Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Makoto Senoo | -- | 2904 | 2023-03-17 21:00:12 | | | |
| 2 | Rita Xu | Meta information modification | 2904 | 2023-03-20 03:15:31 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pitt, K.; Mochida, Y.; Senoo, M. Epithelial Stem Cell Innovation. Encyclopedia. Available online: https://encyclopedia.pub/entry/42313 (accessed on 04 March 2026).
Pitt K, Mochida Y, Senoo M. Epithelial Stem Cell Innovation. Encyclopedia. Available at: https://encyclopedia.pub/entry/42313. Accessed March 04, 2026.
Pitt, Keshia, Yoshiyuki Mochida, Makoto Senoo. "Epithelial Stem Cell Innovation" Encyclopedia, https://encyclopedia.pub/entry/42313 (accessed March 04, 2026).
Pitt, K., Mochida, Y., & Senoo, M. (2023, March 17). Epithelial Stem Cell Innovation. In Encyclopedia. https://encyclopedia.pub/entry/42313
Pitt, Keshia, et al. "Epithelial Stem Cell Innovation." Encyclopedia. Web. 17 March, 2023.
Copy Citation
The field of epithelial stem cell development has been irrevocably shaped by the work of American scientist Howard Green, whose breakthroughs in stem cell culture methods translated to therapeutic practice.
stem cells
epithelia
p63
cultured epithelial autografts
holoclones
1. Introduction
Epithelial tissues such as the skin and digestive tract line the outer surfaces and inner cavities of organs, and are comprised of multilayered sheets or a monolayer of cells, respectively, that are held together primarily by E-cadherin-mediated adhesion forces [1]. Epithelial tissues are widespread throughout the body and include the epidermis, the reproductive and urinary tracts, the digestive tract, the respiratory tract [2], the mammary gland [3], the prostate gland [4], the salivary gland [5], the cornea [6], and the lung [7]. The thymus is a crucial immune organ for T cell development and is also comprised of thymic epithelia [8].
The epidermis is a classic example of epithelial tissue and acts as a barrier against the external environment. Tight junctions, a network of particle strands mainly comprised of transmembrane proteins, form a paracellular barrier against epidermal dehydration. This function is mediated by adhesion molecules, of which claudin-1 is the most prominent [9]. Corneocytes are flat, anucleated cells organized into a continuous sheet of lamellar lipid layers known as the stratum corneum, which aids in the regulation of water release [10].
Epidermal components exhibit immune competence against pathogens through Langerhans cells and tissue-resident macrophages that function as antigen-presenting cells, as well as through keratinocyte secretion of cytokines and chemokines to initiate inflammatory responses [11]. Within the epidermis reside Merkel cells, mechanoreceptors descended from the epithelial lineage that are required for response to tactile stimuli [12]. Hair follicles, sweat glands, and sebaceous glands are also present, as are neural crest-derived melanocytes. The secreted melanin granules from melanocytes are responsible for skin color and lend photoprotection to keratinocytes against UV radiation [13].
Epithelial homeostasis is maintained through self-renewal of the basal layer of the epidermis; detachment of proliferating cells from the basal layer precedes terminal differentiation and eventual detachment from the outer surface of the skin [14]. Epithelial stem cells are responsible for epithelial homeostasis within stratified epithelial and glandular tissues, and their self-renewal is governed by the transcription factor p63 [4]. The p63 gene encodes two major isoforms: TAp63, whose structure contains an N-terminal transactivation domain [15], and ΔNp63, which lacks this domain and is responsible for the formation of gross epithelium and maintenance of epithelial stem cell proliferative potential [8]. The stem cell niche varies among epithelial structures. In the skin, it is the bulge of hair follicles and the interfollicular epidermis [16]. Corneal epithelial stem cells reside in the basal layer of the limbus [6], whereas the lung contains several populations of epithelial stem cells that maintain distinct regions such as the bronchioles and alveoli [7], suggesting the existence of at least two distinct niches within the lung.
2. Early Epithelial Stem Cell Innovations
The progressive advancement of epithelial stem cell research cannot be explained without Howard Green: a decades-long pioneer and visionary in the stem cell field whose epidermal research revolutionized regenerative medicine. When he published his 1963 paper with George J. Todaro that chronicled the growth of mouse embryo-derived cells in culture, the science of cell line establishment and inoculation technique was yet in development. The very first immortal cell line, HeLa, had been isolated in 1951 from cervical cancer patient Henrietta Lacks [17], and none of the publications cited by Todaro and Green dated prior to 1940. The concept of a “feeder” layer was already established: a living yet mitotically arrested layer of cells used to promote the growth of other cells plated atop them. One factor in the early characterization of HeLa lines was determining which variants required a feeder layer and which did not [18].
Todaro and Green created the original 3T3-Swiss albino cell line (referred to at the time as 3T3) in 1962 from primary cultures of Swiss mouse embryonic fibroblasts. Their paper detailed the lengthy process of the primary cell culture, which first required affirmation of the correlation between growth rate and inoculation size. The optimal parameters for cell plating density were also established: too few cells resulted in zero population growth, while too many caused cell crowding and impeded growth by contact inhibition. By the 15 to 30 generation mark, the growth rate was consistent and remained so when those parameters were followed.
Upon reaching this milestone, Todaro and Green declared those cultures to be established lines with significant replating viability and cultured them past a hundred generations with no signs of replicative senescence. The pair concluded that high inoculation size favors cell line establishment, and that the threshold at which cell growth ceases is higher in cells that were continually cultured at high densities than in those continually cultured at low densities, as was the case for the 3T3 lines [19]. The original 3T3-Swiss albino cell line would later see further notable subclones, including the pivotal 3T3-J2.
In the twelve years between the 1963 paper and further developments with the 3T3 lines, various additional insights in epithelial tissue development were made beyond the epidermis. Improved understanding of the thymus was underway, with attempts made to determine the origin of thymocytes: lymphoid cells present in the thymus that undergo transformation into T cells. At the time, the debate over this origin comprised contradictory data [20]. One side included British developmental biologist John Beard [21] and instructor of anatomy E. T. Bell [22], and suggested that a local transformation of epithelial cells in the thymic rudiment was responsible for thymocyte origin. The other included pathologist William Bloom [23] and proposed a migration from extrinsic mesenchymal cells. In 1967, analysis of cellular migration in chick embryos by Moore and Owen at the University of Oxford concluded that thymic development was dependent on an influx of extrinsic and uncommitted stem cells from the blood. This influx allowed lymphoid differentiation in the epithelial anlage [20].
Rheinwald and Green published two papers in 1975 that established the potential of the 3T3 cell lines in feeder technology. One of their works discussed establishment of a keratinizing cell line that was derived from a mouse teratoma. At the time, the term teratoma was considered imprecise because it made no assertions about structure or behavior; the notion of subdividing the word for better specificity according to malignant behavior remained in question. The general consensus, however, was thus: a teratoma was a real tumor with malformed development, grew rapidly, almost always contained malignant elements comprised of stem cells (called embryonal carcinoma cells), rarely metastasized, was highly transplantable, and usually killed the host by causing emaciation [24]. The modern definition of teratoma has solidified into a benign germ cell tumor comprised of various somatic tissues that are derived from all three embryonic germ layers and are arranged haphazardly [25]. Random growth of hair, teeth, and bone is common [26].
The clone that would become Rheinwald and Green’s keratinizing cell line was derived from a solid tumor grown by Leroy Stevens, created through the graft of a mouse embryo onto the testis of an adult mouse that was given to Rheinwald and Green [27]. The tumor was minced, its cells plated, and small colonies were eventually isolated that retained an epithelial nature. The cells from those colonies survived over 100 generations in culture and became established as the cell line XA1.2 alongside further subclones. Feeder layers of 3T3 cells were used to aid cloning, as normal fibroblasts did not grow well atop them while the teratoma-derived XA1.2 thrived. Staining of the cells with dyes to mark keratin fibers as well as light microscopy revealed that the clones were capable of keratinization, and electron microscopy showed the stratified epithelium in the flattened cell layers of the colonies. Because the original teratoma showed no stratified squamous epithelium, Rheinwald and Green concluded that the keratinizing cells had to come from either embryonal carcinoma stem cells differentiating in culture or a small amount of differentiated cells present in the tumors.
3. Modern Advancements in Epithelial Stem Cell Culture
The biological role of the transcription factor p63 remained in dispute at the turn of the 21st century. Craniofacial defects, limb truncation, and dramatic loss of all stratified epithelia were known consequences of homozygous p63 gene disruption in mouse embryos, which spurred belief that p63 was responsible for maintenance of stem cell populations in epithelia [5]. A different perspective posited that p63 was a molecular switch, wherein the ΔNp63 isoform functioned as a dominant negative against the TAp63 isoform to prevent keratinocytes from Ca2+-mediated terminal differentiation in vitro [28]. This perspective was not consistent with the lack of epidermal phenotype in TAp63-specific knockout mice [29].
Researchers then sought to determine if p63 was required for the proliferative potential of thymic epithelial stem/progenitor cells. To enrich immature thymic epithelial cells, researchers used rats as a model and they collected holoclones with the Green method. Researchers transduced these rat thymic epithelial cells with a lentivirus that encoded shRNA against p63 transcripts. The shRNA treatment resulted in reduction of p63 protein levels to less than 5% of control cells as confirmed by Western blot. Though the p63-silenced cells and the control cells showed similar percentages of Ki67-positive cells in the early stages of 3D culture (thymospheres), the former developed smaller colonies than the latter and showed a smaller percentage of Ki67-positive cells at later stages [8].
As well as the effect of p63 on thymic epithelial stem/progenitor cells, the research tested the role of p63 in epidermal stem cells. p63-positive rat epidermal cells generated holoclones, meroclones, and paraclones when grown in clonogenic culture that were similar to the patterns of human epidermal cells in vitro, which enabled their use as a model. Transduction with the lentivirus that expressed shRNA against p63 transcripts showed a 90% reduction of p63 protein levels in these epidermal cells by Western blot, compared to cells transduced with a control shRNA lentivirus. By days 10 and 14, the stem cells with an shRNA-induced reduction of p63 formed far smaller colonies than control cells. The lab concluded that while p63 is not required for stem cell commitment and differentiation during tissue morphogenesis, it is indispensable for maintenance of the proliferative potential of epithelial stem cells [8]. This knowledge enabled greater understanding of the mechanisms underlying epidermal stratification [30].
To discuss the final component of Green’s epidermal keratinocyte propagation technique, researchers must first mention two new individuals: Kazutoshi Takahashi and Shinya Yamanaka. Through an exhaustive process of elimination, it was determined that the transcription factors Oct3/4, Sox2, c-Myc, and Klf4 in tandem with each other are able to reprogram differentiated cells into induced pluripotent stem cells (iPS cells) that exhibit the properties of embryonic stem cells. Though a fifth gene called Nanog was implicated in this process, it was ultimately found disposable for iPS cell induction and maintenance [31].
As with Green’s cultured epithelial autografts, Takahashi and Yamanaka’s groundbreaking discovery came with key caveats. The viral vectors used for transfer and induction of what became known as the Yamanaka factors were found to increase tumorigenicity and cause aberrant transcription, which hampered potential clinical use [32][33]. Justin Ichida and collaborators posited in 2009 that if small molecules could catalyze the reprogramming of differentiated cells into iPS cells through either direct activation of the Yamanaka factors or compensation for their activity, viral vectors would no longer be needed to do so [34]. Of the four factors, Oct3/4 and Sox2 were especially poignant, not only for being essential to the maintenance of embryonic stem cell pluripotency but for regulating other genes necessary for development [35].
Ichida’s paper focused on Sox2, culturing Oct4::GFP transgenic mouse embryonic fibroblasts that were transduced with Oct4, c-Myc, and Klf4. Further, 1 compound from a library of 800 with known pharmacological targets was added to each well of a 96-well plate. Three wells yielded GFP+ colonies, which indicated the growth of mouse embryonic stem cell-like colonies in the absence of Sox2. Because the 800-compound screen included the histone deacetylase inhibitor (HDAC) valproic acid in the cell culture medium, further tests were required to see if the small molecule candidates required an HDAC inhibitor to reprogram differentiated cells. From the three candidates, only the transforming growth factor-β (TGF-β) receptor 1 kinase inhibitor E-616452 was shown to form GFP+ colonies in the HDAC inhibitor’s absence. For its ability to replace both Sox2 and c-Myc (whose absence reduced tumor formation risk), E-616452 was chosen for further characterization and given the name RepSox—Replacement of Sox2 [34].
The knowledge of the TGF-β ligand pathway that leads to the nuclear translocation of SMAD2/3 (Figure 1), the observation of the large fraction of SMAD2/3 in the nuclei of low-proliferative mouse primary epithelial keratinocytes, and the known role of RepSox as a TGF-β inhibitor led to the hypothesis that RepSox could support epithelial keratinocyte proliferation through the suppression of SMAD2/3 nuclear translocation, preventing terminal differentiation [36].
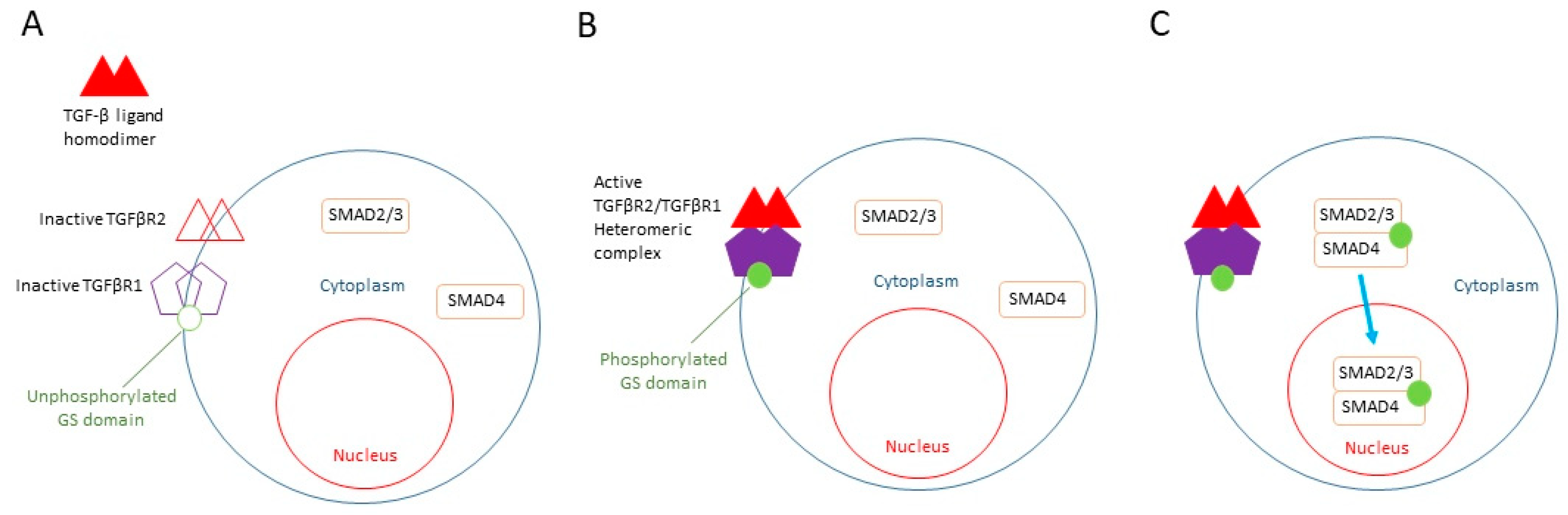
Figure 1. TGF-β signaling pathway. (A) Inactive TGFβR2 (open triangles) and TGFβR1 (open pentagons) present on the cytoplasmic membrane with unphosphorylated GS domain (open circle). Unphosphorylated SMAD2/3 and SMAD 4 (rectangular) in the cytoplasm. Unbound TGFβ ligand homodimer outside of the cell (closed triangles). (B) Binding of TGF-β ligand homodimer to TGFβR2, resulting in the formation of active heteromeric complex of TGFβR2 and TGFβR1 (closed triangles and closed pentagons). This leads to phosphorylation of TGFβR1 in its GS domain (closed circle). (C) Subsequent phosphorylation of SMAD 2/3 complex. Translocation of SMAD 2/3/4 complex into the nucleus.
The data showed that not only did RepSox stimulate proliferative capacity, it enriched p63-positive epithelial stem/progenitor cells derived from the mouse epidermis in tandem with 3T3-J2 co-culture. This allowed for long-term survival of these otherwise rapidly senescent cells. TGF-β signaling thus correlated inversely with the clonal potential of epithelial stem/progenitor cells. When RepSox was removed and the Ca2+ concentration raised, the cells were able to differentiate normally at any given passage cycle up to one year, suggesting the lack of malignant transformation after RepSox expansion. Furthermore, this finding was not restricted to keratinocytes: with RepSox, newborn and 4-week-old mouse epithelial cells from the salivary gland, tongue, esophagus, bladder, thymus, and cornea all survived expansion for at least 60 days. These cultures maintained their epithelial progenitor cells, evidenced in the production of large holoclone-like clones in 3T3-J2 co-culture and in the expression of epithelial progenitor cell-associated genes (CK14, CK8, CK19 Foxa1, and Sox2) measured by quantitative RT-PCR [36]. With this final addition of RepSox to the cFAD medium and 3T3-J2 feeder techniques that Green pioneered (Figure 2), the milestone of long-term culture of mouse epithelial stem cells was reached.
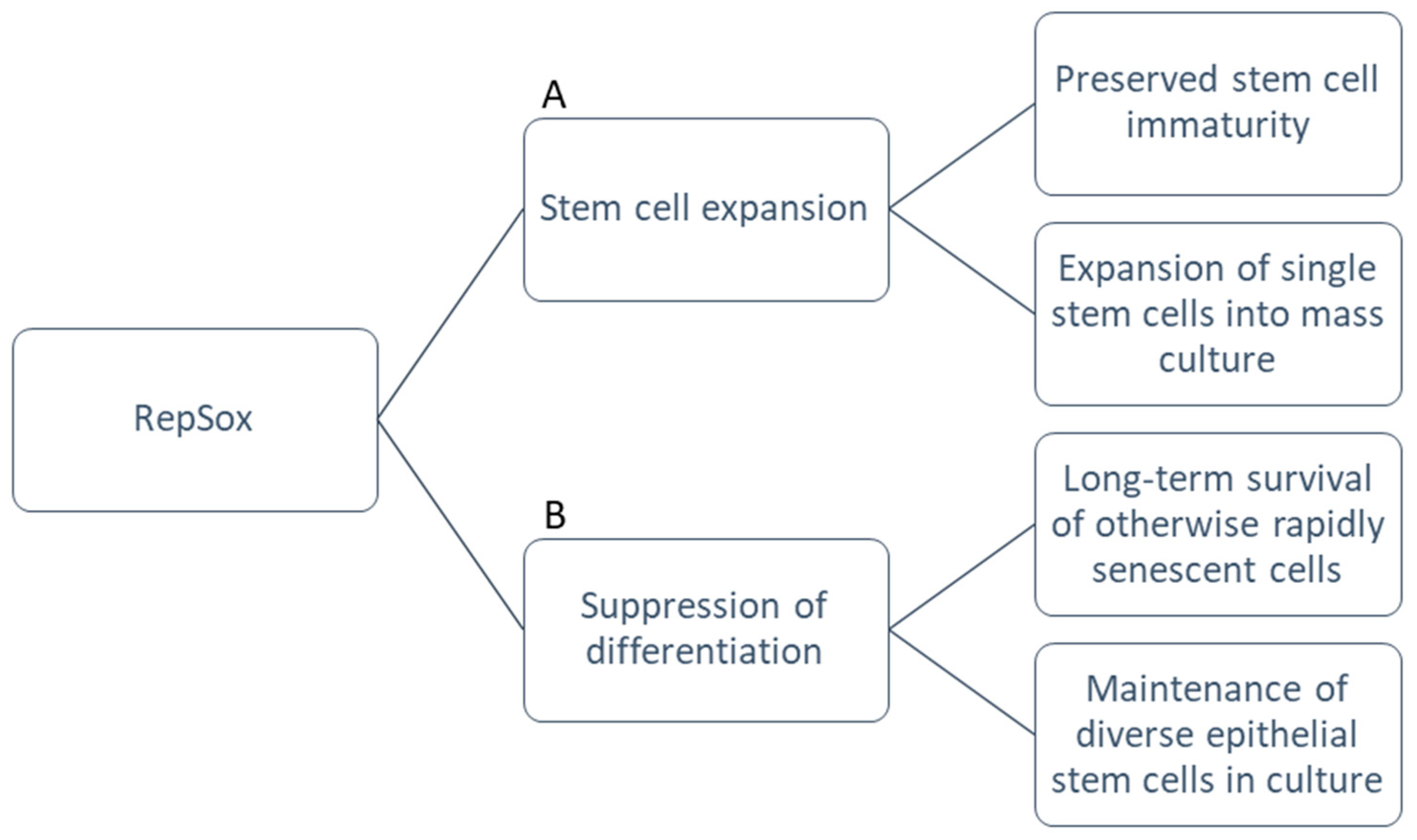
Figure 2. Functional advantages of RepSox. (A) RepSox maintains immaturity of stem cell populations and supports clonogenic potential. This allows rapid expansion of single stem cells into mass cultures for large-scale experiments. (B) RepSox enables long-term survival of cells without senescence or differentiation in diverse epithelial cell types.
The two pediatric burn patients saved by cultured epithelial autografts in 1984 represented a clinical and scientific breakthrough, to say nothing of their chance to live full lives. In 2015, another pediatric patient presented with a dire condition known as Junctional Epidermolysis Bullosa (JEB). As a disease class, Epidermolysis Bullosa is characterized by extreme fragility of the skin that leads to frequent blisters despite minimal injury [37]; epidermal autografts grown on collagen sponges had been used to treat facial erosions in decades prior [38].
Tobias Hirsch and collaborators pursued a gene therapy approach for the patient, who had complete epidermal loss on roughly ~60% of the body surface. As usual, the Green protocol began with a biopsy and expansion of primary keratinocytes from the biopsy sample; Hirsch took this a step further and transduced the primary keratinocytes from the biopsy with a retroviral vector. This vector expressed the wildtype cDNA for the LAMB3 gene, one of three genes jointly responsible for coding the basement membrane protein laminin-332; the mutation of these genes characterizes JEB.
The transgenic keratinocytes maintained consistent percentages of holoclones throughout the mass culture and formed epidermal graft sheets. Ultimately, the treatment team was able to cover the patient’s extant lesions with the transgenic graft sheets. The fully regenerated epidermis spread over time to restore ~80% of the body surface. At biopsies 4, 8, and 21 months after grafting, there was no sign of blistering or erosion and the epidermis had wholly normal morphology [39]. This case represented a novel change to the Green cultured epidermal autograft technique through use of gene therapy, one that not only healed an extant ailment but spurred long-term repair and prevented recurrence.
Though the 3T3-J2 cell line has been a mainstay in epithelial stem cell research since its inception, recent developments have shown the feasibility of other approaches. With the use of RepSox, the lab was successful in replacing mouse 3T3-J2 feeder cells with human feeder cells (dermal fibroblasts and preadipocytes) to culture human epidermal keratinocytes. This effectively ended the reliance on 3T3-J2 feeder layers, and allowed for the creation of completely autologous skin grafts with no reliance on mouse tissues [40]. RepSox continues to show potential to enable future scientific approaches (Figure 3).
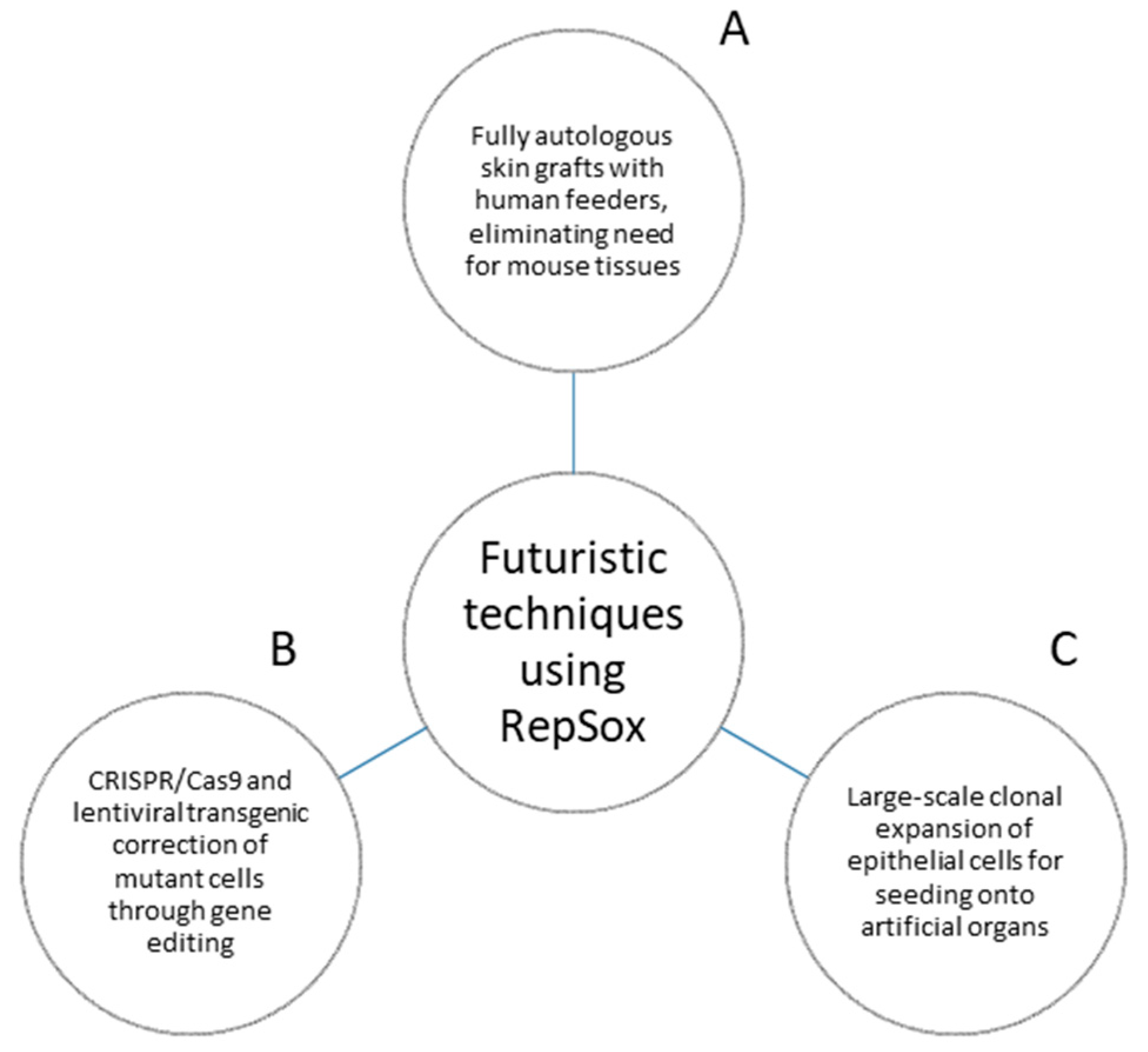
Figure 3. Futuristic techniques using RepSox. (A) RepSox allows for fully humanized autologous skin grafts, eliminating reliance upon mouse tissues. (B) RepSox enables the growth and maintenance of genetically mutant cells corrected by techniques such as CRISPR/Cas9 genetic editing and lentiviral-mediated transgenes. No current examples of CRISPR/Cas9 used with the Green method exist, and the current most advanced technique is a transgenic approach. Ideally, researchers can adapt the same system to CRISPR/Cas9 for greater accuracy in the repair of mutant protein expression. (C) RepSox enables mass expansion of the vast cell quantities required for large-scale seeding onto artificial organs.
References
- Guillot, C.; Lecuit, T. Mechanics of epithelial tissue homeostasis and morphogenesis. Science 2013, 340, 1185–1189.
- Ganz, T. Epithelia: Not just physical barriers. Proc. Natl. Acad. Sci. USA 2002, 99, 3357–3358.
- Stingl, J.; Eirew, P.; Ricketson, I.; Shackleton, M.; Vaillant, F.; Choi, D.; Li, H.I.; Eaves, C.J. Purification and unique properties of mammary epithelial stem cells. Nature 2006, 439, 993–997.
- Melino, G.; Memmi, E.M.; Pelicci, P.G.; Bernassola, F. Maintaining epithelial stemness with p63. Sci. Signal. 2015, 8, re9.
- Yang, A.; Schweitzer, R.; Sun, D.; Kaghad, M.; Walker, N.; Bronson, R.T.; Tabin, C.; Sharpe, A.; Caput, D.; Crum, C.; et al. P63 Is Essential for Regenerative Proliferation in Limb, Craniofacial and Epithelial Development. Nature 1999, 398, 714–718.
- Stepp, M.A.; Zieske, J.D. The corneal epithelial stem cell niche. Ocul. Surf. 2005, 3, 15–26.
- Donne, M.L.; Lechner, A.J.; Rock, J.R. Evidence for lung epithelial stem cell niches. BMC Dev. Biol. 2015, 15, 1–7.
- Senoo, M.; Pinto, F.; Crum, C.P.; McKeon, F. p63 Is Essential for the Proliferative Potential of Stem Cells in Stratified Epithelia. Cell 2007, 129, 523–536.
- Furuse, M.; Hata, M.; Furuse, K.; Yoshida, Y.; Haratake, A.; Sugitani, Y.; Noda, T.; Kubo, A.; Tsukita, S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: A lesson from claudin-1-deficient mice. J. Cell Biol. 2002, 156, 1099–1111.
- Proksch, E.; Brandner, J.M.; Jensen, J.M. The skin: An indispensable barrier. Exp. Dermatol. 2008, 17, 1063–1072.
- Salmon, J.K.; Armstrong, C.A.; Ansel, J.C. Conferences and Reviews The Skin as an Immune Organ. West J. Med. 1994, 160, 142–152.
- Morrison, K.M.; Miesegaes, G.R.; Lumpkin, E.A.; Maricich, S.M. Mammalian Merkel cells are descended from the epidermal lineage. Dev. Biol. 2009, 336, 76–83.
- Agar, N.; Young, A.R. Melanogenesis: A photoprotective response to DNA damage? Mutat. Res.-Fundam. Mol. Mech. Mutagen. 2005, 571, 121–132.
- Tumbar, T.; Guasch, G.; Greco, V.; Blanpain, C.; Lowry, W.E.; Rendl, M.; Fuchs, R. Defining the Epithelial Stem Cell Niche in Skin. Science 2004, 303, 359–363.
- Vanbokhoven, H.; Melino, G.; Candi, E.; Declercq, W. P63, a story of mice and men. J. Investig. Dermatol. 2011, 131, 1196–1207.
- Blanpain, C.; Lowry, W.E.; Geoghegan, A.; Polak, L.; Fuchs, E. Self-Renewal, Multipotency, and the Existence of Two Cell Populations within an Epithelial Stem Cell Niche. Cell 2004, 118, 635–648.
- Lucey, B.P.; Nelson-Rees, W.A.; Hutchins, G.M. Historical Perspective Henrietta Lacks, HeLa Cells, and Cell Culture Contamination. Arch. Pathol. Lab. Med. 2009, 133, 1463–1467.
- Fisher, H.W.; Puck, T.T. On the Functions of X-Irradiated “Feeder” Cells in Supporting Growth of Single Mammalian Cells. Proc. Natl. Acad. Sci. USA 1956, 42, 900–906.
- Todaro, G.; Green, H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J. Cell Biol. 1963, 17, 299–313.
- Moore, M.A.S.; Owen, J.J.T. Experimental Studies on the Development of the Thymus. J. Exp. Med. 1967, 126, 715–726.
- Moss, R.W. The life and times of John Beard, DSc (1858–1924). Integr. Cancer Ther. 2008, 7, 229–251.
- Bell, E.T. The development of the thymus. Am. J. Anat. 1905, 5, 29–62.
- Singer, R. Biographical Memoirs: Volume 62. Natl. Acad. Sci. 1993, 62, 17–29.
- Damjanov, I.; Solter, D. Experimental teratoma. Curr. Top. Pathol. 1974, 59, 69–129.
- Damjanov, I. The road from teratocarcinoma to human embryonic stem cells. Stem Cell Rev. 2005, 1, 273–276.
- Ingale, Y.; Shankar, A.A.; Routray, S.; Agrawal, M.; Kadam, A.; Patil, T. Ectopic Teeth in Ovarian Teratoma: A Rare Appearance. Case Rep. Dent. 2013, 2013, 970464.
- Stevens, L.C. The development of transplantable teratocarcinomas from intratesticular grafts of pre- and postimplantation mouse embryos. Dev. Biol. 1970, 21, 364–382.
- Koster, M.I.; Kim, S.; Mills, A.A.; DeMayo, F.J.; Roop, D.R. P63 Is the Molecular Switch for Initiation of an Epithelial Stratification Program. Genes Dev. 2004, 18, 126–131.
- Suh, E.K.; Yang, A.; Kettenbach, A.; Bamberger, C.; Michaelis, A.H.; Zhu, Z.; Elvin, J.A.; Bronson, R.T.; Crum, C.P.; McKeon, F. P63 Protects the Female Germ Line During Meiotic Arrest. Nature 2006, 444, 624–628.
- Blanpain, C.; Fuchs, E. Epidermal homeostasis: A balancing act of stem cells in the skin. Nat. Rev. Mol. Cell Biol. 2009, 10, 207–217.
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676.
- Nakagawa, M.; Koyanagi, M.; Tanabe, K.; Takahashi, K.; Ichisaka, T.; Aoi, T.; Okita, K.; Mochiduki, Y.; Takizawa, N.; Yamanaka, S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat. Biotechnol. 2008, 26, 101–106.
- Hacein-Bey-Abina, S.; Von Kalle, C.; Schmidt, M.; McCormack, M.P.; Wulffraat, N.; Leboulch, P.; Lim, A.; Osborne, C.S.; Pawliuk, R.; Morillon, E.; et al. LMO2-Associated Clonal T Cell Proliferation in Two Patients after Gene Therapy for SCID-X1. Science 2003, 302, 415–419.
- Ichida, J.K.; Blanchard, J.; Lam, K.; Son, E.Y.; Chung, J.E.; Egli, D.; Loh, K.M.; Carter, A.C.; Di Giorgio, F.P.; Koszka, K.; et al. A Small-Molecule Inhibitor of Tgf-β Signaling Replaces Sox2 in Reprogramming by Inducing Nanog. Cell Stem Cell 2009, 5, 491–503.
- Rizzino, A. Sox2 and Oct-3/4: A versatile pair of master regulators that orchestrate the self-renewal and pluripotency of embryonic stem cells. Wiley Interdiscip. Rev. Syst. Biol. Med. 2009, 1, 228–236.
- Suzuki, D.; Pinto, F.; Senoo, M. Inhibition of TGF-β signaling supports high proliferative potential of diverse p63+ mouse epithelial progenitor cells in vitro. Sci. Rep. 2017, 7, 6089.
- Bardhan, A.; Bruckner-Tuderman, L.; Chapple, I.L.C.; Fine, J.D.; Harper, N.; Has, C.; Magin, T.M.; Marinkovich, M.P.; Marshall, J.F.; McGrath, J.A.; et al. Epidermolysis bullosa. Nat. Rev. Dis. Prim. 2020, 6, 78.
- Carter, D.M.; Lin, A.N.; Varghese, M.C.; Caldwell, D.; Pratt, L.A.; Eisinger, M. Treatment of junctional epidermolysis bullosa with epidermal autografts. J. Am. Acad. Dermatol. 1987, 17, 246–250.
- Hirsch, T.; Rothoeft, T.; Teig, N.; Bauer, J.W.; Pellegrini, G.; De Rosa, L.; Scaglione, D.; Reichelt, J.; Klausegger, A.; Kneisz, D.; et al. Regeneration of the entire human epidermis using transgenic stem cells. Nature 2017, 551, 327–332.
- Suzuki, D.; Pinto, F.; Senoo, M. Inhibition of TGF-β Signaling Promotes Expansion of Human Epidermal Keratinocytes in Feeder Cell Co-culture. Wound Repair Regen. 2017, 25, 526–531.
More
Information
Subjects:
Cell Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
865
Revisions:
2 times
(View History)
Update Date:
20 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





