
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Collins Ngoe Elangwe | -- | 3062 | 2023-03-20 10:36:30 | | | |
| 2 | Jessie Wu | Meta information modification | 3062 | 2023-03-21 01:49:56 | | | | |
| 3 | Jessie Wu | Meta information modification | 3062 | 2023-03-21 01:51:06 | | |
Video Upload Options
Polysaccharide polymers have been used to fabricate wound dressings. The applications of biopolymers, such as chitin, gelatin, pullulan, and chitosan, have greatly expanded in the biomedical field due to their non-toxic, antibacterial, biocompatible, hemostatic, and nonimmunogenic properties. Most of these polymers have been used in the form of foams, films, sponges, and fibers in drug carrier devices, skin tissue scaffolds, and wound dressings. Currently, special focus has been directed towards the fabrication of wound dressings based on synthesized hydrogels using natural polymers. The high-water retention capacity of hydrogels makes them potent candidates for wound dressings as they provide a moist environment in the wound and remove excess wound fluid, thereby accelerating wound healing. The incorporation of pullulan with different, naturally occurring polymers, such as chitosan, in wound dressings is currently attracting much attention due to the antimicrobial, antioxidant and nonimmunogenic properties. Despite the valuable properties of pullulan, it also has some limitations, such as poor mechanical properties and high cost. However, these properties are improved by blending it with different polymers. Additionally, more investigations are required to obtain pullulan derivatives with suitable properties in high quality wound dressings and tissue engineering applications.
1. Introduction
2. Applications of Pullulan-Based Biomaterials as Wound Dressings and Skin Tissue Engineering Scaffolds
| Application | System Used | Drug/Growth Enhancing Factor(s) | Cell Types Treated | Reference |
|---|---|---|---|---|
| Wound healing | Pullulan film | - | Rat skin cells | [23] |
| Wound dressing | Keratin/pullulan/PVA hydrogel membrane | Cefotaxime | Incision on male SD rat skin cells | [24] |
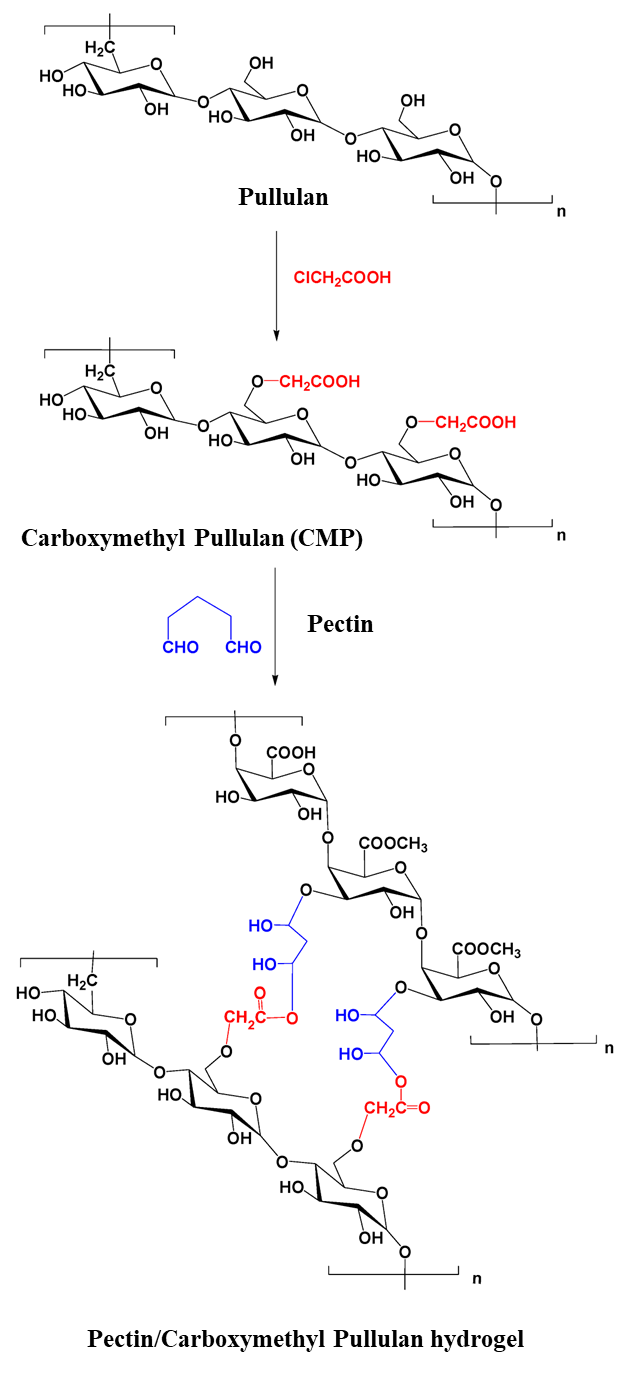
| Wound dressing and antibacterial effect | Carboxymethyl pullulan hydrogels | Gentamicin | In vitro drug release studies in phosphate buffer saline solution | [25] |
| Wound healing | Hyaluronic/pullulan/ grafted-Pluronic F127 hydrogel |
Curcumin | Female rat skin cells | [26] |
| High oxidative stress wound dressing | Pullulan hydrogels | Mesenchymal stromal cell | Anterior and posterior full thickness transverse incisions on skin cells | [27] |
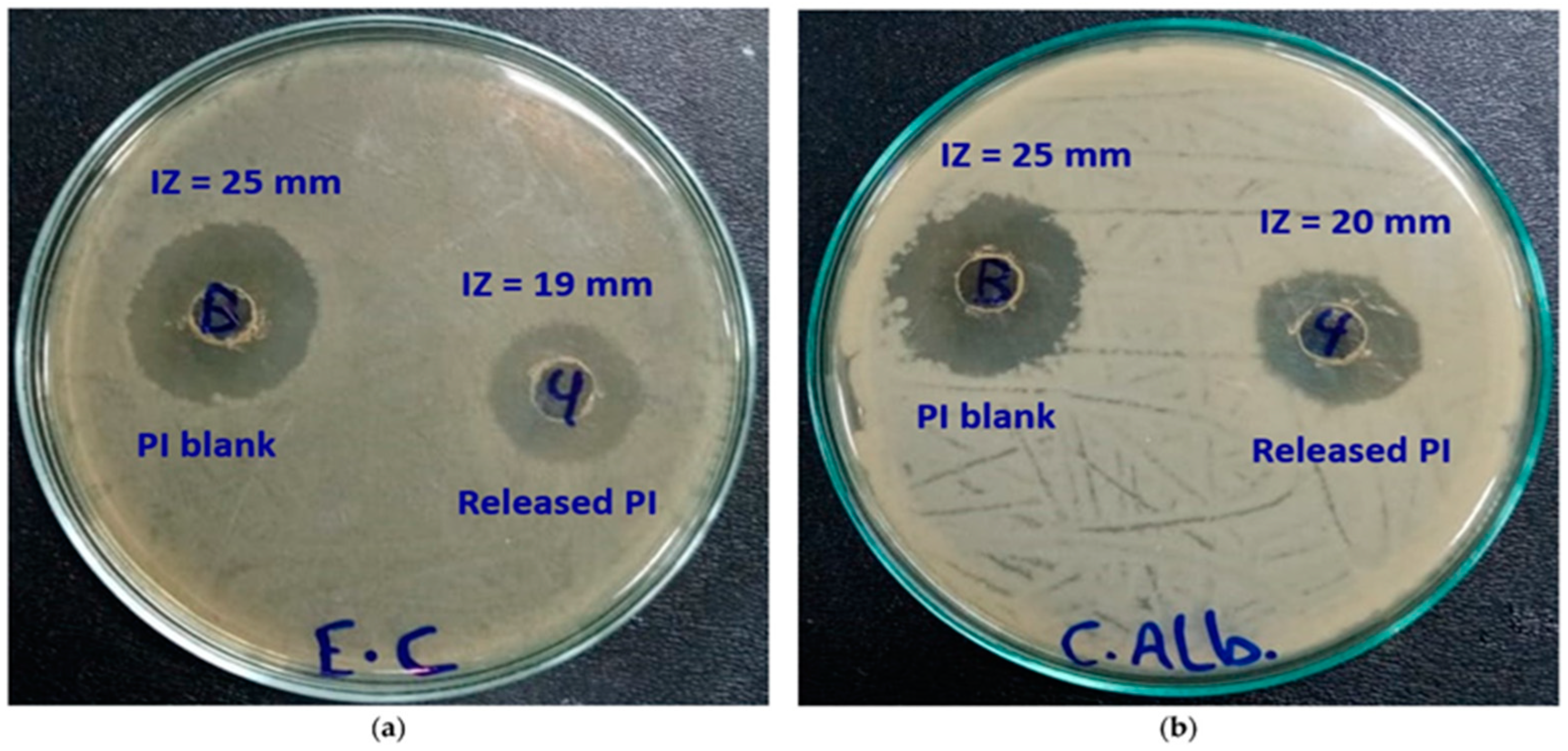
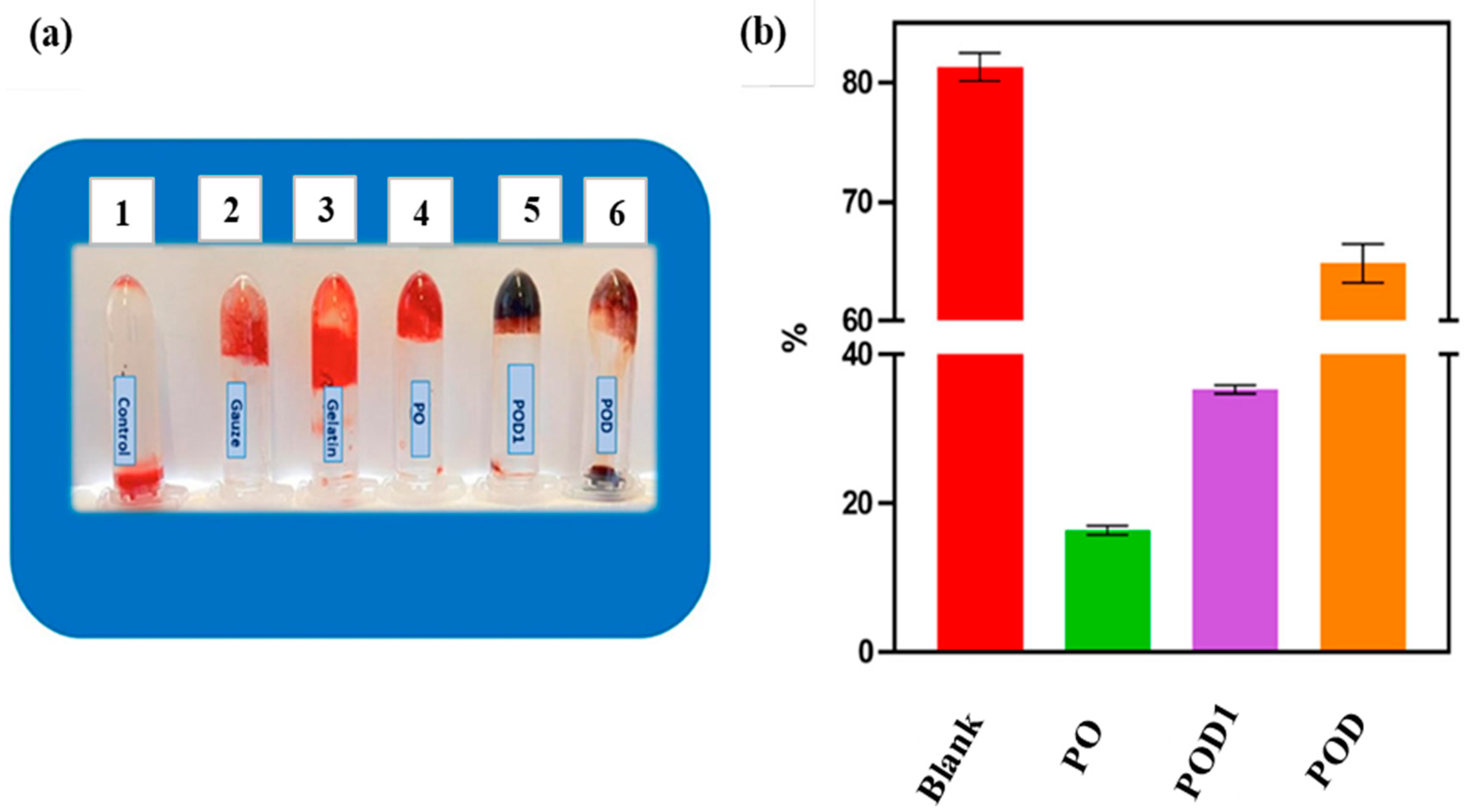
| Wound dressing | Pullulan/dopamine hydrogel | - | In vitro studies of sheep blood | [28] |
| Wound healing | Pullulan/chitosan composite nanofibers | Tannic acid | NIH 3T3 mouse embryonic fibroblast cells | [29] |
| Wound dressing and antioxidant effect | Pullulan/bacterial cellulose hydrogel | Vitamin C and E | In vitro studies in phosphate saline buffer | [30] |
| Early cutaneous wound healing | Pullulan/collagen composite hydrogels | - | Full thickness skin cells incisions | [31] |
| Wound dressing | Collagen/pullulan hydrogel | Polydatin | Wistar rat skin cells | [32] |
| Wound healing | Cholesterol bearing pullulan nanogels | Prostaglandin E1 | Full thickness defect on rat dermal cells | [33] |
| Wound dressing and drug delivery system | Pu-g-p(AA-co-IA) hydrogel film | Ampicillin | In vitro drug release study in phosphate buffer saline solution | [34] |
References
- Firlar, I.; Altunbek, M.; McCarthy, C.; Ramalingam, M.; Camci-Unal, G. Functional Hydrogels for Treatment of Chronic Wounds. Gels 2022, 8, 127.
- Turner, N.J.; Badylak, S.F. The use of biologic scaffolds in the treatment of chronic nonhealing wounds. Adv. Wound Care 2015, 4, 490–500.
- Sivaraj, D.; Chen, K.; Chattopadhyay, A.; Henn, D.; Wu, W.; Noishiki, C.; Magbual, N.J.; Mittal, S.; Mermin-Bunnell, A.M.; Bonham, C.A.; et al. Hydrogel scaffolds to deliver cell therapies for wound healing. Front. Bioeng. Biotechnol. 2021, 9, 660145.
- Naseri-Nosar, M.; Ziora, Z.M. Wound Dressings from Naturally occurring Polymers: A Review on Homopolysac charide-based Composites. Carbohydr. Polym. 2018, 89, 379–398.
- Archana, D.; Dutta, J.; Dutta, P.K. Evaluation of chitosan nano dressing for wound healing: Characterization, in vitro and in vivo studies. Int. J. Biol. Macromol. 2013, 57, 193–203.
- Farzamfar, S.; Naseri-Nosar, M.; Samadian, H.; Mahakizadeh, S.; Tajerian, R.; Rahmati, M.; Vaez, A.; Salehi, M. Taurine-loaded poly (ε-caprolactone)/gelatin electrospun film as a potential wound dressing material: In vitro and in vivo evaluation. J. Bioact. Compat. Polym. 2018, 33, 282–294.
- Savencu, I.; Iurian, S.; Porfire, A.; Bogdan, C.; Tomuța, I. Review of advances in polymeric wound dressing films. React. Funct. Polym. 2021, 168, 105059.
- Bai, M.Y.; Chen, M.C.; Yu, W.C.; Lin, J.Y. Foam dressing incorporating herbal extract: An all-natural dressing for potential use in wound healing. J. Bioact. Compat. Polym. 2017, 32, 293–308.
- Mano, J.; Silva, G.; Azevedo, H.S.; Malafaya, P.; Sousa, R.; Silva, S.; Boesel, L.; Oliveira, J.M.; Santos, T.; Marques, A. Natural origin biodegradable systems in tissue engineering and regenerative medicine: Present status and some moving trends. J. R. Soc. Interface 2007, 4, 999–1030.
- Mogoşanu, G.D.; Grumezescu, A.M. Natural and synthetic polymers for wounds and burns dressing. Int. J. Pharm. 2014, 463, 127–136.
- Jain, A.; Gupta, Y.; Jain, S.K. Perspectives of biodegradable natural polysaccharides for site specific drug delivery to the colon. J. Pharm. Pharm. Sci. 2007, 10, 86–128.
- d’Ayala, G.G.; Malinconico, M.; Laurienzo, P. Marine derived polysaccharides for biomedical applications: Chemical modification approaches. Molecules 2008, 13, 2069–2106.
- Malafaya, P.B.; Silva, G.A.; Reis, R.L. Natural–origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Adv. Drug Deliv. Rev. 2007, 59, 207–233.
- Cui, R.; Zhang, L.; Ou, R.; Xu, Y.; Xu, L.; Zhan, X.-Y.; Li, D. Polysaccharide-Based Hydrogels for Wound Dressing: Design Considerations and Clinical Applications. Front. Bioeng. Biotechnol. 2022, 10, 845735.
- Tang, N.; Zhang, R.; Zheng, Y.; Wang, J.; Khatib, M.; Jiang, X.; Zhou, C.; Omar, R.; Saliba, W.; Wu, W.; et al. Highly Efficient Self-Healing Multifunctional Dressing with Antibacterial Activity for Sutureless Wound Closure and Infected Wound Monitoring. Adv. Mater. 2022, 34, 2106842.
- Duceac, A.I.; Vereștiuc, L.; Coroaba, A.; Arotăriței, D.; Coseri, S. All-polysaccharide hydrogels for drug delivery applications: Tunable chitosan beads surfaces via physical or chemical interactions, using oxidized pullulan. Int. J. Biol. Macromol. 2021, 181, 1047–1062.
- Emam, H.E.; Mohamed, A.L. Controllable Release of Povidone-Iodine from Networked Pullulan Hydrogel. Polymers 2021, 13, 3118.
- Priya, V.S.; Iyappan, K.; Gayathri, V.S.; William, S.; Suguna, L. Influence of pullulan hydrogel on sutureless wound healing in rats. Wound Med. 2016, 14, 1–5.
- Chen, K.; Sivaraj, D.; Davitt, M.F.; Leeolou, M.C.; Henn, D.; Steele, S.R.; Huskins, S.L.; Trotsyuk, A.A.; Kussie, H.C.; Greco, A.H.; et al. Pullulan-Collagen hydrogel wound dressing promotes dermal remodelling and wound healing compared to commercially available collagen dressings. Wound Rep. Reg. 2022, 30, 397–408.
- Barrera, J.A.; Trotsyuk, A.A.; Maan, Z.N.; Bonham, C.A.; Larson, M.M.R.; Mittermiller, P.A.; Henn, D.; Chen, K.; Mays, M.C.J.; Mittal, M.S.; et al. Adipose-derived stromal cells seeded in pullulan-collagen hydrogels improve healing in murine burns. Tissue Eng. Part A 2021, 27, 844–856.
- Nicholas, M.N.; Jeschke, M.G.; Amini-Nik, S. Cellularized bilayer pullulan-gelatin hydrogel for skin regeneration. Tissue Eng. Part A 2016, 9, 754–764.
- Wang, X.; Zhang, D.; Wang, J.; Tang, R.; Wei, B.; Jiang, Q. Succinyl Pullulan-Crosslinked Carboxymethyl Chitosan Sponges for Potential Wound Dressing. Int. J. Polym. Mater. Polym. 2017, 66, 61–70.
- Suguna, L. Wound healing potential of a biodegradable film from pullulan in rats. Clin. Exp. Dermatol. 2014, 5, 63.
- Khaliq, T.; Sohail, M.; Shah, S.A.; Mahmood, A.; Kousar, M.; Jabeen, N. Bioactive and multifunctional keratin-pullulan based hydrogel membranes facilitate re-epithelization in diabetic model. Int. J. Biol. Macromol. Part B 2022, 209, 1826–1836.
- Li, H.; Yang, J.; Hu, X.; Liang, J.; Fan, Y.; Zhang, X. Superabsorbent polysaccharide hydrogels based on pullulan derivate as antibacterial release wound dressing. J. Biomed. Mater. Res. 2011, 98A, 31–39.
- Shah, S.A.; Sohail, M.; Minhas, M.U.; Khan, S.; Hussain, Z.; Mahmood, A.; Kousar, M.; Thu, H.E.; Abbasi, M.; Kashif, M.U.R. Curcumin-laden hyaluronic acid-co-Pullulan-based biomaterials as a potential platform to synergistically enhance the diabetic wound repair. Int. J. Biol. Macromol. 2021, 185, 350–368.
- Wong, V.W.; Rustad, K.C.; Glotzbach, J.P.; Sorkin, M.; Inayathullah, M.; Major, M.R.; Longaker, M.T.; Rajadas, J.; Gurtner, G.C. Pullulan hydrogels improve mesenchymal stem cell delivery into high-oxidative-stress wounds. Macromol. Biosci. 2011, 11, 1458–1466.
- Baron, R.I.; Duceac, I.A.; Morariu, S.; Bostanaru-Iliescu, A.-C.; Coseri, S. Hemostatic Cryogels Based on Oxidized Pullulan/Dopamine with Potential Use as Wound Dressings. Gels 2022, 8, 726.
- Xu, F.; Weng, B.; Gilkerson, R.; Materon, L.A.; Lozano, K. Development of tannic acid/chitosan/pullulan composite nanofibers from aqueous solution for potential applications as wound dressing. Carbohydr. Polym. 2015, 115, 16–24.
- Atila, D.; Karataş, A.; Keskin, D.; Tezcaner, A. Pullulan hydrogel-immobilized bacterial cellulose membranes with dual-release of vitamin C and E for wound dressing applications. Int. J. Biol. Macromol. 2022, 218, 760–774.
- Wong, V.W.; Rustad, K.C.; Galvez, M.G.; Neofytou, E.; Glotzbach, J.P.; Januszyk, M.; Major, M.R.; Sorkin, M.; Longaker, M.T.; Rajadas, J.; et al. Engineered pullulan-collagen composite dermal hydrogels improve early cutaneous wound healing. Tissue Eng. Part A 2011, 17, 631–644.
- Selvakumar, G.; Lonchin, S. Bioactive functional collagen-oxidized pullulan scaffold loaded with polydatin for treating chronic wounds. Biomater. Adv. 2022, 140, 213078.
- Kobayashi, H.; Katakura, O.; Morimoto, N.; Akiyoshi, K.; Kasugai, S. Effects of cholesterol-bearing pullulan (CHP)-nanogels in combination with prostaglandin E1 on wound healing. J. Biomed. Mater. Res. Part B Appl. Biomater. J. 2009, 91, 55–60.
- Mert, H.; Ozkahraman, B.; Damar, H. A novel wound dressing material: Pullulan grafted copolymer hydrogel via UV copolymerization and crosslinking. J. Drug Deliv. Sci. Technol. 2020, 60, 101962.
- Omar, N.A.; Amédée, J.; Letourneur, D.; Fricain, J.-C.; Fenelon, M. Recent Advances of Pullulan and/or Dextran-Based Materials for Bone Tissue Engineering Strategies in Preclinical Studies: A Systematic Review. Front. Bioeng. Biotechnol. 2022, 10, 889481.
- Bae, H.; Ahari, A.F.; Shin, H.; Nichol, J.W.; Hutson, C.B.; Masaeli, M.; Kim, S.H.; Aubin, H.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered pullulan methacrylate hydrogels promote cell proliferation and 3D cluster formation. Soft Matter 2011, 7, 1903–1911.
- Fricain, J.C.; Schlaubitz, S.; Le Visage, C.; Arnault, I.; Derkaoui, S.M.; Siadous, R.; Catros, S.; Lalande, C.; Bareille, R.; Renard, M.; et al. A nano-hydroxyapatite-pullulan/dextran polysaccharide composite macroporous material for bone tissue engineering. Biomaterials 2013, 34, 2947–2959.
- Galvez, M.G.; Wong, V.W.; Chang, E.I.; Major, M.; Carre, L.; Kandimalla, R.; Bhatt, K.A.; Rajadas, J.; Longaker, M.T.; Gurtner, G.C. Pullulan-collagen hydrogel scaffold as a dermal substitute. J. Am. Coll. Surg. 2009, 209, 78.
- Iswariya, S.; Bhanu Keerthi, A.V.; Velswamy, P.; Uma, T.S.; Perumal, P.T. Design and development of a piscine collagen blended pullulan hydrogel for skin tissue engineering. RSC Adv. 2016, 6, 57863–57871.
- Singh, R.S.; Kaur, N.; Rana, V.; Kennedy, J.K. Recent insights on applications of pullulan in tissue engineering. Carbohydr. Polym. 2016, 153, 455–462.
- Atila, D.; Keskin, D.; Tezcaner, A. Cellulose acetate based 3-dimensional electrospun scaffolds for skin tissue engineering applications. Carbohydr. Polym. 2015, 133, 251–261.
- Younas, A.; Dong, Z.; Hou, Z.; Asad, M.; Li, M.; Zhang, N. A chitosan/fucoidan nanoparticle-loaded pullulan microneedle patch for differential drug release to promote wound healing. Carbohydr. Polym. 2023, 306, 120593.
- Singh, S.R.; Kaur, N.; Singh, D.; Purewal, S.S.; Kennedy, F.J. Pullulan in pharmaceutical and cosmeceutical formulations: A review. Int. J. Biol. Macromol. 2023, 231, 123353.
- Zheng, W.; Zhang, Z.; Li, Y.; Wang, L.; Fu, F.; Diao, H.; Liu, X. A novel pullulan oxidation approach to preparing a shape memory sponge with rapid reaction capability for massive hemorrhage. Chem. Eng. J. 2022, 447, 137482.





