Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kaito Iwayama | -- | 1080 | 2023-03-14 04:54:24 | | | |
| 2 | Conner Chen | Meta information modification | 1080 | 2023-03-15 03:29:50 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Iwayama, K.; Seol, J.; Tokuyama, K. Exercise Timing on Fat Oxidation during Exercise. Encyclopedia. Available online: https://encyclopedia.pub/entry/42147 (accessed on 07 February 2026).
Iwayama K, Seol J, Tokuyama K. Exercise Timing on Fat Oxidation during Exercise. Encyclopedia. Available at: https://encyclopedia.pub/entry/42147. Accessed February 07, 2026.
Iwayama, Kaito, Jaehoon Seol, Kumpei Tokuyama. "Exercise Timing on Fat Oxidation during Exercise" Encyclopedia, https://encyclopedia.pub/entry/42147 (accessed February 07, 2026).
Iwayama, K., Seol, J., & Tokuyama, K. (2023, March 14). Exercise Timing on Fat Oxidation during Exercise. In Encyclopedia. https://encyclopedia.pub/entry/42147
Iwayama, Kaito, et al. "Exercise Timing on Fat Oxidation during Exercise." Encyclopedia. Web. 14 March, 2023.
Copy Citation
Due to increasingly diverse lifestyles, exercise timings vary between individuals: before breakfast, in the afternoon, or in the evening. The endocrine and autonomic nervous systems, which are associated with metabolic responses to exercise, show diurnal variations. Moreover, physiological responses to exercise differ depending on the timing of the exercise. The postabsorptive state is associated with greater fat oxidation during exercise compared to the postprandial state. The increase in energy expenditure persists during the post-exercise period, known as “Excess Post-exercise Oxygen Consumption”.
postprandial state
postabsorptive state
whole-room indirect calorimeter
1. Introduction
According to surveys on time use conducted in the United States [1] and Japan [2], more people exercise in the postprandial state in the late afternoon, and few individuals exercise in the postabsorptive state before breakfast (Figure 1). Additionally, most individuals choose to exercise when it best fits their schedule; thus, there are variations in exercise timing among individuals. In professional athletes, daily training is frequently divided into multiple sessions. A recent survey found that 48% of elite endurance athletes reported performing at least some training sessions in the morning in the postabsorptive state [3]. The most common reasons for this included weight loss or body composition goals.
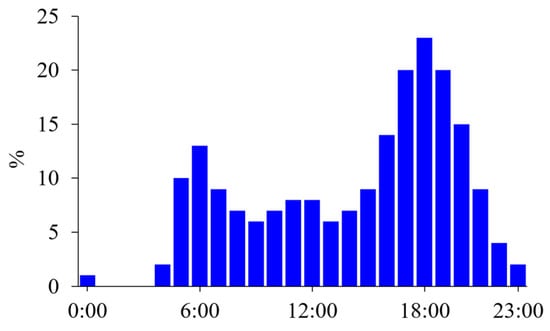
Figure 1. Time use survey on sports, exercise, and recreational activities. Diurnal variations in the percentage (%) of people participating in sports, exercising, or performing recreational activities are shown in 1-h intervals based on the time use survey in a working day in the United States [1]. People mainly exercised after their working hours, but some exercise in the morning, probably before breakfast.
Regular exercise helps to achieve and maintain the desired body composition [4] and reduces the risk of multiple chronic health conditions [5]. Specific recommendations regarding the duration and intensity of exercise necessary to maintain a healthy body are provided by the World Health Organization physical activity guidelines: 150–300 or 75–150 min of moderate- or vigorous-intensity physical activity per week, respectively, or an equivalent combination of moderate- and vigorous-intensity aerobic physical activities per week [6]. The physical Activity Guidelines for Americans also suggest that exercise should be performed at least three days a week to avoid excessive fatigue and an increased risk of injury [7]. However, the preferable time (morning, afternoon, or evening) or nutritional state (postabsorptive or postprandial) when exercise is performed is not specified in the guidelines. The endocrine and autonomic nervous systems associated with metabolic responses to exercise exhibit diurnal variations [8][9]. Moreover, physiological responses to exercise may differ depending on the timing of the exercise. Shibata et al. recently proposed the term “chrono-exercise,” which associates exercise effectiveness with the time when it is performed [10]. Various studies have examined how exercise timing relates to food intake [11], circadian rhythm [12][13], energy substrates [14], body composition [15][16][17][18], training adaptations [19][20], and glucose metabolism [21][22]. Taken together, it appears that there may be a best time to exercise.
The evaluation of the accumulated energy expenditure and substrate oxidation over 24 h is essential in order to discuss the role of exercise on body weight control. The mechanisms underlying the time or nutritional state-specific effects of exercise on 24 h energy metabolism are currently being studied. First, fat oxidation during different exercise protocols was compared using indirect calorimetry with a face mask or mouthpiece. Second, accumulated fat oxidation over 24 h was compared using indirect calorimetry in a whole-room metabolic chamber. Third, the time course of glycogen content in the liver and muscles was determined by conducting a 13C magnetic resonance spectroscopy (MRS) study.
2. Effects of Exercise Timing on Fat Oxidation during Exercise
If the motive of exercise is to reduce body fat, increasing fat oxidation relative to energy expended is critical [19]. Hence, understanding the factors that increase or decrease fat oxidation is key. Exercise intensity is one of the most important determinants affecting fat oxidation during exercise. The relative contribution of fat oxidation to total energy expenditure is greater at lower exercise intensities, and the energy is primarily obtained from the oxidation of free fatty acids in plasma [23]. Additionally, the rate of fat oxidation is increased in low-to-moderate intensity exercise, whereas it is decreased with high-intensity exercise [23]. Therefore, low-intensity exercise rather than high-intensity exercise is recommended to maximize fat oxidation and fat loss.
Fat oxidation during exercise has also been reported to vary depending on the timing and intensity of the exercise. According to Amaro-Gahate et al. [24], fat oxidation during graded exercise was greater between 5 and 8 PM than between 8 and 11 AM. Similarly, Sharma and Agarwal. [25] discovered that fat oxidation during steady-state exercise was higher between 3 and 4 PM than between 9 and 10 AM. Furthermore, serum-free fatty acid levels were significantly higher at 2 h after 60 min of steady-state exercise in the evening (between 5 and 6 PM) than in the morning (between 9 and 10 AM) [26]. To increase fat oxidation, evening exercise has been suggested over morning exercise [27]. Although these studies were conducted under standardized dietary conditions prior to experimental exercise conditions, the relative time between exercise and eating in real life is not fixed. The physical response to exercise is affected not only by the time of day but also by the nutritional status, which can be postprandial or postabsorptive. Particularly, the morning after an overnight fast, which lasts 8–10 h after the last meal, is a characteristic time of the day to spare carbohydrates and increase reliance on fat as a substrate for energy supply [28]. The only time the postabsorptive state occurs in a typical three-meal-a-day lifestyle is before breakfast. Exercise performed in the postabsorptive state is associated with greater fat oxidation than in the postprandial state [29][30].
Based on previous findings, it is possible to determine the optimal exercise conditions for losing body fat. However, the increase in whole-body fat oxidation persists not only during exercise but also after exercise [31]. Moreover, the intensity of exercise may affect post-exercise nutrient oxidation differently. Indeed, when high-intensity exercise is compared to low-intensity exercise, there is evidence of a greater reliance on energy from fat during the post-exercise period [32]. As a result, the energy source after high-intensity exercise shifts to fat-dominant sources; total fat oxidation during exercise remains constant, and a 3 h recovery period is unaffected by exercise intensity [33]. Therefore, to completely understand the implications of exercise for body weight regulation, the long-term effects of exercise on nutrient oxidation must be considered. However, assessing energy metabolism over an extended period, including eating and sleeping, would be difficult with a method that completely covers the mouth with a mask (Figure 2).
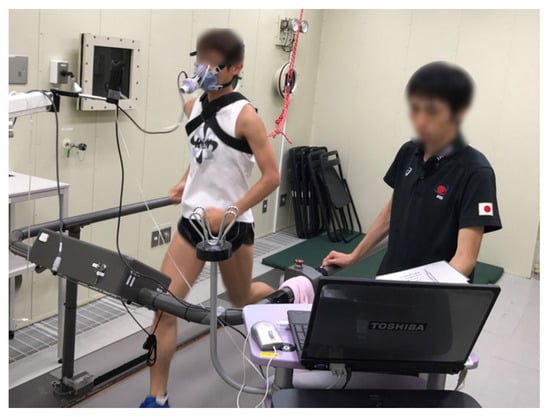
Figure 2. Indirect calorimetry using a face mask. Expired air was collected through a face mask, limiting the measurement duration to a few hours. The expired air was subjected to analysis of O2 and CO2 concentration.
References
- Sports and Exercise. Bureau of Labor Statistics. United States Department of Labor. Available online: http://www.bls.gov/spotlight/2008/sports/ (accessed on 18 January 2023).
- Survey on Time Use and Leisure Activities. Statistics Bureau, Ministry of Internal Affairs and Communications. Available online: https://www.e-stat.go.jp/en/stat-search/files?page=1&toukei=00200533&tstat=000001158160 (accessed on 18 January 2023).
- Heikura, I.A.; Stellingwerff, T.; Burke, L.M. Self-reported periodization of nutrition in elite female and male runners and race walkers. Front. Physiol. 2018, 9, 1732.
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The physical activity guidelines for Americans. JAMA 2018, 320, 2020–2028.
- Rhodes, R.E.; Janssen, I.; Bredin, S.S.D.; Warburton, D.E.R.; Bauman, A. Physical activity: Health impact, prevalence, correlates and interventions. Psychol. Health 2017, 32, 942–975.
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behavior. Br. J. Sport. Med. 2020, 54, 1451–1462.
- US Department of Health and Human Services. Physical Activity Guidelines for Americans, 2nd ed.; US Dept. of Health and Human Services: Wasington, DC, USA, 2018.
- Ishay, Y.; Kolben, Y.; Kessler, A.; Ilan, Y. Role of circadian rhythm and autonomic nervous system in liver function: A hypothetical basis for improving the management of hepatic encephalopathy. Am. J. Physiol. 2021, 321, G400–G412.
- Gabriel, B.M.; Zierath, J.R. Circadian rhythms and exercise—Re-setting the clock in metabolic disease. Nat. Rev. Endocrinol. 2019, 15, 197–206.
- Kim, H.K.; Radak, Z.; Takahashi, M.; Inami, T.; Shibata, S. Chrono-exercise: Time-of-day-dependent physiological responses to exercise. Sport. Med. Health Sci. 2022, in press.
- Bachman, J.J.; Deitrick, R.W.; Hillman, A.R. Exercising in the fasted state reduced 24-hour energy intake in active male adults. J. Nutr. Metab. 2016, 2016, 1984198.
- Tanaka, Y.; Ogata, H.; Kayaba, M.; Ando, A.; Park, I.; Yajima, K.; Araki, A.; Suzuki, C.; Osumi, H.; Zhang, S.; et al. Effect of a single bout of exercise on clock gene expression in human leukocyte. J. Appl. Physiol. 2020, 128, 847–854.
- Yamanaka, Y.; Hashimoto, S.; Takasu, N.N.; Tanahashi, U.; Nishide, S.; Honma, S.; Honma, K. Morning and evening physical exercise differentially regulate the autonomic nervous system during nocturnal sleep in humans. Am. J. Physiol. 2015, 309, R1112–R1121.
- Vieira, A.F.; Costa, R.R.; Macedo, R.C.O.; Coconcelli, L.; Kruel, L.F.M. Effects of aerobic exercise performed in fasted v. fed state on fat and carbohydrate metabolism in adults: A systematic review and meta-analysis. Br. J. Nutr. 2016, 116, 1153–1164.
- Blankenship, J.M.; Rosenberg, R.C.; Rynders, C.A.; Melanson, E.L.; Catenacci, V.A.; Creasy, S.A. Examining the role of exercise timing in weight management: A review. Int. J. Sport. Med. 2021, 42, 967–978.
- Gillen, J.B.; Percival, M.E.; Ludzki, A.; Tarnopolsky, M.A.; Gibala, M.J. Interval training in the fed or fasted state improves body composition and muscle oxidative capacity in overweight women. Obesity 2013, 21, 2249–2255.
- Nybo, L.; Pedersen, K.; Christensen, B.; Aagaard, P.; Brandt, N.; Kiens, B. Impact of carbohydrate supplementation during endurance training on glycogen storage and performance. Acta Physiol. 2009, 197, 117–127.
- Schoenfeld, B.J.; Aragon, A.A.; Wilborn, C.D.; Krieger, J.W.; Sonmez, G.T. Body composition changes associated with fasted versus non-fasted aerobic exercise. J. Int. Soc. Sport. Nutr. 2014, 11, 54.
- Chtourou, H.; Souissi, N. The effect of training at a specific time of day: A review. J. Strength Cond. Res. 2012, 26, 1984–2005.
- Van Proeyen, K.; Szlufcik, K.; Nielens, H.; Ramaekers, M.; Hespel, P. Beneficial metabolic adaptations due to endurance exercise training in the fasted state. J. Appl. Physiol. 2011, 110, 236–245.
- Mancilla, R.; Krook, A.; Schrauwen, P.; Hesselink, M.K.C. Diurnal regulation of peripheral glucose metabolism: Potential effects of exercise timing. Obesity 2020, 28, S38–S45.
- Terada, T.; Wilson, B.J.; Myette-Cote, E.; Kuzik, N.; Bell, G.J.; McCarggar, L.J.; Boule, N.G. Targeting specific interstitial glycemic parameters with highintensity interval exercise and fasted-state exercise in type 2 diabetes. Metabolism 2016, 65, 599–608.
- Romijn, J.A.; Coyle, E.F.; Sidossis, L.S.; Gastaldelli, A.; Horowitz, J.F.; Endert, E.; Wolfe, R.R. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am. J. Physiol. 1993, 265, E380–E391.
- Amaro-Gahete, F.J.; Jurado-Fasoli, L.; Trivino, A.R.; Sanchez-Delgado, G.; De-la-O, A.; Helge, J.W.; Ruiz, J.R. Diurnal variation of maximal fat-oxidation rate in trained male athletes. Int. J. Sport Physiol. Perform. 2019, 14, 1140–1146.
- Sharma, P.; Agarwal, M. Diurnal variation of fat oxidation rate and energy expenditure in an acute bout of endurance exercise by young healthy males. J. Fam. Med. Prim. Care 2022, 11, 240–244.
- Kim, H.K.; Konishi, M.; Takahashi, M.; Tabata, H.; Endo, N.; Numao, S.; Lee, S.K.; Kim, Y.H.; Suzuki, K.; Sakamoto, S. Effects of acute endurance exercise performed in the morning and evening on inflammatory cytokine and metabolic hormone responses. PLoS ONE 2015, 10, e0137567.
- Aoyama, S.; Shibata, S. Time-of-day-dependent physiological responses to meal and exercise. Front. Physiol. 2020, 7, 18.
- Maughan, R.J.; Fallah, J.; Coyle, E.F. The effects of fasting on metabolism and performance. Br. J. Sport. Med. 2010, 44, 490–494.
- Horowitz, J.F.; Mora-Rodriguez, R.; Byerley, L.O.; Coyle, E.F. Lipolytic suppression following carbohydrate ingestion limits fat oxidation during exercise. Am. J. Physiol. 1997, 273, E768–E775.
- Bergman, B.C.; Brooks, G.A. Respiratory gas-exchange ratios during graded exercise in fed and fasted trained and untrained men. J. Appl. Physiol. 1999, 86, 479–487.
- Bielinski, Y.; Schutz, Y.; Jequier, E. Energy metabolism during the postexercise recovery in man. Am. J. Clin. Nutr. 1985, 42, 69–82.
- Bahr, R.; Sejersted, O.M. Effect of intensity of exercise on excess postexercise O2 consumption. Metabolism 1991, 40, 836–841.
- Kuo, C.C.; Fattor, J.A.; Henderson, G.C.; Brooks, G.A. Lipid oxidation in fit young adults during postexercise recovery. J. Appl. Physiol. 2005, 99, 349–356.
More
Information
Subjects:
Others
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
899
Revisions:
2 times
(View History)
Update Date:
15 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




