavia, R.; Falco, A. Poly(methyl vinyl ether-alt-maleic acid) and ethyl monoester as building polymers for drug-loadable electrospun nanofibers. Sci. Rep. 2017, 7, 17205.
- Hoang Thi, T.T.; Pilkington, E.H.; Nguyen, D.H.; Lee, J.S.; Park, K.D.; Truong, N.P. The Importance of Poly(ethylene glycol) Alternatives for Overcoming PEG Immunogenicity in Drug Delivery and Bioconjugation. Polymers 2020, 12, 298.
- Singh, R.; Kumar, N.; Mehrotra, T.; Bisaria, K.; Sinha, S. Chapter 9-Environmental hazards and biodegradation of plastic waste: Challenges and future prospects. In Bioremediation for Environmental Sustainability; Saxena, G., Kumar, V., Shah, M.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 193–214.
- Zeka, K.; Coppa, P.; Corsi, L.; Pajewski, L.A.; Veglio, F.; Continenza, M. In vitro biocompatibility of a new PVP-Hydrogel. Ital. J. Anat. Embryol. 2013, 117, 202.
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-based materials in biomedical applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893.
- Singhvi, M.S.; Zinjarde, S.S.; Gokhale, D.V. Polylactic acid: Synthesis and biomedical applications. J. Appl. Microbiol. 2019, 127, 1612–1626.
- Wang, X.; Niu, D.; Hu, C.; Pei, L.Z. Polyethyleneimine-Based Nanocarriers for Gene Delivery. Curr. Pharm. Des. 2015, 21, 6140–6156.
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A Natural Biopolymer with a Wide and Varied Range of Applications. Molecules 2020, 25, 3981.
- Rehman, W.; Majeed, A.; Mehra, R.; Bhushan, S.; Rani, P.; Saini, K.; Bast, F. Gelatin: A Comprehensive Report Covering its Indispensable Aspects; Nova Science Publishers: New York, NY, USA, 2016; pp. 209–222.
- Kothale, D.; Verma, U.; Dewangan, N.; Jana, P.; Jain, A.; Jain, D. Alginate as Promising Natural Biodegradable Polymer for Pharmaceutical, Food, and Biomedical Applications. Curr. Drug Deliv. 2020, 17, 755–775.
- Sionkowska, A.; Gadomska, M.; Musiał, K.; Piątek, J. Hyaluronic Acid as a Component of Natural Polymer Blends for Biomedical Applications: A Review. Molecules 2020, 25, 4035.
- Zarrintaj, P.; Saeb, M.R.; Jafari, S.H.; Mozafari, M. Chapter 18-Application of compatibilized polymer blends in biomedical fields. In Compatibilization of Polymer Blends; A.R, A., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 511–537.
- Nunes, C.S.; Philipps-Wiemann, P. Chapter 22-Formulation of enzymes. In Enzymes in Human and Animal Nutrition; Nunes, C.S., Kumar, V., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 429–440.
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials 2020, 10, 1403.
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for drug delivery applications: A review. J. Control. Release 2021, 334, 463–484.
- Lee, K.Y.; Jeong, L.; Kang, Y.O.; Lee, S.J.; Park, W.H. Electrospinning of polysaccharides for regenerative medicine. Adv. Drug Deliv. Rev. 2009, 61, 1020–1032.
- John, J.V.; McCarthy, A.; Xie, J. Chapter 9-Electrospun nanofiber matrix for tissue repair and regeneration. In Tissue Engineering; Sharma, C.P., Chandy, T., Thomas, V., Thankam, F.G., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 175–191.
- Davoodi, P.; Gill, E.L.; Wang, W.; Shery Huang, Y.Y. Chapter Two-Advances and innovations in electrospinning technology. In Biomedical Applications of Electrospinning and Electrospraying; Kasoju, N., Ye, H., Eds.; Woodhead Publishing: Cambridge, UK, 2021; pp. 45–81.
- Buzgo, M.; Mickova, A.; Rampichova, M.; Doupnik, M. 11-Blend electrospinning, coaxial electrospinning, and emulsion electrospinning techniques. In Core-Shell Nanostructures for Drug Delivery and Theranostics; Focarete, M.L., Tampieri, A., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 325–347.
- Nikmaram, N.; Roohinejad, S.; Hashemi, S.; Koubaa, M.; Barba, F.J.; Abbaspourrad, A.; Greiner, R. Emulsion-based systems for fabrication of electrospun nanofibers: Food, pharmaceutical and biomedical applications. RSC Adv. 2017, 7, 28951–28964.
- Singh, B.; Kim, K.; Park, M.-H. On-Demand Drug Delivery Systems Using Nanofibers. Nanomaterials 2021, 11, 3411.
- Mira, A.; Sainz-Urruela, C.; Codina, H.; Jenkins, S.I.; Rodriguez-Diaz, J.C.; Mallavia, R.; Falco, A. Physico-Chemically Distinct Nanomaterials Synthesized from Derivates of a Poly(Anhydride) Diversify the Spectrum of Loadable Antibiotics. Nanomaterials 2020, 10, 486.
- Lindner, H.B.; Zhang, A.; Eldridge, J.; Demcheva, M.; Tsichilis, P.; Seth, A.; Vournakis, J.; Muise-Helmericks, R.C. Anti-Bacterial Effects of Poly-N-Acetyl-Glucosamine Nanofibers in Cutaneous Wound Healing: Requirement for Akt1. PLoS ONE 2011, 6, e18996.
- Confederat, L.G.; Tuchilus, C.G.; Dragan, M.; Sha’at, M.; Dragostin, O.M. Preparation and Antimicrobial Activity of Chitosan and Its Derivatives: A Concise Review. Molecules 2021, 26, 3694.
More
Information
Subjects:
Biotechnology & Applied Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
21 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




