An efficient and reliable electrochemical sensing platform based on COOH-fMWCNTs modified GCE (COOH-fMWCNTs/GCE) was designed for the detection of nanomolar concentration of Nile Blue Sulphate (NBS). The photocatalytic degradation of NBS dye was carried out by using TiO2 nanoparticles as photocatalyst in the presence of H2O2.
1. Introduction
Several semiconductors, including titania, vanadium pentoxide, zinc oxide, ferric oxide, cadmium oxide, cadmium sulfide, and alumina, have been used as photocatalysts for the degradation of different colored organic pollutants such as dyes. Among these, TiO2 is the most commonly employed photocatalyst. The current research work focuses on light-assisted catalytic degradation of Nile Blue Sulphate (NBS) dye using TiO2 nanoparticles as it possesses very high mechanical strength, nontoxicity, chemical, and thermal stability [1]. However, owing to its band gap of 3.2 eV, the recombination of the electron-hole pair and absorption in the UV region limits its utility. To hinder the recombination of generated electron-hole pair, H2O2 was used for enhancing the practical utility of TiO2 nanoparticles by abstracting the photogenerated electron and avoiding its recombination with the hole. Different free radicals like •OH, •O2−/•OOH (reactive oxygen species) are generated from H2O2 at the surface of the catalyst that enhance the TiO2 capability of degrading the organic dye molecule in wastewater [2].
2. Square Wave Voltammetric Analysis of NBS
Compared to CV, SWV is much more sensitive and can remarkably differentiate between the faradaic and non-Faradaic current
[3]. It is a reliable electroanalytical technique that produces results with enhanced resolution. Therefore, in the present work, SWV was employed for the electrochemical sensing of NBS. The intensity of peak current response of NBS at bare and modified GCE was investigated in PBS of pH 6.0 at a scan rate of 100 mV s
−1 keeping frequency and pulse amplitude 20 Hz and 20 mV, respectively. The oxidation peak of NBS was obtained at 0.86 and 0.84 V at bare and MWCNTs modified GCE respectively. After modification with COOH-
fMWCNTs, it was shifted to a lower potential of 0.82 V. This negative shift in the potential shows the electrocatalytic behavior of the sensing platform. The peak current of NBS was also enhanced to 8.63 µA and 11.7 µA at MWCNTs and COOH-
fMWCNTs respectively, as presented in
Figure 1a
[4][5]. The current value for COOH-
fMWCNTs/GCE is almost twice that of the bare which supports the proposition of availability of more active sites for interaction of analyte. The surface features of the electrodes were distinguished from contact angle analysis as shown in
Figure 1b. The contact angle (angle measured in liquid) of the water droplet on bare GCE with a value of 80° shows a slight hydrophilic character of the GCE surface. The hydrophilicity of GCE was increased when modified with MWCNTs as demonstrated by the 60° contact angle of the water droplet. Modification of the GCE surface with COOH-
fMWCNTs led to additional enhancement in hydrophilicity as witnessed by further reduction in contact angle (45°) of water droplet. Hence, analyte in the droplet of aqueous solution placed at the GCE surface modified with COOH-
fMWCNTs should have closer accessibility to the electrode due to more hydrophilic nature of the surface. This behaviour is consistent with the most intense oxidation signal of the NBS at COOH-
fMWCNTs/GCE as obvious from observation of
Figure 1a which is a direct consequence of maximum surface coverage.
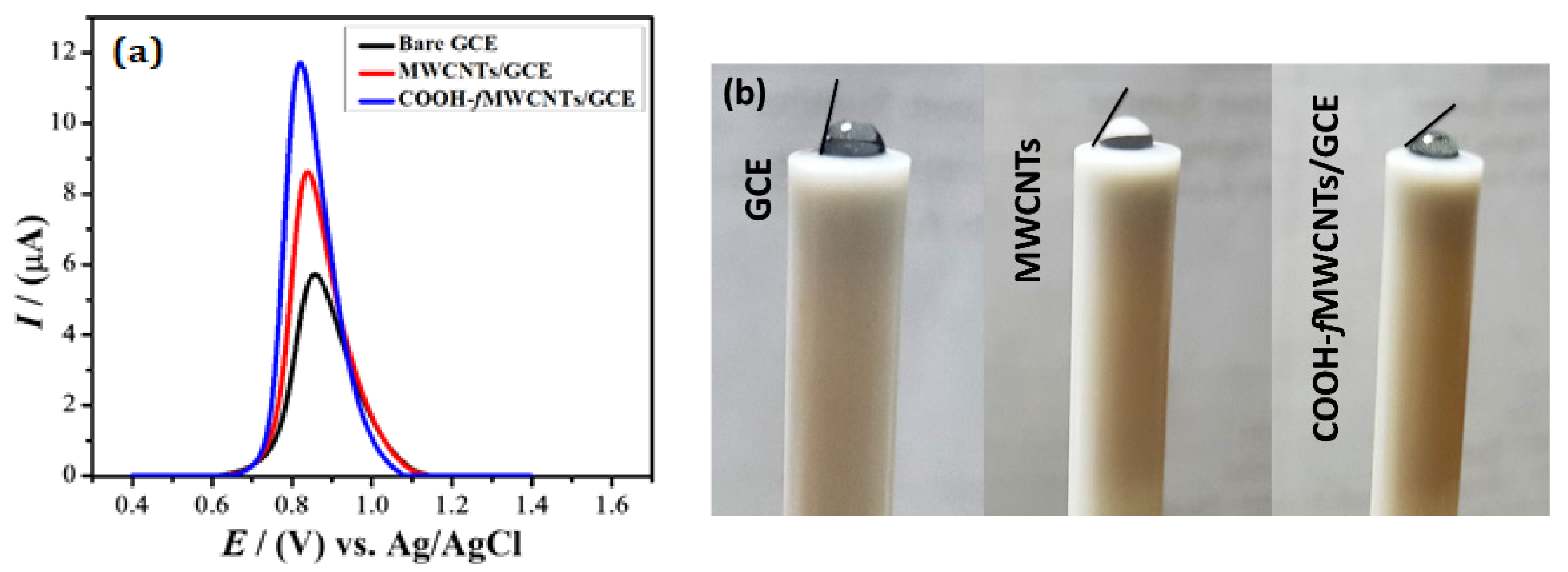
Figure 1. (a) SWVs response of 10 µM NBS at bare GCE, MWCNTS/GCE, and COOH-fMWCNTs/GCE in PBS of pH 6.0. (b) Picture recorded after 30 s placement of the same volume of water droplet on bare GCE, MWCNTs/GCE and COOH-fMWCNTs/GCE.
This enhancement in peak current in SWV response is in good agreement with EIS and CV findings, and this is because of the increase in the electrode’s electroactive surface area by using COOH-
fMWCNTs, as a modifier. Here, the modifier acts as a bridge between the analyte and the transducer (GCE) to facilitate electron transfer. Thus, the NBS transfers electrons through COOH-
fMWCNTs to GCE for boosting the oxidation current via the electron hopping mechanism. Moreover, the boosted surface area results in enhanced interaction between the dye molecules and modifier that leads to increased preconcentration of dye molecules near the electrode surface. The preconcentration of the dye molecule at the modified electrode surface may also be the reason for peak current enhancement
[4][6].
3. Spectroscopic Studies of NBS Photocatalytic Degradation
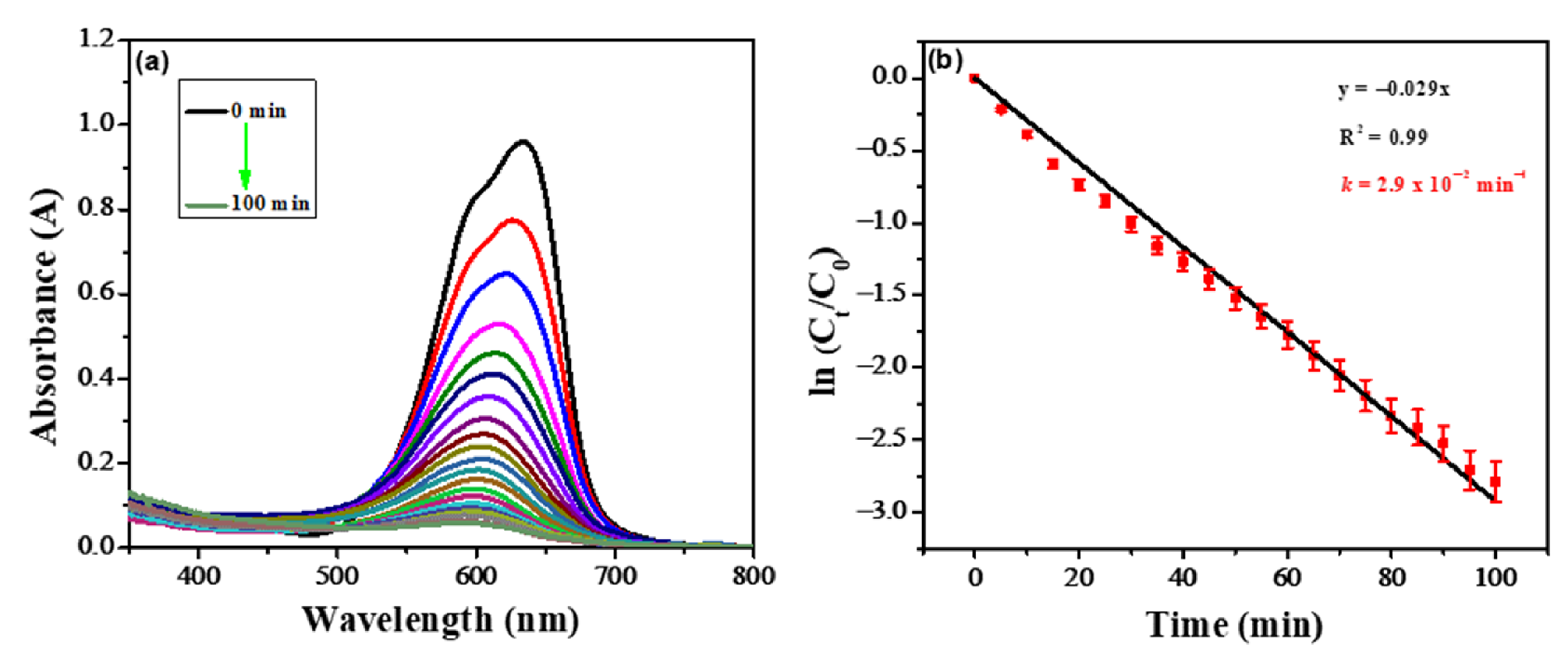
A UV-visible spectrophotometer was also used for studying the photocatalytic degradation of NBS. The TiO2 nanoparticles and H2O2 were used as photocatalysts and oxidizing agents respectively. The corresponding absorption spectra at different time intervals are depicted in Figure 2a. The dye molecule’s color is because of the conjugation system in the structure. Electron-hole pair generation occurred as the photocatalyst was exposed to sunlight. The generation of electron-hole pair and hydroxyl radical leads to dye degradation by breaking up the conjugation system in the dye molecule. It is evident from UV-visible spectra that over the time, the photocatalytic degradation process increased as the dye molecule was degraded in the presence of sunlight. The presence of H2O2 facilitates the production of hydroxyl radicals which leads to the mineralization of the organic molecule.
Figure 2. (a) UV-Vis spectra of the photocatalytic degradation of NBS at different time intervals. (b) The photocatalytic degradation kinetics of NBS using UV-visible spectroscopic data.
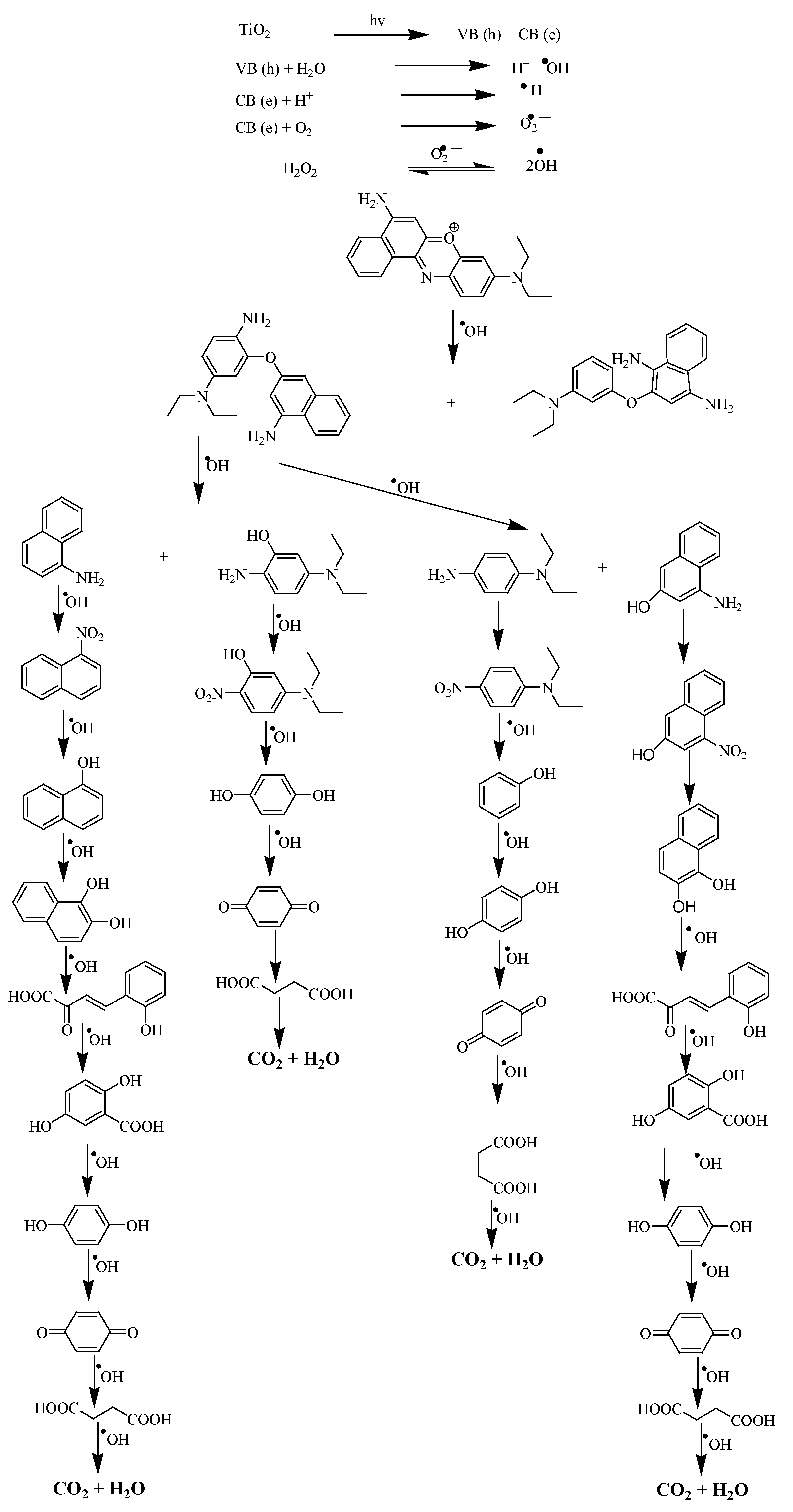
The proposed degradation mechanism as depicted in Scheme 1 of NBS shows attack of hydroxyl free radicals generated by TiO
2 and H
2O
2 at a double bond present in the ring, hence, with the breakdown of conjugation of the ring, the color variation from being intense to dull, was observed (
Figure 3). Scheme 1 is the proposed mechanism, based upon the obtained electrochemical and UV-Vis spectroscopic data and also in the light of reported literature, of the degradation of NBS and other dyes using methods other than the researchers' developed method
[5][7][8]. The final product is the mineralization of the NBS to CO
2 and H
2O.
Scheme 1. Proposed mechanism for the degradation of NBS.
Figure 3. Naked eye evidence of the decolorization of NBS dye.
Using spectroscopic results, the percent degradation of NBS was calculated using the following equation.
The spectroscopic data were also used to explore the kinetics of the photocatalytic degradation process
[9][10][11]. The reaction order and rate were confirmed from the plot between ln [
Ct/
C0] as a function of time, as shown in
Figure 2b. The rate of reaction (
k) was calculated from the equation i.e.,
lnCtC0=−kt. Where
Ct is the absorption at time t,
C0 is the absorption at zero time, and
k is the rate constant. The straight line of the plot between ln [
Ct/
C0] as a function of time exhibited that the reaction follows first-order kinetics. The rate of reaction calculated from spectroscopic data is 0.029 min
−1. The order of reaction and rate of reaction calculated from electrochemical and spectroscopic data are in good agreement revealing that the designed sensor is also efficient for monitoring photocatalytic degradation of NBS.
4. Conclusions
The toxicity of dyes is a threat to life in water and on land. Thus, in order to address abatement of the dyes and overcome issues pertaining to environmental degradation, the current work presents the development of a sensor for the nanomolar detection of Nile Blue Sulphate (NBS) dye. Moreover, it presents employing photocatalyst for the photocatalytic degradation of NBS followed by analytical methods for monitoring the extent and degradation kinetics. Acid-functionalized MWCNTs were immobilized on the surface of the GCE to fabricate an efficient electrochemical sensing platform. Various experimental parameters such as accumulation potential, accumulation time, supporting electrolyte, and pH of solution were optimized to obtain intense signal of the target analyte at the designed probe. Peak current versus concentration plot was found to have a wide linear range with LOD value of 1.21 nM under optimized experimental conditions for NBS. After detection, photocatalytic degradation experiments for NBS were conducted using TiO2 nanoparticles in the presence of hydrogen peroxide.