Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hsin-Hua Nien | -- | 2651 | 2023-03-10 03:39:12 | | | |
| 2 | Jessie Wu | + 2 word(s) | 2653 | 2023-03-10 03:48:03 | | | | |
| 3 | Jessie Wu | + 2 word(s) | 2655 | 2023-03-10 03:51:24 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Nien, H.; Hsieh, C.; Shueng, P.; Tien, H. Total Skin Treated by Helical Tomotherapy. Encyclopedia. Available online: https://encyclopedia.pub/entry/42061 (accessed on 07 February 2026).
Nien H, Hsieh C, Shueng P, Tien H. Total Skin Treated by Helical Tomotherapy. Encyclopedia. Available at: https://encyclopedia.pub/entry/42061. Accessed February 07, 2026.
Nien, Hsin-Hua, Chen-Hsi Hsieh, Pei-Wei Shueng, Hui-Ju Tien. "Total Skin Treated by Helical Tomotherapy" Encyclopedia, https://encyclopedia.pub/entry/42061 (accessed February 07, 2026).
Nien, H., Hsieh, C., Shueng, P., & Tien, H. (2023, March 10). Total Skin Treated by Helical Tomotherapy. In Encyclopedia. https://encyclopedia.pub/entry/42061
Nien, Hsin-Hua, et al. "Total Skin Treated by Helical Tomotherapy." Encyclopedia. Web. 10 March, 2023.
Copy Citation
Helical tomotherapy (HT) is a rotational intensity-modulated radiotherapy with a unique gantry mechanical design that can deliver highly conformal dose distributions to provide an alternative approach for total body irradiation or total marrow irradiation.
total skin irradiation
technique
lesion
1. Introduction
With special designs, such as virtual bolus, complete block and direction block techniques, helical tomotherapy (HT) delivers photon beams with highly conformal dose distribution to convex or concave shape targets while effectively protecting organs at risk (OAR) compared with traditional photon beam radiotherapy. Additionally, the technique allows patients to remain in a comfortable and accurate position with better support during long treatment periods. Several studies have demonstrated that HT is a feasible tool for circular target treatment areas, such as the chest wall and scalp [1][2][3][4][5][6][7]. Accurate dose calculation and delivery of tomotherapy have also been verified [1][8][9]. Therefore, HT has been investigated for use in total skin irradiation, and several techniques have been reported: helical irradiation of the total skin (HITS) [10][11], helical arc radiotherapy of total skin (HEARTS) [12] or total skin helical tomotherapy (TSHT) [13], helical skin radiation therapy (HSRT) [14], and helical intensity modulated radiation therapy (HI) [15].
2. Clinical Application
Helical tomotherapy (HT) for total skin irradiation has been investigated with phantoms since 2009 [10][16][17][18]. Hsieh et al. applied the first HITS technique with central core complete block (CCCB) in clinical treatment in 2013. To ensure the skin surface dose for HITS, a diving suit was proposed for the whole-body bolus effect, and a complete response was reported [11]. After the report of this successful treatment, the number of investigations and evaluations of HITS gradually increased [11][12][13][14][15][18][19][20][21][22][23][24]. However, given the hematologic adverse effects caused by HITS [11], the HITS technique was revised to develop helical arc radiotherapy of total skin (HEARTS) and avoid toxicity. The distance from the PTV to the central core complete block (CCCB) was modified from 2.5 cm to 2.2 cm. The delivery method was a helical arc with tangential delivery to restrict the photon beams to be obliquely incident to the total skin [12].
Helical tomotherapy to the total skin is not only applied for curative intent but also for palliative therapy [20][24], and most patients receiving this treatment are diagnosed with mycosis fungoides (MF). In addition to MF, HEARTS is also delivered to patients with other diagnoses, such as therapy-refractory cutaneous CD4+ T-cell lymphoma, refractory acute myelogenous leukemia with extensive cutaneous involvement, and primary cutaneous T-cell lymphoma [11][12][14][19].
The clinical prescribed dose varies, including a conventional high-dose level of 26 Gy–36 Gy [11][15], a moderate-dose level of 20 Gy [14][18][22], a low-dose level of 10–14 Gy [12][13][14][18][19][21][22][23][24], and an ultralow dose of 4 Gy [20]. The overall response rate is 100%. Complete response was reported in most cases, as shown in Table 1. Significant improvement of previous lesion-related itching symptoms was also demonstrated [20]. Disease-free duration varied from 2 months to 1.5 years after treatment completion according to the accessible data. Both skin-related and systemic adverse effects were reported. Bone marrow suppression should be carefully evaluated in total skin helical tomotherapy.
Table 1. The reported dose regimens and treatment response of total skin helical tomotherapy.
| Study | Patient Number | Total Dose Prescribed | Fractions | Fraction Size | Overall Durations | Treatment Response |
|---|---|---|---|---|---|---|
| Hsieh et al. [11] | 1 | 30 Gy | In 40 Fx with HITS | 0.75 Gy | interrupted at 20 fractions, with one week resting, four times per week |
CR |
| Buglione et al. [15] | 1 | 27 Gy to UH body 26 Gy to LH body 22.05 Gy to scalp and eyelids |
15 Fx to UH body 13 Fx to LH body 15 Fx to scalp and eyelids |
1.8 Gy to UH body 2.0 Gy to LH body 1.47 Gy to scalp and eyelids |
5 days a week 23 days split in between |
CR |
| 1 | 28.8 Gy to UH body 28.8 Gy to LH body |
16 Fx to UH body 16 Fx to LH body |
1.8 Gy to UH body 1.8 Gy to LH body |
5 days a week 15 days split in between |
CR | |
| 1 | 30.4 Gy to UH body 30 Gy to LH body |
16 Fx to UH body 15 Fx to LH body |
1.9 Gy to UH body 2.0 Gy to LH body |
5 days a week 8 days split in between |
CR | |
| Haraldsson et al. [18] | 1 | 20 Gy | 10 Fx | 2.0 Gy | Daily, no reported duration |
- |
| Kitaguchi et al. [14] | 6 | 20 Gy | in 10 Fx | 2 Gy | Sequentially treat different parts: Trunk and arms; head and neck; legs no reported frequency or duration |
CR: 6 |
| Okuma et al. 2017 [22] | 6 | 10–20 Gy | 10 Fx | 1.0–2.0 Gy | Over 14 days | - |
| Hsieh et al. [12] | 1 | 21 Gy to lesions 15 Gy to total skin |
15 Fx | SIB-HEARTS 1.4 Gy to lesions 1 Gy to total skin |
No reported frequency or duration | CR |
| Yonekura et al. [24] | 1 | 34 Gy local RT followed by 12 Gy TSHT |
17 Fx for local RT 6 Fx for TSHT |
2.0 Gy | Over 6 days | CR |
| Sarfehnia et al. [19] | 1 | 14 Gy TSHT followed by 10 Gy TBI |
7 Fx for TSHT 5 Fx for TBI |
2.0 Gy | Daily, no reported duration |
- |
| Haraldsson et al. [23] | 1 | 12 Gy | 6 Fx | 2.0 Gy | Over 30 days | CR |
| Haraldsson et al. [18] | 1 | 12 Gy | 6 Fx | 2.0 Gy | Daily, no reported duration |
- |
| Schaff et al. [13] | 1 | 12 Gy | 8 Fx | 1.5 Gy | 4 days per week | PR |
| 1 | 12 Gy | 6 Fx | 2.0 Gy | Daily, no reported duration |
PR | |
| Okuma et al. [21] | 3 | 10 Gy | 10 Fx | 1.0 Gy | Delivered to three parts (trunk, head and neck, legs), irradiate only one part per day no reported frequency or duration |
- |
| Kitaguchi et al. [14] | 2 | 10 Gy | 10 Fx | 1.0 Gy | Sequentially deliver to three parts: Trunk and arms; head and neck; legs; no reported frequency or duration |
CR: 1 PR: 1 |
| De Bari et al. [20] | 1 | 4 Gy | 2 Fx | 2.0 Gy | No reported frequency or duration | Improved clinical severe itching symptom |
Fx: fractions; HITS: Helical irradiation of the total skin; HEARTS: Helical arc radiotherapy of the total skin; SIB: Simultaneous integrated boost; RT: radiotherapy; UH body: upper hemi body; LH body: lower hemi body; TSHT: total skin helical tomotherapy; TBI: total body irradiation; CR: complete response; PR: partial response.
3. Bolus and Skin Surface Dose
The skin-sparing effect of photon beams draws attention to the dose distribution of skin targets. Piotrowski et al. reported an excellent homogenous dose distribution to the surface area for helical tomotherapy, with 90.8–110.2% of the prescribed dose [16]. According to previous experience in total body irradiation, a virtual bolus setting is suggested for targets close to the skin for setup error compensation and the overfluence peak generated by inverse planning avoidance [25][26]. Lin et al. evaluated the dose effects contributed by different thicknesses of hypothetic boluses and various actual bolus thicknesses. The surface dose is increased as the hypothetic bolus increased. With 10 mm of hypothetic bolus, the measurement dose on the phantom surface was 89.5%, 111.4%, 116.9%, and 117.7% of the prescribed dose with 0, 1, 2, and 3 mm of actual bolus, respectively. Hsieh et al. proposed a 3 mm diving suit as a bolus for the entire body and Polyflex II tissue-equivalent material at the ears, fingers, and toes. A hypothetical bolus of 1.0–1.5 cm was set at different regions to prevent overhit in inverse planning. The results revealed good and even 95% to 125% distributed doses in the skin of the entire body [11]. Haraldsson et al. applied a 7 mm neoprene bolus and revealed a significantly higher surface dose (57% compared to the setting without a bolus [18]. Haraldsson’s team also demonstrated that 7 mm neoprene is equivalent to a 3 mm thick water bolus. A slightly soaked neoprene wet suit is equivalent to a 4.2 mm thick water bolus [17]. For the clinical treatment of total skin by HEARTS or other similar techniques, the measured skin surface dose was reported as a maximum underdose of 17.2% for an actual bolus applied and 26% without an actual bolus, as shown in Table 2. Rapid relapse was reported by Schaff et al. (2 months) and Kitaguchi et al. (relapse soon), and both studies delivered radiotherapy by helical tomotherapy without an actual bolus. Although the patient number was limited, the effect of skin surface dose variation on local control warrants further investigation.
Table 2. Skin dose measurement during clinical treatment.
| Study | Hypothetic Bolus | Actual Bolus | Measured Equipment | Measured Skin Dose |
|---|---|---|---|---|
| Sarfehnia et al. 2014 [19] |
Not mentioned | No bolus | Gafchromic EBT3 film | Maximum under 25% from TPS |
| Buglione et al. 2018 [15] | OPTT exist | No bolus | Gafchromic EBT3 film | 85–120% |
| Kitaguchi et al. 2021 [14] | Yes | No bolus | Glass luminescent radiation dosimeter | 74–130% |
| Hsieh et al. 2013 [11] | 1.0–1.5 cm | -A 3 mm diving suit -Polyflex II tissue equivalent material: Ears, fingers, toes -Conformal bolus: Trunk lesions |
Radiochromic EBT2 film | 95–125% |
| Haraldsson et al. 2018 [23] | Not mentioned | Custom fit, neoprene diving suit of 7 mm thickness |
Gafchromic EBT3 film | Median difference from TPS: 4% (SD 11%) |
| Haraldsson et al. 2019 [18] | 8 mm, 0.4 g/cm3 | -A 7 mm neoprene wetsuit, hood, gloves, and socks of neoprene -A 5 mm water equivalent bolus: Eye lids, forehead |
Radiochromic EBT3 film | Mean difference from TPS: Patient 1: 5.3% (SD 11.9%) Patient 2: 1.5% (SD 9.0%) |
| Hsieh et al. 2019 [12] | 1.0–1.5 cm | Diving suit, gloves, socks, head hood | Radiochromic EBT3 film | 93–154% |
OPTT: PTV portion outside body contour; TPS: treatment planning system.
4. Clinical Adverse Effects and Management
Eight studies reported adverse treatment effects, and seven studies provided hematologic examination results [11][12][13][14][15][23][24]. Total skin irradiation is a skin-directed therapy, and treatment adverse effects should theoretically primarily consist of skin toxicity. However, systemic effects are also observed during or after HEARTS or other similar treatment techniques.
4.1. Clinical Adverse Effects
The reported skin-directed adverse effects of helical tomotherapy include dermatitis, erythema and epitheliolysis, alopecia, onycholysis, nail changes, paronychia, plantar foot pain, and edema of the fingers and toes. Other adverse effects include grade 1–2 mucositis, xerostomia, fatigue, nausea, fever, watery eyes, and body weight loss. Each symptom was present in a small number of diverse patients. One episode of epistaxis was reported, and the symptom self-resolved 40 min later [13]. Dermatitis, alopecia, and mucositis are the most common skin toxicities. Erythema and epitheliolysis were noted in nonhomogenous dose distribution regions, such as the axillary area, inguinal area, and fingers [15]. Edema of the fingers and toes was only reported by one study [21]. Hair loss usually resolves within 3 months after completion of treatment.
Bone marrow suppression, including anemia, leukopenia, and thrombocytopenia, was present in all seven available hematologic examination results studies. The presentation of leukopenia and thrombocytopenia is more prominent than that of anemia. Grade 3–4 leukopenia and thrombocytopenia were reported in most cases. The nadir of leukopenia and thrombocytopenia usually occurred 1–2 months after the completion of HITS. Each reported individual patient toxicity data point is plotted in Figure 1 and listed in Table 3. Thrombocytopenia tends to persist for longer than leukopenia. Kitaguchi et al. applied HSRT to treat the head and neck, trunk and arms, and leg in 24 patients. Eight patents received three sequential portions of irradiation as total skin radiotherapy. However, one planned HSRT of the head and neck was aborted due to remission of the head and neck lesion during earlier leg irradiation. One patient who received HSRT expired 10 months later due to a graft-versus-host reaction after transplant. According to the study, no cytopenia was noted for head and neck and leg HSRT, and bone marrow suppression symptoms mainly presented in patients who received helical skin radiotherapy at the trunks and arms [14].
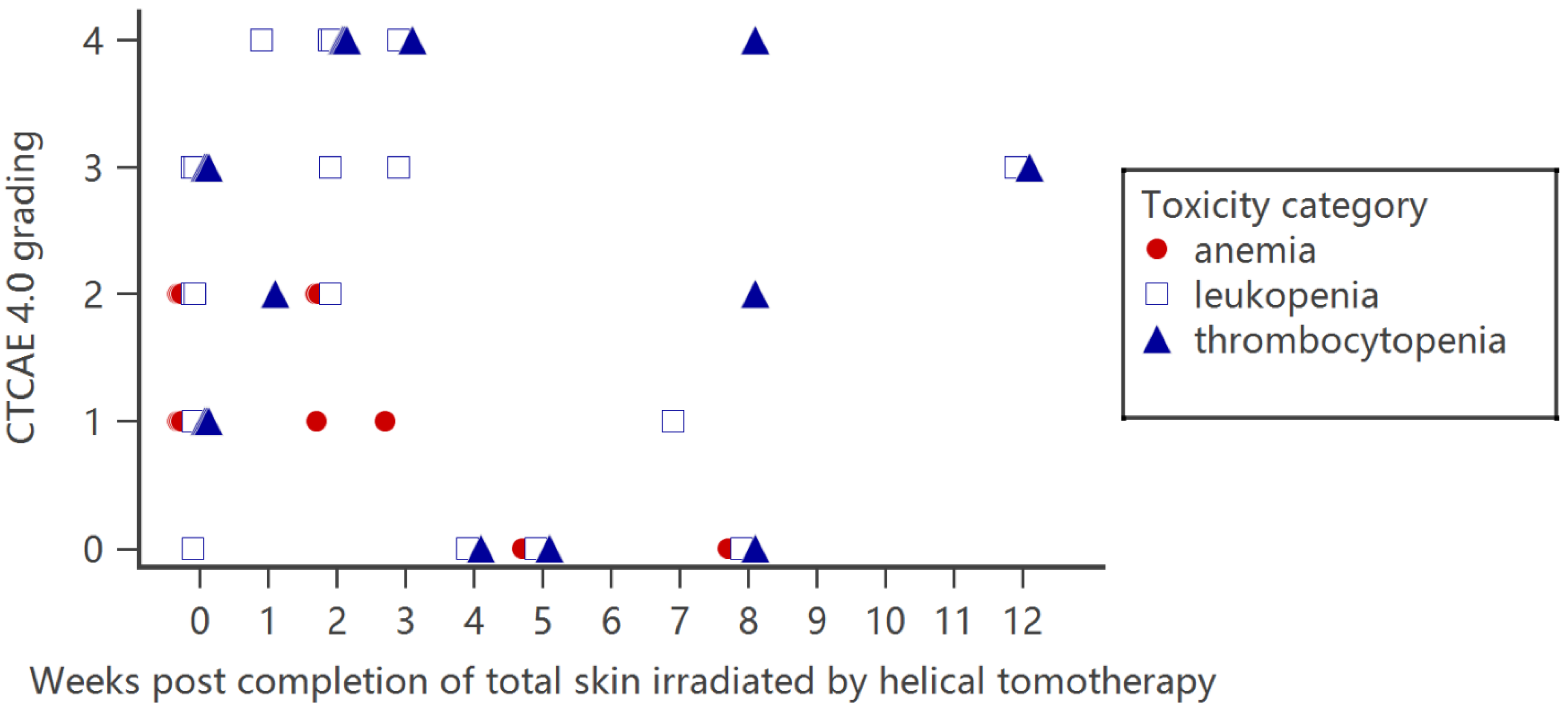
Figure 1. Hematopoietic toxicity severity and presentation time for patients who received total skin irradiation by helical tomotherapy. Each data point represents individual patient toxicity data reported in the articles.
Table 3. Dose regimen, correlated bone/bone marrow dose evaluation, and hematopoietic toxicity for patients treated by helical arc radiotherapy of total skin (HEARTS) or other similar techniques.
| Study | Patient Number | Total Dose Prescribed | Mean Dose Evaluation of Bone/Bone Marrow (Gy) | Hematopoietic Toxicity Evaluation Time | Anemia (Grade) | Leukopenia (Grade) | Thrombocytopenia (Grade) |
|---|---|---|---|---|---|---|---|
| Hsieh et al. [11] | 1 | 30 Gy/40 Fx HITS (0.75 Gy/Fx) interrupted at 20 fractions, with one week resting, 4 times per week |
Cervical, thoracic, lumbar spine, sacrum, iliac bone : 5.8, 6.3, 4.0, 4.8, R 8.9/L 8.5 |
During RT: | 1 | 3 | 1 |
| 2 ms later: | 4 | 4 | 4 | ||||
| The 3rd month after RT: | 3 | 3 | |||||
| Hsieh et al. [12] | Revised plan | 30 Gy HEARTS |
Cervical, thoracic, lumbar spine, sacrum, iliac bone : 3.6, 3.6, 3.3, 4.0, R 6.1/L 6.2 |
- | - | - | - |
| Revised plan | 12 Gy low-dose HEARTS |
Cervical, thoracic, lumbar spine, sacrum, iliac bone : 1.5, 1.4, 1.3, 1.6, R 2.4/L 2.5 |
- | - | - | - | |
| Revised plan | 25 Gy/12 Gy SIB-HEARTS |
Cervical, thoracic, lumbar spine, sacrum, iliac bone : 1.9, 1.5, 1.3, 1.4, R 2.1/L 4.0 |
- | - | - | - | |
| 1 | 21 and 15 Gy/15 Fx (1.4 and 1 Gy/Fx) SIB-HEARTS |
Cervical, thoracic, lumbar spine, sacrum, iliac bone : 2.2, 2.3, 1.9, 3.0, R 3.6/L 3.1 |
During RT: | 1 | 1 | 1 | |
| Day 17 post RT: | 4 | 4 | |||||
| Day 21 post RT: | 4 (Nadir) | ||||||
| Day 47 post RT: | 1 | ||||||
| Day 60 post RT: | 2 | ||||||
| Haraldsson et al. [18] | 1 | 12 Gy/6 Fx (2.0 Gy/Fx) |
Bone: 4.2 | - | - | - | - |
| 1 | 20 Gy/10 Fx (2.0 Gy/Fx) |
Bone: 7.7 | - | - | - | - | |
| Okuma et al. [21] | 3 | 10 Gy/10 Fx (1.0 Gy/Fx) |
Bone: 2.27 | ||||
| Kitaguchi et al. [14] | 6 | 20 Gy/10 Fx (2.0 Gy/Fx) sequentially treat different parts: Trunk and arms; head and neck; legs no reported frequency or duration |
Bone in head and neck, trunk and arms, legs group: 12.5, 7.8, 10.6 | No mentioned evaluation time | 0 (1/6, 16.7%) | 0 (0/6, 0%) | 0 (0/6, 0%) |
| 1 (1/6, 16.7%) | 1 (0/6, 0%) | 1 (2/6, 33.3%) | |||||
| 2 (2/6, 33.3%) | 2 (1/6, 16.7%) | 2 (0/6, 0%) | |||||
| 3 (2/6, 33.3%) | 3 (5/6, 83.3%) | 3 (2/6, 33.3%) | |||||
| 4 (0/6, 0%) | 4 (0/6, 0%) | 4 (2/6, 33.3%) | |||||
| 2 | 10 Gy in 10 Fx sequentially treat different parts: Trunk and arms; head and neck; legs no reported frequency or duration |
No presented data | 0 (0/2, 0%) | 0 (0/2, 0%) | 0 (0/2, 0%) | ||
| 1 (1/2, 50%) | 1 (0/2, 0%) | 1 (0/2, 0%) | |||||
| 2 (0/2, 0%) | 2 (1/2, 50%) | 2 (1/2, 50%) | |||||
| 3 (1/2, 50%) | 3 (1/2, 50%) | 3 (1/2, 50%) | |||||
| 4 (0/2, 0%) | 4 (0/2, 0%) | 4 (0/2, 0%) | |||||
| Buglione et al. [15] | 1 | 27 Gy/15 Fx to UH (1.8 Gy/Fx) 26 Gy/13 Fx to LH (2.0 Gy/Fx) 5 days a week 23 days split in between |
Bone marrow: 8.5 | No mentioned evaluation time | Gr 2 twice during the LH and UH RT; Recovered within 2 ms after RT | 2, Recovered within 2 ms after RT |
3 |
| 1 | 28.8 Gy/16 Fx to UH (1.8 Gy/Fx) 28.8 Gy/16 Fx to LH (1.8 Gy/Fx) 5 days a week 15 days split in between |
Bone marrow: 10.1 | At the end of both UH/LH body RT | 1, Recovered within 2 ms after RT |
3, Recovered within 2 ms after RT |
1 | |
| 1 | 30.4 Gy/16 Fx to UH (1.9 Gy/Fx) 30 Gy/15 Fx to LH (2.0 Gy/Fx) 5 days a week 8 days split in between |
Bone marrow: 12.0 | At the end of RT | 2, Recovered within 2 ms after RT |
1, Recovered within 2 ms after RT |
3, Prolonged thrombocytopenia, recovered within 6 ms | |
| Schaff et al. [13] | 1 | 12 Gy/8 Fx (2.0 Gy/Fx) 4 days a week |
Bone marrow (not including arms): 1.66 (including arms): 2.62 |
At the end of RT | 1 | 1 | |
| 2 weeks after RT | 2 | 2 | 4 | ||||
| 1 | (Local HT) 20 Gy/10 Fx to scalp/buttocks/neck/axilla 10 Gy/5 Fx to back |
Bone marrow (not including arms): 2.3 (Including arms): 3.56 |
2 weeks after RT | 1 | 3 | 4 |
Fx: fractions; HITS: Helical irradiation of the total skin; HEARTS: Helical arc radiotherapy of the total skin; SIB Simultaneous integrated boost; RT: radiotherapy; UH body: upper hemi body; LH body: lower hemi body; ms: months.
4.2. Bone Marrow Dose Evaluation
The mean dose delivered to the bone marrow was evaluated. The mean dose in the bone marrow correlates with the total prescribed dose. With the HEARTS technique, the mean dose of each part of the bone marrow at 30 Gy was much lower than that at 30 Gy with the HITS technique [12]. The 30 Gy HEARTS technique provided a lower mean bone marrow dose compared with other HITS techniques using a total prescribed dose exceeding 20 Gy [12][14][15][18]. Low-dose HITS at 10–12 Gy was prescribed as an effective clinical treatment with fewer adverse effects. The mean bone marrow dose of 10–12 Gy HITS ranged from 1.66 to 4.2 [12][13][18][21]. However, grade 4 thrombocytopenia occurred even when the mean bone marrow dose was as low as 1.66 Gy [13].
4.3. Management
Bone marrow suppression by HEARTS or other parallel techniques is similar to that in patients who receive total body irradiation (TBI). The possible reasons for hematopoietic syndrome in patients treated by HEARTS or other similar techniques could be that hematopoietic progenitor cells are more radiosensitive than pluripotent stem cells and are easily depleted by irradiation [27][28][29]. Additionally, pluripotent stem cells required approximately 30 days to reconstitute neutrophils and platelets [30]. Therefore, prior to recovery after HEARTS or other similar techniques, the care experience for bone marrow suppression due to TBI and accidental radiation exposure can also be applied to these patients. For patients under bone marrow suppression, supportive and specific care according to each patient’s clinical symptoms are needed. Granulocyte colony-stimulating factor (G-CSF) is critical for neutrophil regeneration, and thrombopoietin is critical for megakaryocyte progenitor cell regeneration [31]. Colony-stimulating factors, including granulocyte macrophage colony stimulating factor, G-CSF, and the pegylated form of G-CSF, can be administered to patients experiencing neutropenia. Cytokine treatment not only mitigates symptoms but also has opportunities to shorten symptom duration [32][33]. Blood transfusion with packed red blood cells and platelets is needed for patients with severe bone marrow suppression. A 25 Gy irradiated leukoreduced cellular production is suggested to prevent transfusion-associated graft-versus-host disease, which may be difficult to distinguish under bone marrow suppression conditions [33][34]. Allogenic/syngeneic stem cell transplantation is a treatment option for patients with persistent bone marrow suppression despite treatments [32]. Amiofostine, an FDA-approved radiation protector, has been primarily demonstrated to prevent radiation-induced mucositis, xerostomia, dysphagia, pulmonary fibrosis, or pneumonitis without altering the tumor treatment effect, which may benefit these patients [35][36][37][38]. Blood transfusion and antibiotics can decrease the mean lethal dose [33]. Other supportive care, including parenteral nutrition, antioxidants, oral glutamine, and yeast-derived 1,3/1,6 glucopolysaccharide, can be applied for maintenance. (Figure 2) The reported recovery times ranged from 2 weeks to 1 year [11][12][15][23][24].
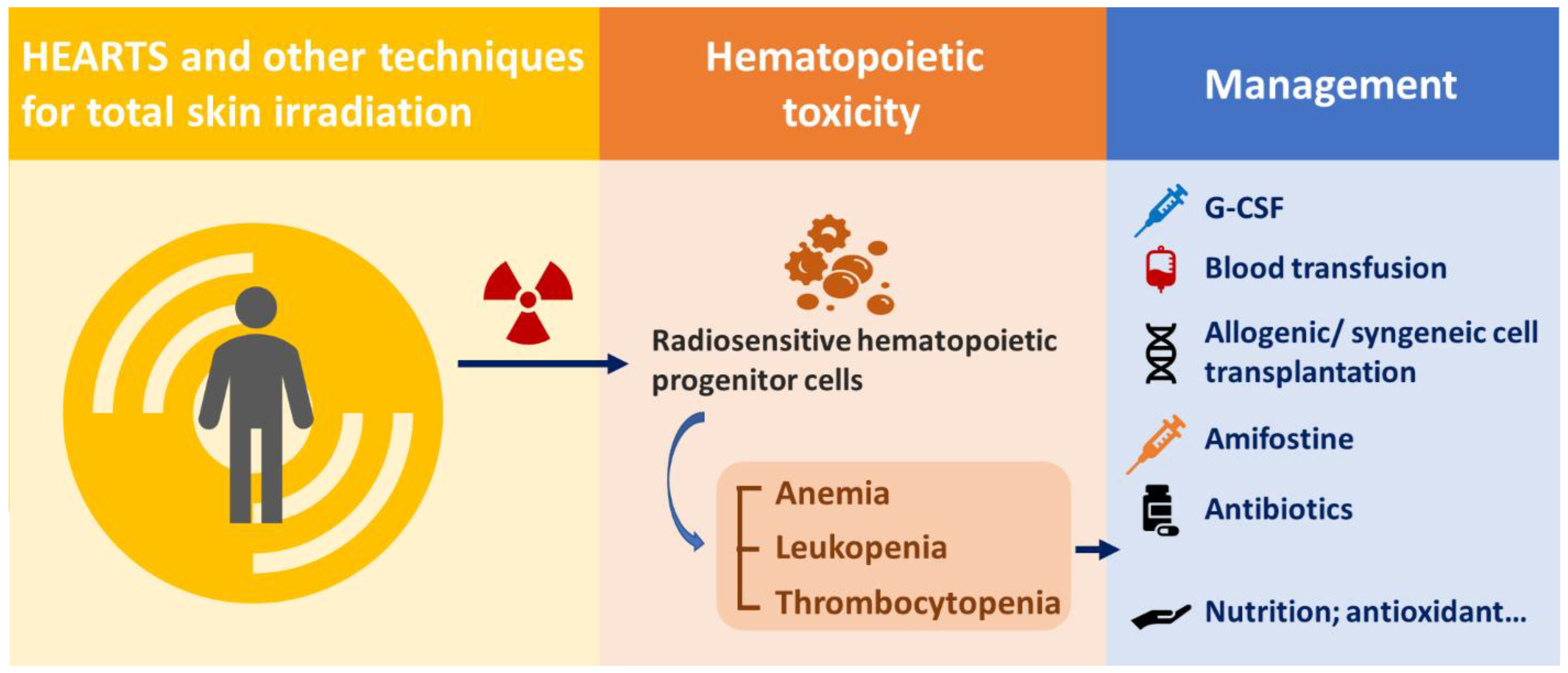
Figure 2. Management of hematopoietic syndrome caused by HEARTS and other techniques for total skin irradiation.
References
- Hardcastle, N.; Soisson, E.; Metcalfe, P.; Rosenfeld, A.; Tomé, W.A. Dosimetric verification of helical tomotherapy for total scalp irradiation. Med. Phys. 2008, 35, 5061–5068.
- Suzuki, G.; Masui, K.; Watanabe, S.; Yamazaki, H.; Takenaka, T.; Asai, J.; Maruyama, A.; Yamada, K. A successful approach for angiosarcoma of the scalp using helical tomotherapy and customized surface mold brachytherapy: A case report. Medicine 2021, 100, e28210.
- Cuccia, F.; Figlia, V.; Palmeri, A.; Verderame, F.; Casto, A.L.; Mannino, M.; Ferrera, G. Helical Tomotherapy® is a Safe and Feasible Technique for Total Scalp Irradiation. Rare Tumors 2017, 9, 7–8.
- Katayama, S.; Hantschke, M.; Lissner, S.; Lindel, K.; Oetzel, D.; Herfarth, K.; Debus, J.; Sterzing, F. Helical tomotherapy of the complete scalp and the ipsilateral lymph nodes in a case of scalp angiosarcoma. Ear Nose Throat J. 2014, 93, E24–E28.
- Takenaka, R.; Haga, A.; Nawa, K.; Hideomi, Y.; Nakagawa, K. Improvement of the robustness to set up error by a virtual bolus in total scalp irradiation with Helical TomoTherapy. Radiol. Phys. Technol. 2019, 12, 433–437.
- Shiau, A.-C.; Hsieh, C.-H.; Tien, H.-J.; Yeh, H.-P.; Lin, C.-T.; Shueng, P.-W.; Wu, L.-J. Left-Sided Whole Breast Irradiation with Hybrid-IMRT and Helical Tomotherapy Dosimetric Comparison. BioMed Res. Int. 2014, 2014, e741326.
- Yeh, H.-P.; Huang, Y.-C.; Wang, L.-Y.; Shueng, P.-W.; Tien, H.-J.; Chang, C.-H.; Chou, S.-F.; Hsieh, C.-H. Helical tomotherapy with a complete-directional-complete block technique effectively reduces cardiac and lung dose for left-sided breast cancer. Br. J. Radiol. 2020, 93, 20190792.
- Kelly, D.; Lollar, D.; Sen, A. Dosimetric Evaluation of Helical Tomotherapy Low-Dose Total Body Irradiation (TBI) for Mini Allogeneic Transplants. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, S683.
- Song, J.H.; Jung, J.Y.; Park, H.W.; Lee, G.W.; Chae, S.M.; Kay, C.S.; Son, S.H. Dosimetric comparison of three different treatment modalities for total scalp irradiation: The conventional lateral photon-electron technique, helical tomotherapy, and volumetric-modulated arc therapy. J. Radiat. Res. 2015, 56, 717–726.
- Lin, C.-T.; Shiau, A.-C.; Tien, H.-J.; Yeh, H.-P.; Shueng, P.-W.; Hsieh, C.-H. An Attempted Substitute Study of Total Skin Electron Therapy Technique by Using Helical Photon Tomotherapy with Helical Irradiation of the Total Skin Treatment: A Phantom Result. BioMed Res. Int. 2013, 2013, 108794.
- Hsieh, C.-H.; Shueng, P.-W.; Lin, S.-C.; Tien, H.-J.; Shiau, A.-C.; Chou, Y.-H.; Wu, M.-H.; Wang, J.-Y.; Chen, C.-K.; Chen, Y.-J. Helical Irradiation of the Total Skin with Dose Painting to Replace Total Skin Electron Beam Therapy for Therapy-Refractory Cutaneous CD4+ T-Cell Lymphoma. BioMed Res. Int. 2013, 2013, 717589.
- Hsieh, C.-H.; Tien, H.-J.; Yu, Y.-B.; Wu, Y.-H.; Shueng, P.-W.; Lu, Y.-F.; Wang, S.-Y.; Wang, L.-Y. Simultaneous integrated boost with helical arc radiotherapy of total skin (HEARTS) to treat cutaneous manifestations of advanced, therapy-refractory cutaneous lymphoma and leukemia—Dosimetry comparison of different regimens and clinical application. Radiat. Oncol. 2019, 14, 17.
- Schaff, E.M.; Rosenberg, S.A.; Olson, S.J.; Howard, S.P.; Bradley, K.A. Bone marrow suppression as a complication of total skin helical tomotherapy in the treatment of mycosis fungoides. Radiat. Oncol. 2018, 13, 67.
- Kitaguchi, M.; Yamashita, H.; Takenaka, R.; Okuma, K.; Nawa, K.; Nakagawa, K. Helical Skin Radiation Therapy Including Total Skin Radiation Therapy Using Tomotherapy for Primary Cutaneous Lymphoma with Bone Marrow Suppression as a Related Adverse Event. Pr. Radiat. Oncol. 2021, 11, e308–e321.
- Buglione, M.; Spiazzi, L.; Urpis, M.; Baushi, L.; Avitabile, R.; Pasinetti, N.; Borghetti, P.; Triggiani, L.; Pedretti, S.; Saiani, F.; et al. Light and shadows of a new technique: Is photon total-skin irradiation using helical IMRT feasible, less complex and as toxic as the electrons one? Radiat. Oncol. 2018, 13, 158.
- Piotrowski, T.; Kazmierska, J. Could We use Helical Tomotherapy for Total Skin Irradiation? A Study of the Dose Distribution in the Rando Alderson Phantom. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, S706.
- Haraldsson, A.; Engström, P. Total skin irradiation with helical Tomotherapy: Planning and dosimetry feasibility aspects. Radiother. Oncol. 2017, 123, S954–S955.
- Haraldsson, A.; Engleson, J.; Bäck, S.J.; Engelholm, S.; Engström, P.E. A Helical tomotherapy as a robust low-dose treatment alternative for total skin irradiation. J. Appl. Clin. Med. Phys. 2019, 20, 44–54.
- Sarfehnia, A.; Poon, E.; Davis, S.D.; Fleming, A.; Mitchell, D.; Freeman, C.R. A novel approach to total skin irradiation using helical TomoTherapy. Pr. Radiat. Oncol. 2014, 4, 330–335.
- De Bari, B.; Zeverino, M.; Durham, A.; Bourhis, J.; Moeckli, R.; Ozsahin, M. Palliative short course total skin irradiation with helical tomotherapy for primary cutaneous T-cell lymphoma. Strahlenther. Onkol. 2016, 192, 859–860.
- Okuma, K.; Haga, A.; Imae, T.; Takenaka, R.; Sugaya, M.; Nakagawa, K. Total skin irradiation using helical tomotherapy: A novel experience and report of three cases. Radiother. Oncol. 2016, 119, S649.
- Okuma, K.; Haga, A.; Imae, Y.; Takahashi, W.; Nakagawa, K. Technical results of total skin irradiation using helical TomoTherapy. Radiother. Oncol. 2017, 123, S619.
- Haraldsson, A.; Engleson, J.; Engelholm, S.; Enström, P. Total Skin Irradiation using helical tomotherapy: First experience. Radiother. Oncol. 2018, 127, S1204–S1205.
- Yonekura, K.; Ichiki, M.; Takeda, K.; Uchiyama, N.; Nishida, H.; Dokiya, T. Successful treatment of tumor stage mycosis fungoides with total skin helical tomotherapy. J. Dermatol. 2022, 49, 289–293.
- Moliner, G.; Izar, F.; Ferrand, R.; Bardies, M.; Ken, S.; Simon, L. Virtual bolus for total body irradiation treated with helical tomotherapy. J. Appl. Clin. Med. Phys. 2015, 16, 164–176.
- Simon, L.; Moliner, G.; Izar, F.; Jenny, C.; Chea, M.; Bardiès, M.; Ferrand, R. Use of a virtual bolus for TBI in tomotherapy. Phys. Medica 2014, 30, e143.
- Anno, G.H.; Baum, S.J.; Withers, H.R.; Young, R.W. Symptomatology of Acute Radiation Effects in Humans after Exposure to Doses of 0.5-30 Gy. Health Phys. 1989, 56, 821–838.
- Dainiak, N. Hematologic consequences of exposure to ionizing radiation. Exp. Hematol. 2002, 30, 513–528.
- Takahashi, K.; Monzen, S.; Hayashi, N.; Kashiwakura, I. Correlations of Cell Surface Antigens with Individual Differences in Radiosensitivity in Human Hematopoietic Stem/Progenitor Cells. Radiat. Res. 2010, 173, 184–190.
- Rosen, E.M.; Day, R.; Singh, V.K. New Approaches to Radiation Protection. Front. Oncol. 2014, 4, 381.
- Farese, A.M.; Booth, C.; Tudor, G.L.; Cui, W.; Cohen, E.P.; Parker, G.A.; Hankey, K.G.; MacVittie, T.J. The Natural History of Acute Radiation-induced H-ARS and Concomitant Multi-organ Injury in the Non-human Primate: The MCART Experience. Health Phys. 2021, 121, 282–303.
- Pandey, B.N.; Kumar, A.; Tiwari, P.; Mishra, K.P. Radiobiological basis in management of accidental radiation exposure. Int. J. Radiat. Biol. 2010, 86, 613–635.
- Goans, R.E.; Waselenko, J.K. Medical management of radiological casualties. Health Phys. 2005, 89, 505–512.
- Waselenko, J.K.; MacVittie, T.J.; Blakely, W.F.; Pesik, N.; Wiley, A.L.; Dickerson, W.E.; Tsu, H.; Confer, D.L.; Coleman, C.N.; Seed, T.; et al. Medical Management of the Acute Radiation Syndrome: Recommendations of the Strategic National Stockpile Radiation Working Group. Ann. Intern. Med. 2004, 140, 1037–1051.
- Antonadou, D.; Pepelassi, M.; Synodinou, M.; Puglisi, M.; Throuvalas, N. Prophylactic use of amifostine to prevent radiochemotherapy-induced mucositis and xerostomia in head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2002, 52, 739–747.
- Gu, J.; Zhu, S.; Li, X.; Wu, H.; Li, Y.; Hua, F. Effect of Amifostine in Head and Neck Cancer Patients Treated with Radiotherapy: A Systematic Review and Meta-Analysis Based on Randomized Controlled Trials. PLoS ONE 2014, 9, e95968.
- Vujaskovic, Z.; Feng, Q.F.; Rabbani, Z.N.; Samulski, T.V.; Anscher, M.S.; Brizel, D.M. Assessment of the protective effect of amifostine on radiation-induced pulmonary toxicity. Exp. Lung Res. 2002, 28, 577–590.
- Uzal, C.; Durmus-Altun, G.; Caloglu, M.; Ergülen, A.; Altaner, S.; Yigitbasi, N.O. The protective effect of amifostine on radiation-induced acute pulmonary toxicity: Detection by 99mTc-DTPA transalveolar clearances. Int. J. Radiat. Oncol. Biol. Phys. 2004, 60, 564–569.
More
Information
Subjects:
Primary Health Care
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
975
Revisions:
3 times
(View History)
Update Date:
10 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




