Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Deok-Chun Yang | -- | 3461 | 2023-03-09 03:31:35 | | | |
| 2 | Rita Xu | Meta information modification | 3461 | 2023-03-09 06:26:50 | | | | |
| 3 | Rita Xu | + 19 word(s) | 3480 | 2023-03-13 09:42:06 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Morshed, M.N.; Ahn, J.C.; Mathiyalagan, R.; Rupa, E.J.; Akter, R.; Karim, M.R.; Jung, D.H.; Yang, D.U.; Yang, D.C.; Jung, S.K. P. ginseng in Reactive Oxygen Species-Mediated Diseases. Encyclopedia. Available online: https://encyclopedia.pub/entry/42002 (accessed on 07 February 2026).
Morshed MN, Ahn JC, Mathiyalagan R, Rupa EJ, Akter R, Karim MR, et al. P. ginseng in Reactive Oxygen Species-Mediated Diseases. Encyclopedia. Available at: https://encyclopedia.pub/entry/42002. Accessed February 07, 2026.
Morshed, Md Niaj, Jong Chan Ahn, Ramya Mathiyalagan, Esrat Jahan Rupa, Reshmi Akter, Md. Rezaul Karim, Dae Hyo Jung, Dong Uk Yang, Deok Chun Yang, Seok Kyu Jung. "P. ginseng in Reactive Oxygen Species-Mediated Diseases" Encyclopedia, https://encyclopedia.pub/entry/42002 (accessed February 07, 2026).
Morshed, M.N., Ahn, J.C., Mathiyalagan, R., Rupa, E.J., Akter, R., Karim, M.R., Jung, D.H., Yang, D.U., Yang, D.C., & Jung, S.K. (2023, March 09). P. ginseng in Reactive Oxygen Species-Mediated Diseases. In Encyclopedia. https://encyclopedia.pub/entry/42002
Morshed, Md Niaj, et al. "P. ginseng in Reactive Oxygen Species-Mediated Diseases." Encyclopedia. Web. 09 March, 2023.
Copy Citation
Reactive oxygen species (ROS)-the byproduct of regular cell activity formed by various cellular components—play a significant role in pathological and physiological conditions. Alternatively, antioxidants are compounds that reduce or scavenge reactive species in cells. An asymmetry between the antioxidant defense system and ROS from intracellular and extracellular sources cause chronic diseases such as cancer, inflammation, tumorigenesis, cardiovascular and neurogenerative diseases. P. ginseng and its derivatives are some of the antioxidant-rich sources involved in the regulation of many oxidative-stress-related pathways.
ROS
antioxidant
oxidative stress
Panax ginseng
1. Introduction
Ginseng comes from the genus Panax of the Araliaceae family with nine different species such as Panax ginseng (Korean ginseng), Panax notoginseng (Chinese ginseng), Panax japonicum (Japanese ginseng), and Panax quinquefolius (American ginseng) [1]. Among all ginseng, four types of P. ginseng can be categorized according to how they are processed, for example, fresh ginseng, white ginseng (air-dried), red ginseng (steamed), and sun ginseng [2]. The word “ginseng” is derived from the Chinese word “rénshēn” which means “human” as the roots of ginseng are shaped like the human leg [3]. Ginseng has long been recognized as ‘the king of herbs’ due to its ability to improve fitness and relax the mind [4]. P. ginseng is endemic to Korea and China and has been used in traditional treatment [5]. However, consumers from Korea and others prefer Korean ginseng due to its current medical research findings of being useful in boosting blood circulation and better cognition, acting as a mind booster, and it being supposed to bolster one’s soul, increase the body’s immune system, and control diabetes, along with possessing anti-aging and anticancer properties [6][7]. P. ginseng contains a vast amount of secondary metabolites such as phenolic acids (gallic acid, caffeic acid, coumaric acid, salicylic acid, cinnamic acid, maltol, etc.), flavonoids, acid polysaccharides, amino acids, phytosterol, carbohydrates, minerals, ginseng oil, and certain vitamins [4][8]. P. ginseng has attracted the interest of researchers worldwide due to its pharmacological efficacy and potent medical applications.
Instead of entire ginseng and other components, a majority of researches have been conducted on specific ginsenosides to treat a variety of medical problems [9]. Ginsenosides are mainly triterpene saponins of ginseng. To date, more than 218 ginsenosides (major types: Rc, Rb1, Rb2, Rg1, Rd, and Re; minor types: Rh1, Rh2, and Rg3) have been identified from different parts of ginseng (leaves, roots, berries, and flower buds) and these metabolites have become popular for research. Ginsenosides are the therapeutically active components obtained from ginseng and are widely recognized for their oxidative stress [10], apoptosis [11], inflammation [12], angiogenesis [13], anticancer [14], and cancer metastatic properties associated with cell proliferation. Ginsenosides are divided into two groups based on the glycon structure: oleanane and dammarane [15]. According to the chemical structure, dammarane-type ginsenosides can be further classified into two categories: protopanaxadiol (PPD) and protopanaxatriol (PPT) [16], whereas the minor categories depending on aglycone moieties include ocotillol and oleanane [17]. Ginsenosides Rc, Rb2, Rh2, Rg3, Rh4, Ck, Rk1, Rk3, and Rd are strong bioactive components that have been shown to greatly inhibit the proliferation of cancer cells by regulating ROS in mitochondria [18]. The following are listed in decreasing order of how well ginsenosides scavenge intracellular ROS: Rb2 > Rc > Rg2 > Rh2 > Rh1 > Rf > Rg3 > Rg1 > Rb1 > Re > Rd [19]. Different studies have shown that the transformation of ginseng referred to as ginsenosides have stronger activity than crude ginseng [20].
P. ginseng and ginsenosides have excellent ROS-regulating activity in various disease families such as sensor impairment, cardiovascular diseases, neurogenerative diseases, cancer, diabetes, inflammation, and vice-versa.
2. ROS, Oxidative Stress, and Antioxidants
Reactive oxygen species (ROS) including hydrogen peroxide (H2O2), superoxide (O2∙), and hydroxyl (HO∙) radicals were initially recognized as potentially hazardous by-products; they are now acknowledged to serve significant roles as secondary messengers in numerous intercellular pathways [21]. ROS are produced during ATP production by the electron transport chain and NADPH (nicotinamide adenine dinucleotide phosphate) oxidase system [22]. Moreover, our bodies usually produce huge amounts of ROS due to our daily lifestyles including extended working circumstances, sitting for a long time, wearing restrictive clothing, using illicit substances often, eating unhealthily, and smoking or drinking too much alcohol [23]. ROS positively impact on immunological activity and intracellular signaling at mild to moderate levels [24]. A higher concentration of ROS can cause oxidative stress, DNA damage, redox homeostasis, tumor progression, and drug resistance which are related to the development of various diseases. ROS play a crucial role in cell proliferation, differentiation, and the control of signal transduction at certain levels (Figure 1) [22]. According to Sies (1985), “Oxidative stress is defined as the imbalance of pro-oxidant and the antioxidant protective capacity that promotes ROS or RNS which might cause potential damage” [25]. Undeniably, oxidative stress is associated with more than 100 diseases as a source or outcome [26][27]. It is well known that oxidative stress leads to cell death by damaging important bio-compounds such as proteins, DNA, and lipids [28]. Oxidative stress acts as a contributor to many chronic diseases such as cancer, neuro-generative disease, inflammation, cardiovascular disease, etc. [29].
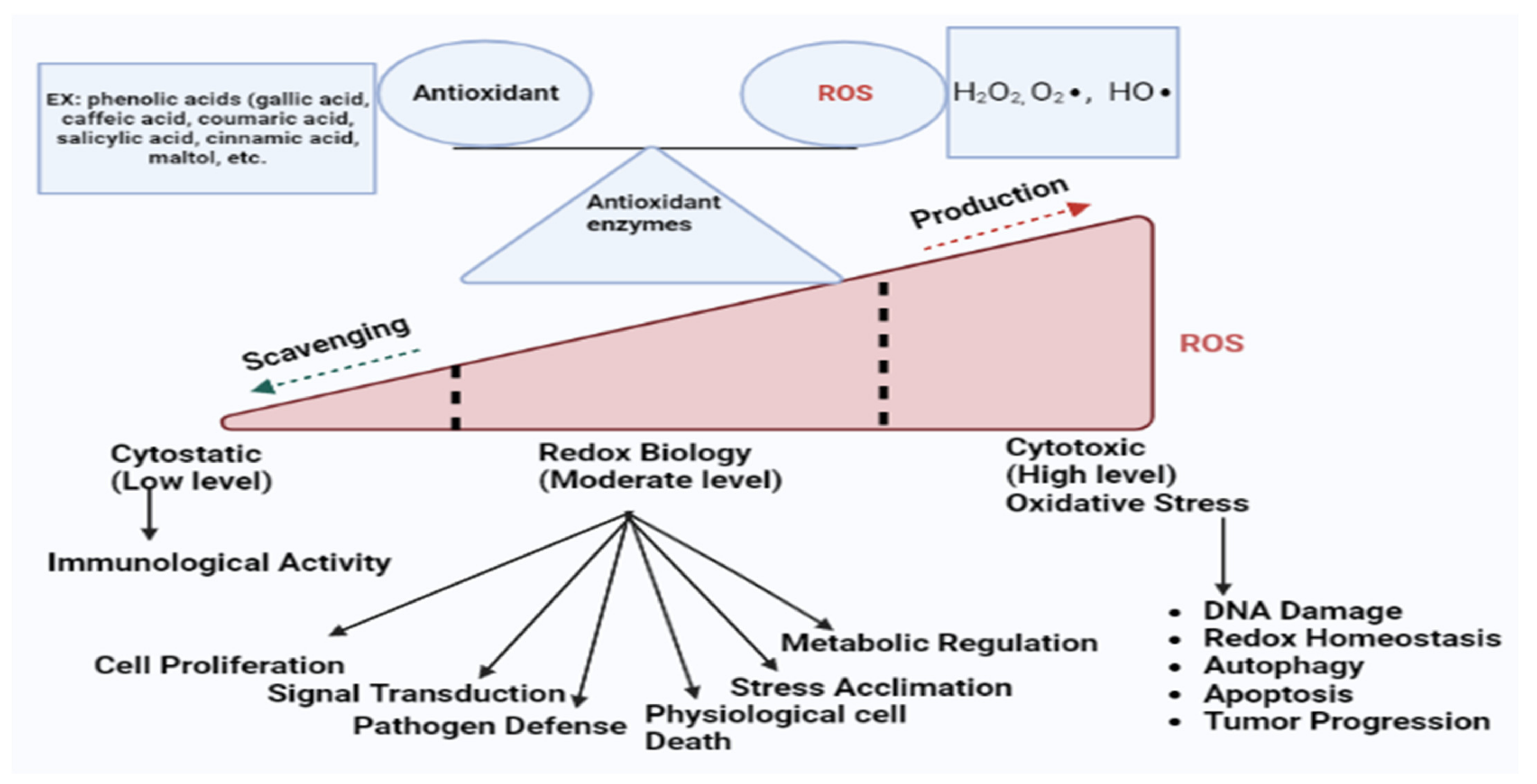
Figure 1. In normal cells, the balance between antioxidants and ROS remain at equilibrium due to the chemical reactions of antioxidant enzymes (SOD, CAT, GPx, and GST). However, the overproduction or scavenging of ROS breaks this equilibrium system. At the moderate or basal state, ROS perform as secondary messengers in several intracellular pathways that are essential for healthy cells. However, higher concentrations of ROS can cause oxidative stress, DNA damage, redox homeostasis, autophagy, apoptosis, tumor progression, and drug resistance which are related to the development of various diseases.
On the other hand, an antioxidant is a compound that reduces or scavenges reactive species or blocks the oxidation in cells [30]. In other words, antioxidants have the power to stop or delay the oxidation reaction to regulate the excessive production of oxidants [31]. Thiols and polyphenols are common examples of antioxidants for their reducing behavior [32]. Plants and animals consist of two types of antioxidants: non-enzymatic (vitamin E, C, carotenoids, lipoic acid, and others) and enzymatic (catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx), Glutathione-S-Transferase (GST), Glutathione reductase (GR), etc.). These enzymatic antioxidants have important functions in regulating cell homeostasis [33]. In a nutshell, the antioxidant mechanisms are (a) inhibiting the production of reactive species, (b) scavenging oxidants, (c) restoring the damaged molecule, (d) blocking the formation of harmful secondary metabolites and inflammation mediators, and (e) developing and boosting the natural antioxidant defense system. These defensive mechanisms work together to prevent oxidative stress in the body [34]. However, in redox biology, superoxide dismutase quickly turns into H2O2 and O2 via the SOD enzyme. The Fenton reaction can decrease metal ions to produce OH. from H2O2, which is issued in systemic inflammation [35][36]. OH. are reactive and so damages macromolecules. Antioxidant enzymes (catalase, glutathione peroxidase) can detoxify H2O2 to avoid the production of OH. (Figure 2).
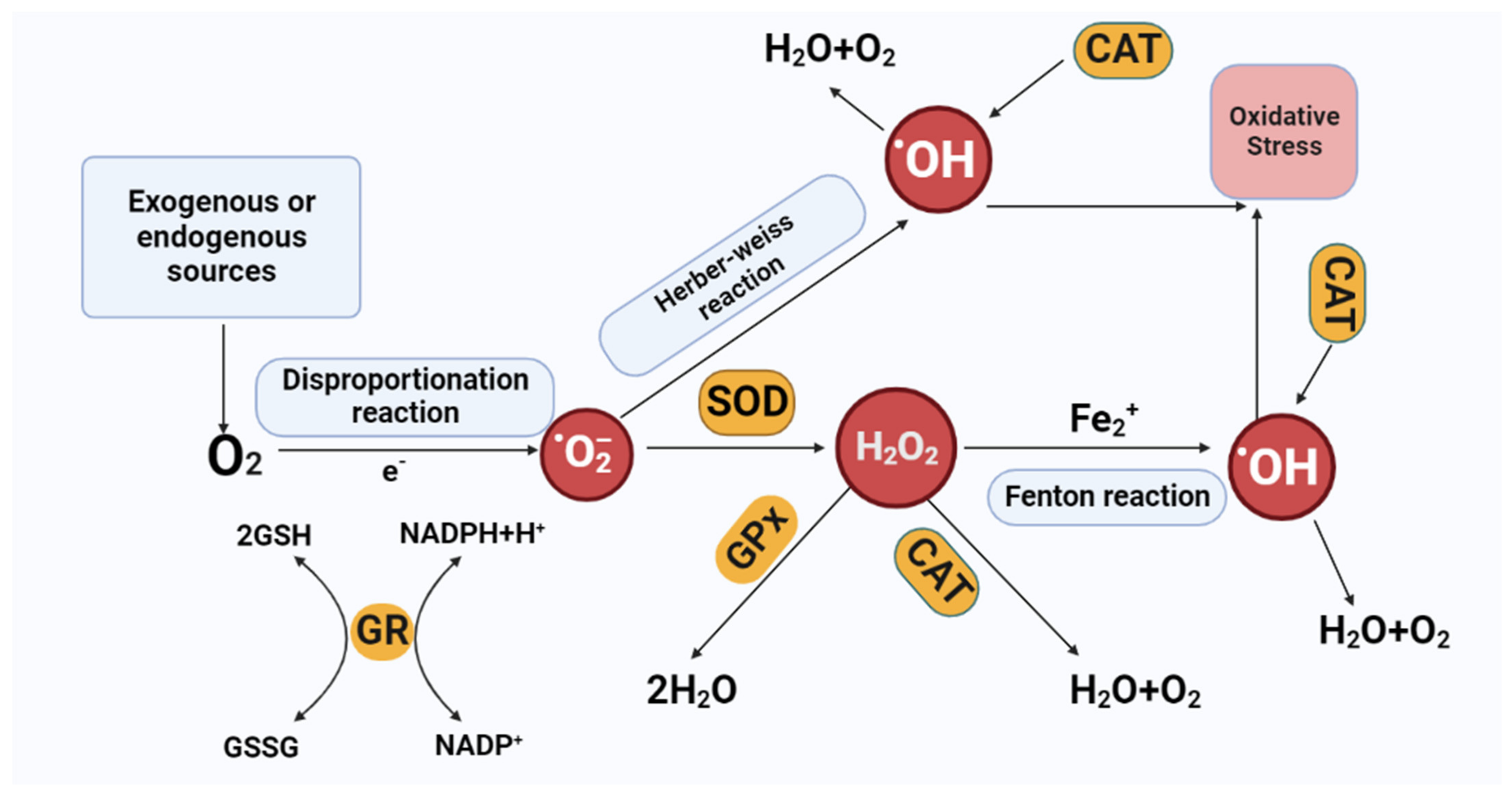
Figure 2. Scavenge and production of reactive oxygen species (ROS). Organic and inorganic constituents can produce, convert, and scavenge ROS. Antioxidant enzymes (SOD, CAT, GPx, and GR) intercept ROS through the chemical reactions. Oxidases convert oxygen to O2·−, which is then dismutated to H2O2 via SOD. H2O2 can be converted to H2O via CAT or GPx or to hydroxyl radical (·OH) after reaction with Fe2+. Abbreviations: SOD-Superoxide dismutase, CAT-catalase, GPx-glutathione Peroxidase, GR-glutathione reductase. GSH-glutathione. GSSG-glutathione disulfide. O2•−-superoxide anion. H2O2-hydrogen peroxide, (HO∙)-hydroxyl, ROS-reactive oxygen species.
Natural antioxidants have numerous biological effects including preventing ROS production and inhibiting free radicals [37]. The administration of natural sources of an antioxidant such as ginseng, pomegranate, curcumin, sesame, garlic, peppermint, and olive leaves demonstrated beneficial effects on ROS-mediated diseases in both animal and human studies [38][39].
3. P. ginseng in ROS-Mediated Diseases
In mammalian cells, mitochondria are significant for pathophysiological processes including oxidative phosphorylation (OXPHOS), the formation of cell development, and mediating key events that determine cell functions and states. According to some reviews, many diseases such as I/R injury, cardiovascular diseases, neurogenerative diseases, cancer, and metabolic disorders have been linked to mitochondrial dysfunction [40]. Several studies have demonstrated that P. ginseng regulates mitochondrial ROS, apoptosis, dynamics, biogenesis, and mitophagy to have a pharmaceutical impact. The overexpression of mitochondrial ROS leads to a wide variety of disorders and many of them lead to causes of death [41] which have been summarized below.
3.1. Antioxidant Activities of P. ginseng in Sensor Impairment
3.1.1. Ototoxicity
Age-related hearing loss is considered to be an ROS-mediated disorder. Other causes of hearing loss are noise, antibiotics (Aminoglycoside, cisplatin) consumption, and immune-mediated hearing loss [42]. Many studies have exhibited that ginseng is helpful to prevent ototoxicity caused by different sources. Aminoglycosides including gentamicin react with iron in the inner ear and produce ROS with damage to hair cells and neurons. Choung et al. showed that ginsenoside Rb1 and Rb2 are effective against aminoglycoside-induced hearing loss by attenuating ROS generation and IL-6 inhibition [43]. The organ of Corti reaches its maximum intensities of ROS and RNS generation after seven to ten days of noise insult [44]. Additionally, ginsenoside Ck and Rg2 have therapeutic effects against noise-induced hearing loss in mice by reducing the levels of ROS and RNS [45]. Ginseng extract protects against cisplatin-induced ototoxicity of the auditory cell line (HEI-OC1) due to its anti-apoptotic and anti-oxidative stress effects [46].
3.1.2. Ocular Disease
It is believed that oxidative stress is involved in many age-related eye illnesses including retinal degeneration and cataract, glaucoma, and diabetes retinopathy, etc.; cataract is an age-related loss of transparency of the eye lens because of the formation of protein complexes in the lens [47][48][49]. ROS and ultraviolet radiations damage crystalline proteins during aging, resulting in the insoluble protein clumps of the lens being opaque which interferes with vision. Park et al. recognized that ginsenoside CK blocked ROS production via Nrf2/HO-1 activation in the H2O2-stimulated ARPE-19 cell line to prevent cataracts [49]. After cataracts, glaucoma is the second leading reason for blindness which is associated with intra-ocular pressure and a loss of vision [50]. An overproduction of ROS leads to apoptosis in retinal ganglion cells that cause glaucoma [51]. Several studies showed evidence that ginseng supplements and ginsenosides are very useful against glaucoma. Ginsenoside Rb1 protects retinal ganglion cells against apoptosis caused by H2O2-induced oxidative stress [52]. Moreover, patients with glaucoma who consumed 3 g of Korean red ginseng daily for 4 weeks observed an improvement in their daytime visual acuity and ocular pain [53]. Eight weeks of consumption of KRG reduces the symptoms of dry eye in glaucoma patients by improving the tear film stability [54].
3.2. Antioxidant Activities of P. ginseng in Neurogenerative Diseases
The central nervous system (CNS) is extremely vulnerable to oxidative injury due to its utilization of a high pace of oxygen [55]. An overproduction of ROS and inadequate antioxidant defense systems have been connected to the pathophysiology of numerous neurogenerative disorders such as Huntington’s disease (HD), Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS), and Alzheimer’s disease (AD) [56]. Neuroprotection inhibits or delays the neurogenerative process to minimize neuronal death [57]. In this case, secondary metabolites of plants which are rich in antioxidant content can protect the nerve cells from free-radical-induced oxidative stress to prevent neurogenerative diseases [58]. The research into and recognition of the potential impact of Panax ginseng on ROS-mediated neurogenerative diseases are growing day by day.
3.2.1. Parkinson’s Disease
Parkinson’s disease (PD) is a chronic neurodegenerative condition that affects approximately 2% of people over 60 years old worldwide. PD depends on the interplay between various genetic and environmental factors and is marked by the development and accumulation of misfolded-α-synuclein [57]. The hallmark symptoms of PD include motor disorders (rigidity, tremor, and bradykinesia) and non-motor disorders (depression, sleep disturbance, and autonomic dysfunction) resulting from the gradual deterioration of the dopaminergic pathway [59]. Several studies have depicted that ginseng and its bioactive components, ginsenosides, have therapeutic actions on PD. P. ginseng extracts can inhibit ROS generation, eliminate Bax/Bcl2, increase the cytochrome C release, and stimulate caspase-3 expression to alleviate cell death [60]. Ginsenoside Rg1 suppressed the oxidative stress to mediate the neuroprotective action in MPTP (1-methyl-4-phenyl-1,2,3,6-twtrahydropyridine)-induced substantia nigra [61][62]. Rg1 activates total superoxide dismutase (SOD) and inhibits glutathione reduction, reducing c-Jun and N-terminal kinase (JNK) in the substantia nigra of C57BL/6 mice [61]. Furthermore, Rg1 decreases ROS production and mitochondrial cytochrome C and blocks the activation of caspase 3 and the formulation of the iNOS protein and NO in PC12 cells [63]. Again, Rg1 attenuates ROS generation and NF-ĸB translocation in MPP+-induced MES23.5 cells for reducing the expression of DMT1-IRE [64]. Ginsenoside Re shows neuroprotective action against the neurotoxicity of substantia nigra. Ginsenoside Re increases the Bcl-2 mRNA and Bcl-2 protein expression decreases the iNOS, Bax, and Bax mRNA and inhibits the cleavage of caspase-3 to protect the SN neuron from MPTP-induced apoptosis [65].
3.2.2. Alzheimer’s Disease
Alzheimer’s disease (AD) is a cognitive condition defined by the accumulation of senile plaques, the development of neurofibrillary tangle, and finally the death of neurons. The improper degradation of the amyloid precursor protein (APP) is the primary mechanism causing AD progression [66][67]. Several changes in molecular and cellular pathways including mitochondrial dysfunction, antioxidant decreases, oxidative stress increases, synaptic impairment, and amyloid Aβ clearance capacity are present in the AD brain [68][69][70]. Many studies have distinguished that P. ginseng extract, powder, and ginsenosides were applied to AD in in vivo and in vitro studies. The total saponins of ginseng consumption for seven months revealed a remarkable reduction in memory loss by inhibiting oxidative stress and increasing the proteins associated with plasticity in aged mice [71]. Ginsenoside Rb1 shields neurons from Aβ1-42 neurotoxicity via an antioxidant mechanism [72]. Rb1 pre-treatment in PC12 cells for 1 day inhibits the overproduction of ROS and lipid peroxidation enhances the activation of caspase-3 and Bcl-2/bax for promoting cell survival [73]. In H2O2-induced PC12 cells, Rg1 prevents NF-κB/P65, ERK1/2, and Akt stimulation [74]. Rg1 can protect PC12 cells from cytotoxicity caused by Aβ25-35 by preventing β-secretase activities [75]. However, the ref. [76] experiment found for the first time that ginsenoside Rk3 can trigger the intracellular ROS level and Aβ-induced neuronal injury by stimulating the AMPK pathway and the upregulation of Nrf2. Interestingly, this research also confirmed that the pharmacological activity of ginsenoside Rk3 is better than the control drug donepezil in case of the treatment of AD. Recently, a new ginseng component gintonin has been discovered that is effective in reducing the severity of AD-related neuropathies [77].
3.2.3. Others
P. ginseng and its active components are also effective in Huntington’s disease (HD), amyotrophic lateral sclerosis (ALS), depression, neuroprotection, and improvement in cognition [78]. Wang et al. recognized that ginseng sesquiterpenoids down-regulate the NF-κB and BDNF/TrkB signaling pathways, and increase SOD production in the hippocampus of the ICR mice model. The result exhibited that the SP of ginseng shows antidepressant activity via Sirt1/NF-κB and BDNF/TrkB pathways [79]. Water extract of Korean red ginseng acts as a neuroprotector through the regulation of the Nrf2 signaling pathway [80]. Ginsenoside Rb1 exhibits strong antioxidant activity in the treatment of many neurological diseases including strokes [81]. Ginsenosides Rg3 and Rg1 are useful in cognition improvement via regulating the NF-κB and PI3K/Akt signaling pathways in mice models [82][83]. It has been reported that P. ginseng fibrous root (GFR) shows good antioxidant activity to scavenge free radicals. GFR enhanced the expression of antioxidant enzymes to trigger the intracellular ROS which ultimately accelerated Alzheimer’s and other neurogenerative diseases [84].
3.3. Antioxidant Activities of P. ginseng in Cardiovascular Diseases
Cardiovascular disease (CVD) is a critical challenge among 50% of the world’s population [85]. CVD includes hypertension, coronary artery disease, massive heart failure, and peripheral vascular disease, etc., which are common and affect newborns, children, and adults of both sexes [86]. Research shows that the enhancement of reactive oxygen species and oxygen consumption is one of the crucial factors in CVD outbreak. For example, heart failure can be caused by ROS-inducing cardiac apoptosis and necrosis [87][88], as well as increased oxidant generation via the NADH/NADPH oxidant, and superoxide-generated endothelium breakdown results in hypertension and coronary artery disease [89][90]; furthermore, an overproduction of ROS and oxidant-mediated myocyte apoptosis and necrosis causes myocardial infarction [91][92]. An excessive amount of free oxygen from heart ischemia causes myocardial damage; however, ginseng consumption increases blood flow by inhibiting free oxygen and myocardial damage [93]. Ginsenoside Rb1 can inhibit the production of ROS to reduce homocysteine which causes endothelial dysfunction [94]. Ginsenoside Re protects the myocardial cell from oxidative damage and increases myocardial cell viability in heart ischemia [95]. Total ginsenosides enhance coronary artery perfusion flow by activating the PI3K/Akt-eNOS signaling pathway that ultimately produces NO levels [96]. Ginsenoside-Rb1 administration increases eNOS expression which also increases NO levels and decreases super oxides in the porcine coronary artery via the vasodilating mechanism [94]. In addition, the saponin fractions of Korean red ginseng decrease blood pressure and prompt reflex tachycardia due to their hypotensive effect and the mechanism of NO donation [97]. In another study, total ginsenosides were effective against right ventricular hypertrophy, promoted systolic pressure, and reduced pulmonary pressure by controlling the ERK-1/MAPK signaling pathway [98]. Hong et al. demonstrated that the consumption of PPT-rich ginseng enhances the activation of eNOS, stimulates NO formulation, and improves the thickness of the vessel walls to attenuate hypertension [99]. Previous studies have depicted that ginsenoside Rg1 inhibited Bcl-2 and caspase-3 expression during myocardial infarction via ischemia to lower myocardial cell death and decreased left ventricular hypertrophy [100]. In another study, ginsenoside CK reduced the burden of myocardial infraction by increasing the protein kinase B(Akt) and nitrogen oxide synthetase (eNOS) followed by ischemia via the Akt/PI3K pathway [101]. Although it has also been reported that ginsenoside Rg3 mitigates myocardial ischemia-reperfusion injury (MIRI) via the AKT/eNOS and Bcl-2/Bax signaling pathways [102], ginsenoside Rd reduces MIRI via the Nrf-2/HO-1 pathway [103], and ginsenoside Rb1 inhibits cardiomyocyte autophagy via the PI3K/Akt/mTOR pathway, thus controlling MIRI [104]. Recently, ref. [105] confirmed that ginsenoside Rh2 provides anti-inflammatory and antioxidant activity on MIRI via the regulation of the Nrf2/HO-1/NLRP3 signaling pathway.
3.4. Antioxidant Activities of P. ginseng in Cancer
According to growing data, ROS are implicated in multiple steps of tumorigenesis from the initiation of the tumor to metastasis [106]. Excessive amounts of ROS in the cell or the defective antioxidant defense mechanisms quicken the cellular damage and initiate carcinogenesis [107]. It has been reported that the cancer cell initiates more ROS than their counterpart [108]. ROS play a dual role in cancer. First, the overabundance of ROS instigates autophagy, apoptosis, and cell cycle arrest signals [109][110]. Second, ROS can influence the initiation, growth, and transmission of cancer via the activation of the signaling pathways which affect cell proliferation, survivability, angiogenesis, and metastasis [106]. In general, when ROS are low to moderate, they may contribute to the initiation of a tumor, and a high amount of ROS causes massive cell damage and death, typically at the early stages of tumor formation [111]. So, it may be possible to destroy cancer cells specifically by raising oxidative stress through exogenous ROS production without significantly harming normal cells [112].
3.5. Antioxidant Activities of P. ginseng in Other Diseases
ROS play a crucial role in the development of diabetes, kidney diseases, aging, etc. [113]. Mitochondrial DNA damaged by ROS is the primary cause of aging. Oxidative damage causes mitochondrial dysfunction and the translation and multiplication of mitochondrial DNA that promotes ROS production which damages mtDNA [114]. However, ginsenoside Rd consumption for one month quickens the cellular senescence to increase the antioxidant enzyme GPx and GR in mitochondria [115]. Moreover, ginsenoside Rb2 increases SOD, CAT activities, and also blood albumin which reduces oxidative stress from the skin cell [116]. According to Ramesh et al., Korean red ginseng reduces MDA levels, creatinine, AST, ALT, and urine nitrogen at higher levels Furthermore, KRG induces SOD, GPx, GST, GR, and CAT activity in the lungs and heart [117]. Additionally, P. ginseng derivative syringaresinol (SYR) shows antioxidant activity and stimulated autophagy in H2O2-induced Hacat cells, therefore inhibiting the mRNA expression of MMP-2 and MMP-9 related to skin aging [118]. SYR may have therapeutic potential to treat diabetic cardiomyopathy by reducing oxidative stress, fibrosis, and inflammation [119].
Furthermore, Korean red ginseng prevented the blood glucose levels in STZ-induced diabetic rats [120]. In addition, it was recognized that ginsenoside Rd consumption via acute renal failure in rats increases SOD and catalase in renal tissue and serum [121]. Recent studies have shown that ginseng triggers pro-inflammatory cytokine (IL-6, IL-1β, and TNF-α) expression as well as activates ROS-mediated pathways to show antifatigue activity through anti-oxidation and anti-inflammatory activity [122]. Research has shown that P. ginseng plays as anti-inflammatory, immunostimulatory, neuroprotective, hepatoprotective, antiplatelet, antidiabetic, and anti-angiogenesis roles. Korean ginseng and its ginsenosides are effective in various anti-inflammatory diseases including colitis, gastritis, and hepatitis. Han et al. depicted that ginseng shows anti-inflammatory activity by preventing Akt [123]. Moreover, Rg1 could be a useful approach for preventing acute liver damage by stimulating the Nrf2 signaling pathway [124]. Additionally, ginseng can regulate streptozotocin-induced diabetes by increasing antioxidant enzymes [125]. Moreover, ref. [126] depicted that fermented black ginseng (P. ginseng) can reduce ROS levels in H2O2-induced Hacat cells via its antioxidant activity compared to black and white ginseng. Furthermore, FBG shows higher anti-wrinkle and anti-melanogenic activity than BG and WG [127].
References
- Angelova, N.; Kong, H.W.; Van Der Heijden, R.; Yang, S.Y.; Choi, Y.H.; Kim, H.K.; Wang, M.; Hankemeier, T.; Van Der Greef, J.; Xu, G. Recent methodology in the phytochemical analysis of ginseng. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 2008, 19, 2–16.
- Bae, J.-S.; Park, H.-S.; Park, J.-W.; Li, S.-H.; Chun, Y.-S. Red ginseng and 20(S)-Rg3 control testosterone-induced prostate hy-perplasia by deregulating androgen receptor signaling. J. Nat. Med. 2012, 66, 476–485.
- Nocerino, E.; Amato, M.; Izzo, A.A. The aphrodisiac and adaptogenic properties of ginseng. Fitoterapia 2000, 71, S1–S5.
- Metwaly, A.M.; Lianlian, Z.; Luqi, H.; Deqiang, D. Black ginseng and its saponins: Preparation, phytochemistry and pharmacological effects. Molecules 2019, 24, 1856.
- Abbas, M.; Haq, M.; Anwar, F. Pharmaceutical and Medicinal Applications of Panax Ginseng and Ginsenosides and Their Theuropatic Role in Different. Nat. Volatiles Essent. Oils 2022, 9, 745–765.
- Kwak, G.-Y.; Ying, Z.; Awais, M.; Akter, R.; Xu, F.; Baik, S.; Jung, D.; Yang, D.C.; Wenying, Y. Discrimination of Panax ginseng from counterfeits using single nucleotide polymorphism: A focused review. Front. Plant Sci. 2022, 2116, 903306.
- Adil, M.; Jeong, B.R. In vitro cultivation of Panax ginseng CA Meyer. Ind. Crops Prod. 2018, 122, 239–251.
- Chung, I.-M.; Lim, J.-J.; Ahn, M.-S.; Jeong, H.-N.; An, T.-J.; Kim, S.-H. Comparative phenolic compound profiles and antiox-idative activity of the fruit, leaves, and roots of Korean ginseng (Panax ginseng Meyer) according to cultivation years. J. Ginseng Res. 2016, 40, 68–75.
- Buettner, C.; Yeh, G.Y.; Phillips, R.S.; Mittleman, M.A.; Kaptchuk, T.J. Systematic review of the effects of ginseng on cardio-vascular risk factors. Ann. Pharmacother. 2006, 40, 83–95.
- Mao, Q.; Zhang, P.-H.; Wang, Q.; Li, S.-L. Ginsenoside F2 induces apoptosis in humor gastric carcinoma cells through reactive oxygen species-mitochondria pathway and modulation of ASK-1/JNK signaling cascade in vitro and in vivo. Phytomedicine 2014, 21, 515–522.
- Li, L.; Wang, Y.; Guo, R.; Li, S.; Ni, J.; Gao, S.; Gao, X.; Mao, J.; Zhu, Y.; Wu, P.; et al. Ginsenoside Rg3-loaded, reactive oxygen species-responsive polymeric nanoparticles for alleviating myocardial ischemia-reperfusion injury. J. Control. Release 2020, 317, 259–272.
- Shin, M.-S.; Song, J.H.; Choi, P.; Lee, J.H.; Kim, S.-Y.; Shin, K.-S.; Ham, J.; Kang, K.S. Stimulation of innate immune function by Panax ginseng after heat processing. J. Agric. Food Chem. 2018, 66, 4652–4659.
- Zeng, D.; Wang, J.; Kong, P.; Chang, C.; Li, J.; Li, J. Ginsenoside Rg3 inhibits HIF-1α and VEGF expression in patient with acute leukemia via inhibiting the activation of PI3K/Akt and ERK1/2 pathways. Int. J. Clin. Exp. Pathol. 2014, 7, 2172–2178.
- Chung, K.-S.; Cho, S.-H.; Shin, J.-S.; Kim, D.-H.; Choi, J.-H.; Choi, S.Y.; Rhee, Y.K.; Hong, H.-D.; Lee, K.-T. Ginsenoside Rh2 induces cell cycle arrest and differentiation in human leukemia cells by upregulating TGF-β expression. Carcinogenesis 2012, 34, 331–340.
- He, Y.; Hu, Z.; Li, A.; Zhu, Z.; Yang, N.; Ying, Z.; He, J.; Wang, C.; Yin, S.; Cheng, S. Recent advances in biotransformation of saponins. Molecules 2019, 24, 2365.
- Qi, L.-W.; Wang, H.-Y.; Zhang, H.; Wang, C.-Z.; Li, P.; Yuan, C.-S. Diagnostic ion filtering to characterize ginseng saponins by rapid liquid chromatography with time-of-flight mass spectrometry. J. Chromatogr. A 2012, 1230, 93–99.
- Ahuja, A.; Kim, J.H.; Kim, J.-H.; Yi, Y.-S.; Cho, J.Y. Functional role of ginseng-derived compounds in cancer. J. Ginseng Res. 2018, 42, 248–254.
- Jin, Y.; Huynh, D.T.N.; Nguyen, T.L.L.; Jeon, H.; Heo, K.-S. Therapeutic effects of ginsenosides on breast cancer growth and metastasis. Arch. Pharmacal Res. 2020, 43, 773–787.
- Chae, S.; Kang, K.A.; Youn, U.; Park, J.S.; Hyun, J.W. A comparative study of the potential antioxidant activities of ginsenosides. J. Food Biochem. 2010, 34, 31–43.
- He, B.; Chen, D.; Zhang, X.; Yang, R.; Yang, Y.; Chen, P.; Shen, Z. Oxidative Stress and Ginsenosides: An Update on the Molecular Mechanisms. Oxidative Med. Cell. Longev. 2022, 2022, 9299574.
- Dröge, W. The plasma redox state and ageing. Ageing Res. Rev. 2002, 1, 257–278.
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic ap-proach? Nat. Rev. Drug Discov. 2009, 8, 579–591.
- Bindu, M.; Annamalai, P. Combined Effect of Alcohol and Cigarette Smoke on Lipid Peroxidation and Antioxidant Status in Rats; CSIR: New Delhi, India, 2004; pp. 40–44.
- Chan, L.Y.; Kwok, H.H.; Chan, R.W.Y.; Peiris, M.J.S.; Mak, N.K.; Wong, R.N.S.; Chan, M.C.W.; Yue, P.Y.K. Dual functions of ginsenosides in protecting human endothelial cells against influenza H9N2-induced inflammation and apoptosis. J. Ethnopharmacol. 2011, 137, 1542–1546.
- Khansari, N.; Shakiba, Y.; Mahmoudi, M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat. Inflamm. Allergy Drug Discov. 2009, 3, 73–80.
- Luo, Z.; Xu, X.; Sho, T.; Zhang, J.; Xu, W.; Yao, J.; Xu, J. ROS-induced autophagy regulates porcine trophectoderm cell apoptosis, proliferation, and differentiation. Am. J. Physiol. Cell Physiol. 2019, 316, C198–C209.
- Devasagayam, T.P.; Steenken, S.; Obendorf, M.S.; Schulz, W.A.; Sies, H. Formation of 8-hydroxy (deoxy) guanosine and gen-eration of strand breaks at guanine residues in DNA by singlet oxygen. Biochemistry 1991, 30, 6283–6289.
- Gutteridge, J.M.; Halliwell, B. Invited review free radicals in disease processes: A compilation of cause and consequence. Free. Radic. Res. Commun. 1993, 19, 141–158.
- Halliwell, B. Reactive oxygen species and the central nervous system. J. Neurochem. 1992, 59, 1609–1623.
- Bhatia, V.; Tandon, R.K. Stress and the gastrointestinal tract. J. Gastroenterol. Hepatol. 2005, 20, 332–339.
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74.
- Alzoghaibi, M.A. Concepts of oxidative stress and antioxidant defense in Crohn’s disease. World J. Gastroenterol. WJG 2013, 19, 6540.
- Pammi, S.S.S.; Suresh, B.; Giri, A. Antioxidant potential of medicinal plants. J. Crop Sci. Biotechnol. 2023, 26, 13–26.
- Duarte, T.L.; Lunec, J. Review: When is an antioxidant not an antioxidant? A review of novel actions and reactions of vitamin C. Free Radic Res. 2005, 39, 671–686.
- Cheung, C.C.; Zheng, G.J.; Li, A.M.; Richardson, B.J.; Lam, P.K. Relationships between tissue concentrations of polycyclic aromatic hydrocarbons and antioxidative responses of marine mussels, Perna viridis. Aquat. Toxicol. 2001, 52, 189–203.
- Halliwell, B. Biochemistry of oxidative stress. Biochem. Soc. Trans. 2007, 35, 1147–1150.
- Kehrer, J.P.; Klotz, L.-O. Free radicals and related reactive species as mediators of tissue injury and disease: Implications for health. Crit. Rev. Toxicol. 2015, 45, 765–798.
- Halliwell, B. Free radicals and antioxidants: Updating a personal view. Nutr. Rev. 2012, 70, 257–265.
- Adwas, A.A.; Elsayed, A.; Azab, A.; Quwaydir, F. Oxidative stress and antioxidant mechanisms in human body. J. Appl. Bio-Technol. Bioeng. 2019, 6, 43–47.
- Murphy, M.P.; Hartley, R.C. Mitochondria as a therapeutic target for common pathologies. Nat. Rev. Drug Discov. 2018, 17, 865–886.
- Huang, Q.; Gao, S.; Zhao, D.; Li, X. Review of ginsenosides targeting mitochondrial function to treat multiple disorders: Current status and perspectives. J. Ginseng Res. 2021, 45, 371–379.
- Pak, J.H.; Kim, Y.; Yi, J.; Chung, J.W. Antioxidant therapy against oxidative damage of the inner ear: Protection and precon-ditioning. Antioxidants 2020, 9, 1076.
- Choung, Y.H.; Kim, S.W.; Tian, C.; Min, J.Y.; Lee, H.K.; Park, S.N.; Lee, J.B.; Park, K. Korean red ginseng prevents gentamicin-induced hearing loss in rats. Laryngoscope 2011, 121, 1294–1302.
- Yamashita, D.; Jiang, H.-Y.; Schacht, J.; Miller, J.M. Delayed production of free radicals following noise exposure. Brain Res. 2004, 1019, 201–209.
- Hong, B.N.; Kim, S.Y.; Yi, T.H.; Kang, T.H. Post-exposure treatment with ginsenoside compound K ameliorates auditory functional injury associated with noise-induced hearing loss in mice. Neurosci. Lett. 2011, 487, 217–222.
- Im, G.J.; Chang, J.W.; Choi, J.; Chae, S.W.; Ko, E.J.; Jung, H.H. Protective effect of Korean red ginseng extract on cisplatin ototoxicity in HEI-OC1 auditory cells. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2010, 24, 614–621.
- Moreau, K.L.; King, J.A. Protein misfolding and aggregation in cataract disease and prospects for prevention. Trends Mol. Med. 2012, 18, 273–282.
- Truscott, R.J.W.; Friedrich, M.G. The etiology of human age-related cataract. Proteins don’t last forever. Biochim. Biophys. Acta BBA Gen. Subj. 2016, 1860, 192–198.
- Park, C.; Cha, H.-J.; Song, K.-S.; Kim, H.-S.; Bang, E.; Lee, H.; Jin, C.-Y.; Kim, G.-Y.; Choi, Y.H. Nrf2-mediated activation of HO-1 is required in the blocking effect of compound K, a ginseng saponin metabolite, against oxidative stress damage in ARPE-19 human retinal pigment epithelial cells. J. Ginseng Res. 2022, in press.
- Quigley, H.A.; Broman, A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267.
- Sim, R.H.; Sirasanagandla, S.R.; Das, S.; Teoh, S.L. Treatment of Glaucoma with Natural Products and Their Mechanism of Action: An Update. Nutrients 2022, 14, 534.
- Liu, Z.; Chen, J.; Huang, W.; Zeng, Z.; Yang, Y.; Zhu, B. Ginsenoside Rb1 protects rat retinal ganglion cells against hypoxia and oxidative stress. Mol. Med. Rep. 2013, 8, 1397–1403.
- Lee, K.; Yang, H.; Kim, J.Y.; Choi, W.; Seong, G.J.; Kim, C.Y.; Lee, J.M.; Bae, H.W. Effect of red ginseng on visual function and vision-related quality of life in patients with glaucoma. J. Ginseng Res. 2021, 45, 676–682.
- Bae, H.W.; Kim, J.H.; Kim, S.; Kim, M.; Lee, N.; Hong, S.; Seong, G.J.; Kim, C.Y. Effect of Korean Red Ginseng supplementation on dry eye syndrome in glaucoma patients–A randomized, double-blind, placebo-controlled study. J. Ginseng Res. 2015, 39, 7–13.
- Sayre, L.M.; Perry, G.; Smith, M.A. Oxidative Stress and Neurotoxicity. Chem. Res. Toxicol. 2008, 21, 172–188.
- Li, J.; O, W.; Li, W.; Jiang, Z.G.; Ghanbari, H.A. Oxidative stress and neurodegenerative disorders. Int. J. Mol. Sci. 2013, 14, 24438–24475.
- Braak, H.; Del Tredici, K.; Rüb, U.; de Vos, R.A.; Jansen Steur, E.N.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211.
- Kang, M.H.; Ji, Y.-J.; Han, Y.M.; Jang, G.Y.; Kim, D.H.; Lee, J.H.; Kim, G.-S.; Choi, S.J.; Kim, H.D. Sasa borealis Ethanol Extract Protects PC12 Neuronal Cells against Oxidative Stress. Appl. Sci. 2022, 12, 6155.
- Schapira, A.H. Molecular and clinical pathways to neuroprotection of dopaminergic drugs in Parkinson disease. Neurology 2009, 72, S44–S50.
- Hu, S.; Han, R.; Mak, S.; Han, Y. Protection against 1-methyl-4-phenylpyridinium ion (MPP+)-induced apoptosis by water extract of ginseng (Panax ginseng C.A. Meyer) in SH-SY5Y cells. J. Ethnopharmacol. 2011, 135, 34–42.
- Chen, X.C.; Zhou, Y.C.; Chen, Y.; Zhu, Y.G.; Fang, F.; Chen, L.M. Ginsenoside Rg1 reduces MPTP-induced substantia nigra neuron loss by suppressing oxidative stress. Acta Pharmacol. Sin. 2005, 26, 56–62.
- Shi, C.; Zhang, Y.X.; Zhang, Z.F. Effect of phosphorylated-ERK1/2 on inducible nitric oxide synthase expression in the sub-stantia nigra of mice with MPTP-induced Parkinson disease. Nan Fang Yi Ke Da Xue Xue Bao 2009, 29, 60–63.
- Chen, X.C.; Zhu, Y.G.; Zhu, L.A.; Huang, C.; Chen, Y.; Chen, L.M.; Fang, F.; Zhou, Y.C.; Zhao, C.H. Ginsenoside Rg1 attenuates dopamine-induced apoptosis in PC12 cells by suppressing oxidative stress. Eur. J. Pharmacol. 2003, 473, 1–7.
- Xu, H.; Jiang, H.; Wang, J.; Xie, J. Rg1 protects the MPP+-treated MES23. 5 cells via attenuating DMT1 up-regulation and cellular iron uptake. Neuropharmacology 2010, 58, 488–494.
- Xu, B.-B.; Liu, C.-Q.; Gao, X.; Zhang, W.-Q.; Wang, S.-W.; Cao, Y.-L. Possible mechanisms of the protection of ginsenoside Re against MPTP-induced apoptosis in substantia nigra neurons of Parkinson’s disease mouse model. J. Asian Nat. Prod. Res. 2005, 7, 215–224.
- Selkoe, D.J.; Schenk, D. Alzheimer’s disease: Molecular understanding predicts amyloid-based therapeutics. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 545.
- Tanzi, R.E.; Bertram, L. The latest suspect. Nature 2008, 454, 707–708.
- Bazan, N.G.; Palacios-Pelaez, R.; Lukiw, W.J. Hypoxia signaling to genes. Mol. Neurobiol. 2002, 26, 283–298.
- Frank, B.; Gupta, S. A review of antioxidants and Alzheimer’s disease. Ann. Clin. Psychiatry 2005, 17, 269–286.
- Finch, C.E.; Morgan, T.E. Systemic inflammation, infection, ApoE alleles, and Alzheimer disease: A position paper. Curr. Alzheimer Res. 2007, 4, 185–189.
- Zhao, H.; Li, Q.; Zhang, Z.; Pei, X.; Wang, J.; Li, Y. Long-term ginsenoside consumption prevents memory loss in aged SAMP8 mice by decreasing oxidative stress and up-regulating the plasticity-related proteins in hippocampus. Brain Res. 2009, 1256, 111–122.
- Qian, Y.-H.; Han, H.; Hu, X.-D.; Shi, L.-L. Protective effect of ginsenoside Rb1 on β-amyloid protein (1-42)-induced neuro-toxicity in cortical neurons. Neurol. Res. 2009, 31, 663–667.
- Xie, X.; Wang, H.-T.; Li, C.-L.; Gao, X.-H.; Ding, J.-L.; Zhao, H.-H.; Lu, Y.-L. Ginsenoside Rb1 protects PC12 cells against β-amyloid-induced cell injury. Mol. Med. Rep. 2010, 3, 635–639.
- Liu, Q.; Kou, J.-P.; Yu, B.-Y. Ginsenoside Rg1 protects against hydrogen peroxide-induced cell death in PC12 cells via inhibiting NF-κB activation. Neurochem. Int. 2011, 58, 119–125.
- Wang, Y.-H.; Du, G.-H. Ginsenoside Rg1 inhibits β-secretase activity in vitro and protects against Aβ-induced cytotoxicity in PC12 cells. J. Asian Nat. Prod. Res. 2009, 11, 604–612.
- She, L.; Xiong, L.; Li, L.; Zhang, J.; Sun, J.; Wu, H.; Ren, J.; Wang, W.; Zhao, X.; Liang, G. Ginsenoside Rk3 ameliorates Aβ-induced neurotoxicity in APP/PS1 model mice via AMPK signaling pathway. Biomed. Pharmacother. 2023, 158, 114192.
- Bouzroud, S.; El Maaiden, E.; Sobeh, M.; Merghoub, N.; Boukcim, H.; Kouisni, L.; El Kharrassi, Y. Biotechnological Approaches to Producing Natural Antioxidants: Anti-Ageing and Skin Longevity Prospects. Int. J. Mol. Sci. 2023, 24, 1397.
- Hou, W.; Wang, Y.; Zheng, P.; Cui, R. Effects of ginseng on neurological disorders. Front. Cell. Neurosci. 2020, 14, 55.
- Wang, W.; Liu, X.; Liu, J.; Cai, E.; Zhao, Y.; Li, H.; Zhang, L.; Li, P.; Gao, Y. Sesquiterpenoids from the root of Panax ginseng attenuates lipopolysaccharide-induced depressive-like behavior through the brain-derived neurotrophic fac-tor/tropomyosin-related kinase B and sirtuin type 1/nuclear factor-κB signaling pathways. J. Agric. Food Chem. 2018, 66, 265–271.
- Liu, L.; Kelly, M.G.; Wierzbicki, E.L.; Escober-Nario, I.C.; Vollmer, M.K.; Doré, S. Nrf2 plays an essential role in long-term brain damage and neuroprotection of Korean red ginseng in a permanent cerebral ischemia model. Antioxidants 2019, 8, 273.
- Dong, X.; Zheng, L.; Lu, S.; Yang, Y. Neuroprotective effects of pretreatment of ginsenoside R b1 on severe cerebral ischemia-induced injuries in aged mice: Involvement of anti-oxidant signaling. Geriatr. Gerontol. Int. 2017, 17, 338–345.
- Kim, J.; Shim, J.; Lee, S.; Cho, W.-H.; Hong, E.; Lee, J.H.; Han, J.-S.; Lee, H.J.; Lee, K.W. Rg3-enriched ginseng extract ameliorates scopolamine-induced learning deficits in mice. BMC Complement. Altern. Med. 2016, 16, 316.
- Garbelli, R.; De Bock, F.; Medici, V.; Rousset, M.; Villani, F.; Boussadia, B.; Arango-Lievano, M.; Jeanneteau, F.; Daneman, R.; Bartolomei, F. PDGFRβ+ cells in human and experimental neuro-vascular dysplasia and seizures. Neuroscience 2015, 306, 18–27.
- Zhu, Y.; Wang, Z.; Yu, S.; Zhao, C.; Xu, B.; Liu, R.; Xu, L.; Guo, Y. Neuroprotective Effect of Ginseng Fibrous Root Enzymatic Hydrolysate against Oxidative Stress. Molecules 2022, 27, 7824.
- Kritharides, L.; Brown, A.; Brieger, D.; Ridell, T.; Zeitz, C.; Jeremy, R.; Tonkin, A.; Walsh, W.; White, H. Overview and de-terminants of cardiovascular disease in indigenous populations. Heart Lung Circ. 2010, 19, 337–343.
- Pratt, C. Alternative prevention and treatment of cardiovascular disease, part 2. Prim. Care Clin. Off. Pract. 2010, 37, 339–366.
- Ing, D.J.; Zang, J.; Dzau, V.J.; Webster, K.A.; Bishopric, N.H. Modulation of cytokine-induced cardiac myocyte apoptosis by nitric oxide, Bak, and Bcl-x. Circ. Res. 1999, 84, 21–33.
- Von Harsdorf, R.d.; Li, P.-F.; Dietz, R. Signaling pathways in reactive oxygen species–induced cardiomyocyte apoptosis. Circulation 1999, 99, 2934–2941.
- Kanani, P.M.; Sinkey, C.A.; Browning, R.L.; Allaman, M.; Knapp, H.R.; Haynes, W.G. Role of oxidant stress in endothelial dysfunction produced by experimental hyperhomocyst (e) inemia in humans. Circulation 1999, 100, 1161–1168.
- B Britten, M.; M Zeiher, A.; Schächinger, V. Clinical importance of coronary endothelial vasodilator dysfunction and thera-peutic options. J. Intern. Med. 1999, 245, 315–327.
- Ferrari, R.; Agnoletti, L.; Comini, L.; Gaia, G.; Bachetti, T.; Cargnoni, A.; Ceconi, C.; Curello, S.; Visioli, O. Oxidative stress during myocardial ischaemia and heart failure. Eur. Heart J. 1998, 19, B2–B11.
- Anversa, P.; Cheng, W.; Liu, Y.; Leri, A.; Redaelli, G.; Kajstura, J. Apoptosis and myocardial infarction. Basic Res. Cardiol. 1998, 93, s008–s012.
- Lim, K.H.; Ko, D.; Kim, J.-H. Cardioprotective potential of Korean Red Ginseng extract on isoproterenol-induced cardiac injury in rats. J. Ginseng Res. 2013, 37, 273.
- Zhou, W.; Chai, H.; Lin, P.H.; Lumsden, A.B.; Yao, Q.; Chen, C. Ginsenoside Rb1 blocks homocysteine-induced endothelial dysfunction in porcine coronary arteries. J. Vasc. Surg. 2005, 41, 861–868.
- Xie, J.-T.; Shao, Z.-H.; Vanden Hoek, T.L.; Chang, W.-T.; Li, J.; Mehendale, S.; Wang, C.-Z.; Hsu, C.-W.; Becker, L.B.; Yin, J.-J.; et al. Antioxidant effects of ginsenoside Re in cardiomyocytes. Eur. J. Pharmacol. 2006, 532, 201–207.
- Yi, X.Q.; Li, T.; Wang, J.R.; Wong, V.K.W.; Luo, P.; Wong, I.Y.F.; Jiang, Z.H.; Liu, L.; Zhou, H. Total ginsenosides increase coronary perfusion flow in isolated rat hearts through activation of PI3K/Akt-eNOS signaling. Phytomedicine 2010, 17, 1006–1015.
- Jeon, B.H.; Kim, C.S.; Park, K.S.; Lee, J.W.; Park, J.B.; Kim, K.-J.; Kim, S.H.; Chang, S.J.; Nam, K.Y. Effect of Korea red ginseng on the blood pressure in conscious hypertensive rats. Gen. Pharmacol. Vasc. Syst. 2000, 35, 135–141.
- Qin, N.; Gong, Q.-h.; Wei, L.-w.; Wu, Q.; Huang, X.-n. Total Ginsenosides Inhibit the Right Ventricular Hypertrophy Induced by Monocrotaline in Rats. Biol. Pharm. Bull. 2008, 31, 1530–1535.
- Hong, S.Y.; Kim, J.Y.; Ahn, H.Y.; Shin, J.-H.; Kwon, O. Panax ginseng Extract Rich in Ginsenoside Protopanaxatriol Attenuates Blood Pressure Elevation in Spontaneously Hypertensive Rats by Affecting the Akt-Dependent Phosphorylation of Endo-thelial Nitric Oxide Synthase. J. Agric. Food Chem. 2012, 60, 3086–3091.
- Zhu, D.; Wu, L.; Li, C.R.; Wang, X.W.; Ma, Y.J.; Zhong, Z.y.; Zhao, H.B.; Cui, J.; Xun, S.F.; Huang, X.L. Ginsenoside Rg1 protects rat cardiomyocyte from hypoxia/reoxygenation oxidative injury via antioxidant and intracellular calcium homeostasis. J. Cell. Biochem. 2009, 108, 117–124.
- Tsutsumi, Y.M.; Tsutsumi, R.; Mawatari, K.; Nakaya, Y.; Kinoshita, M.; Tanaka, K.; Oshita, S. Compound K, a metabolite of ginsenosides, induces cardiac protection mediated nitric oxide via Akt/PI3K pathway. Life Sci. 2011, 88, 725–729.
- Wang, Y.; Hu, Z.; Sun, B.; Xu, J.; Jiang, J.; Luo, M. Ginsenoside Rg3 attenuates myocardial ischemia/reperfusion injury via Akt/endothelial nitric oxide synthase signaling and the B-cell lymphoma/B-cell lymphoma-associated X protein pathway. Mol. Med. Rep. 2015, 11, 4518–4524.
- Qin, G.-W.; Lu, P.; Peng, L.; Jiang, W. Ginsenoside Rb1 inhibits cardiomyocyte autophagy via PI3K/Akt/mTOR signaling pathway and reduces myocardial ischemia/reperfusion injury. Am. J. Chin. Med. 2021, 49, 1913–1927.
- Fan, Z.-X.; Yang, C.-J.; Li, Y.-H.; Yang, J.; Huang, C.-X. Ginsenoside Rh2 attenuates myocardial ischaemia-reperfusion injury by regulating the Nrf2/HO-1/NLRP3 signalling pathway. Exp. Ther. Med. 2023, 25, 35.
- Sodrul, I.M.; Wang, C.; Chen, X.; Du, J.; Sun, H. Role of ginsenosides in reactive oxygen species-mediated anticancer therapy. Oncotarget 2018, 9, 2931.
- Klaunig, J.E.; Xu, Y.; Isenberg, J.S.; Bachowski, S.; Kolaja, K.L.; Jiang, J.; Stevenson, D.E.; Walborg Jr, E.F. The role of oxidative stress in chemical carcinogenesis. Environ. Health Perspect. 1998, 106, 289–295.
- Cairns, R.A.; Harris, I.S.; Mak, T.W. Regulation of cancer cell metabolism. Nat. Rev. Cancer 2011, 11, 85–95.
- Ichijo, H.; Nishida, E.; Irie, K.; Dijke, P.t.; Saitoh, M.; Moriguchi, T.; Takagi, M.; Matsumoto, K.; Miyazono, K.; Gotoh, Y. In-duction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science 1997, 275, 90–94.
- Moon, D.-O.; Kim, M.-O.; Choi, Y.H.; Hyun, J.W.; Chang, W.Y.; Kim, G.-Y. Butein induces G2/M phase arrest and apoptosis in human hepatoma cancer cells through ROS generation. Cancer Lett. 2010, 288, 204–213.
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947.
- Liu, J.; Wang, Z. Increased oxidative stress as a selective anticancer therapy. Oxidative Med. Cell. Longev. 2015, 2015, 294303.
- De Giani, A.; Oldani, M.; Forcella, M.; Lasagni, M.; Fusi, P.; Di Gennaro, P. Synergistic Antioxidant Effect of Prebiotic Ginseng Berries Extract and Probiotic Strains on Healthy and Tumoral Colorectal Cell Lines. Int. J. Mol. Sci. 2023, 24, 373.
- Yokozawa, T.; Satoh, A.; Cho, E.J. Ginsenoside-Rd attenuates oxidative damage related to aging in senescence-accelerated mice. J. Pharm. Pharmacol. 2004, 56, 107–113.
- Oh, M.H.; Chung, H.Y.; Yong, H.S.; Kim, K.W.; Oura, H.; Yokozawa, T. Effects of ginsenoside Rb2 on the antioxidants in SAM-R/1 mice. Korean Biochem. J. 1992, 25, 492–497.
- Choi, W.; Kim, H.S.; Park, S.H.; Kim, D.; Hong, Y.D.; Kim, J.H.; Cho, J.Y. Syringaresinol derived from Panax ginseng berry attenuates oxidative stress-induced skin aging via autophagy. J. Ginseng Res. 2022, 46, 536–542.
- Li, G.; Yang, L.; Feng, L.; Yang, J.; Li, Y.; An, J.; Li, D.; Xu, Y.; Gao, Y.; Li, J. Syringaresinol protects against type 1 diabetic cardiomyopathy by alleviating inflammation responses, cardiac fibrosis, and oxidative stress. Mol. Nutr. Food Res. 2020, 64, 2000231.
- Ramesh, T.; Kim, S.-W.; Hwang, S.-Y.; Sohn, S.-H.; Yoo, S.-K.; Kim, S.-K. Panax ginseng reduces oxidative stress and restores antioxidant capacity in aged rats. Nutr. Res. 2012, 32, 718–726.
- Jung, C.-H.; Seog, H.-M.; Choi, I.-W.; Choi, H.-D.; Cho, H.-Y. Effects of wild ginseng (Panax ginseng C.A. Meyer) leaves on lipid peroxidation levels and antioxidant enzyme activities in streptozotocin diabetic rats. J. Ethnopharmacol. 2005, 98, 245–250.
- Yokozawa, T.; Wu Liu, Z. The role of ginsenoside-Rd in cisplatin-induced acute renal failure. Ren. Fail. 2000, 22, 115–127.
- Lu, G.; Liu, Z.; Wang, X.; Wang, C. Recent advances in panax ginseng CA Meyer as a herb for anti-fatigue: An effects and mechanisms review. Foods 2021, 10, 1030.
- Han, S.Y.; Kim, J.; Kim, E.; Kim, S.H.; Seo, D.B.; Kim, J.-H.; Shin, S.S.; Cho, J.Y. AKT-targeted anti-inflammatory activity of Panax ginseng calyx ethanolic extract. J. Ginseng Res. 2018, 42, 496–503.
- Ning, C.; Gao, X.; Wang, C.; Huo, X.; Liu, Z.; Sun, H.; Yang, X.; Sun, P.; Ma, X.; Meng, Q. Hepatoprotective effect of ginsenoside Rg1 from Panax ginseng on carbon tetrachloride-induced acute liver injury by activating Nrf2 signaling pathway in mice. Environ. Toxicol. 2018, 33, 1050–1060.
- Ramadhania, Z.M.; Yang, D.U.; Moektiwardojo, M.; Han, Y.; Park, J.K.; Rupa, E.J.; Yang, D.C.; Lee, S.J.; Kang, S.C. Enhanced Anti-Skin Aging Effects of Fermented Black Ginseng (Panax ginseng CA Meyer) by Aspergillus niger KHNT-1. Appl. Sci. 2023, 13, 550.
- Lakshmi, T.; Anitha, R.; Geetha, R. Panax ginseng–a universal panacea in the herbal medicine with diverse pharmacological spectrum–a review. Asian J. Pharm. Clin. Res. 2011, 4, 14–18.
- Kim, H.-J.; Lee, S.-G.; Chae, I.-G.; Kim, M.-J.; Im, N.-K.; Yu, M.-H.; Lee, E.-J.; Lee, I.-S. Antioxidant effects of fermented red ginseng extracts in streptozotocin-induced diabetic rats. J. Ginseng Res. 2011, 35, 129.
- Patel, S.; Rauf, A. Adaptogenic herb ginseng (Panax) as medical food: Status quo and future prospects. Biomed. Pharma Cotherapy 2017, 85, 120–127.
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. A review of ginseng species in different regions as a multipurpose herb in traditional Chinese medicine, modern herbology and pharmacological science. J. Med. Plants Res. 2019, 13, 213–226.
More
Information
Subjects:
Biotechnology & Applied Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
3 times
(View History)
Update Date:
13 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




