Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mona Shareif Hashim | -- | 3407 | 2023-03-03 07:07:05 | | | |
| 2 | Catherine Yang | Meta information modification | 3407 | 2023-03-03 07:17:35 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Osaili, T.M.; Dhanasekaran, D.K.; Zeb, F.; Faris, M.E.; Naja, F.; Radwan, H.; Cheikh Ismail, L.; Hasan, H.; Hashim, M.; Obaid, R.S. Therapeutic Effects of Essential Oils. Encyclopedia. Available online: https://encyclopedia.pub/entry/41837 (accessed on 01 March 2026).
Osaili TM, Dhanasekaran DK, Zeb F, Faris ME, Naja F, Radwan H, et al. Therapeutic Effects of Essential Oils. Encyclopedia. Available at: https://encyclopedia.pub/entry/41837. Accessed March 01, 2026.
Osaili, Tareq M., Dinesh Kumar Dhanasekaran, Falak Zeb, Moezalislam E. Faris, Farah Naja, Hadia Radwan, Leila Cheikh Ismail, Hayder Hasan, Mona Hashim, Reyad Shaker Obaid. "Therapeutic Effects of Essential Oils" Encyclopedia, https://encyclopedia.pub/entry/41837 (accessed March 01, 2026).
Osaili, T.M., Dhanasekaran, D.K., Zeb, F., Faris, M.E., Naja, F., Radwan, H., Cheikh Ismail, L., Hasan, H., Hashim, M., & Obaid, R.S. (2023, March 03). Therapeutic Effects of Essential Oils. In Encyclopedia. https://encyclopedia.pub/entry/41837
Osaili, Tareq M., et al. "Therapeutic Effects of Essential Oils." Encyclopedia. Web. 03 March, 2023.
Copy Citation
EEssential oils (EOs) have been known for their therapeutic potential against many health issues. EOs may contribute to the regulation and modulation of various biomarkers and cellular pathways responsible for metabolic health as well as the development of many diseases, including cancer, obesity, diabetes, cardiovascular diseases, and bacterial infections.
essential oils
anti-inflammatory
anti-cancer
metabolic health
1. Antioxidant, Anti-Inflammatory, and Anti-Cancer Activities of Essential Oils
Recent cumulative research suggests that EOs exhibit anti-inflammatory, antioxidant, and anticancer properties in cell and animal models [1][2][3]. Inflammation is a normal response to tissue damage caused by stimuli that could be biological, chemical, or physical [4]. EOs decrease the prerequisite compounds that exaggerate the inflammation process, namely, reactive oxygen species (ROS) and nitrogen species, NF-κB, and proinflammatory cytokines [5]. A comprehensive review of EOs revealed that oils extracted from lemon (Citrus Limon) fruit peels and leaves exerted antioxidant and radical scavenging activities under in vitro conditions [6]. Moreover, it was observed that the monoterpene hydrocarbon fraction was positively correlated with antioxidant activity [6]. Citrus EOs, namely, mandarin, wilking, and clementine, exhibited antioxidant activity by acting as free radical scavengers in a dose-dependent manner [7]. Edema is a pathological feature of inflammation [8]. Carrageenan, a pro-inflammatory agent, was used to induce paw edema in rats, while bergamot EO was applied for its anti-inflammatory effect. It was observed that the bergamot EO significantly decreased the inflammatory markers, namely, prostaglandin (PGE2) and nitrite/nitrate levels, as well as interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF-α) [8]. On a similar note, sesame seed oil decreased lipid peroxidation and nitric oxide production besides increasing antioxidant enzymes such as superoxide dismutase (SOD), glutathione (GSH), GSH peroxidase (GPx), and catalase (CAT) by binding the fatty acids in sesame seed with COX-2 and PGE2 [9]. The COX-2 enzyme activates pain (a symptom of inflammation) mechanisms in the human body. The phytochemical components inherent to sesame seed oil, like sesamol, sesamin, and sesamolin, are responsible for their antioxidant and anti-inflammatory properties [10]. A clinical trial was conducted to investigate the effect of sesame oil on 40 hypertensive male and female patients aged 35–60 years [11]. The results demonstrated that sesame EO decreased lipid peroxidation and increased antioxidant activity [11]. Likewise, eugenol—a bioactive compound in clove EO—decreased rat paw swelling and synovial immune cell infiltration [12]. EOs of the oregano flowering plant (O. vulgare) have also been recognized for their antioxidant potential [13][14]. In contrast, it was also observed that bergamot EO increased intracellular ROS production induced by N-formyl-Met-Leu-Phe in the presence of extracellular Ca2+ chelators. Thereby, the pro-inflammatory potential of EOs also needs to be carefully considered for any future clinical applications [15].
Cancer may significantly increase the fatality rate of the disease by compromising the immune system. The abundant bioactive components in EO may offer immune-boosting activity in the treatment of cancer [16]. Navel orange EO exhibited good anti-cancer potential against lung cancer and prostate cancer [17]. Similarly, non-volatile fractions of bergamot EO, such as coumarins and furocoumarins, showed anti-mutagenic activity [18]. EOs from Croton flavens leaf were observed to be effective on human lung carcinoma and human colon adenocarcinoma cell lines, respectively [19]. Another study identified that an EO from Curcumae rhizoma (CR) inhibited the growth of colon cancer by improving tumor vessel structure, reducing angiogenesis in tumors, and normalizing tumor vessels in both in vitro and in vivo models [20]. Similarly, a recent study demonstrated that oregano EO-based nanoemulsion increased prostate cancer cell death through apoptosis and decreased lipid droplet accumulation via targeting HMGCR, FASN, and SREPB1 signals in vitro [21].
The ability of the EOs to exhibit these bioactive properties could be attributed to their composition of terpenes, hydrocarbons, alcohols, aldehydes, ketones, and esters [22][23][24]. Usually, the method of extraction used to obtain the EO is determined according to the purpose of use. Due to the lipophilic nature of EOs and their low molecular weight, they can easily cross cell membranes or even alter the membrane composition and increase/decrease membrane fluidity [25]. Consequently, changes within the membrane structure lead to the leakage of ions and cytoplasmic molecules, reduced ATP production, and loss of mitochondrial potential, eventually resulting in cell death [26]. Moreover, EOs, upon entering the cell membrane, activate apoptosis and necrosis pathways, resulting in cell death, cell cycle arrest, and loss of function of essential organelles [26]. EOs encourage the condensation and fragmentation of nuclei and chromatin, causing a loss of cristae in the mitochondria, which eventually leads to cell apoptosis [27]. In conclusion, EOs may reduce inflammation, oxidative stress, and cancer development through targeted actions both at the physiological and cellular levels, which are summarized in Figure 1.
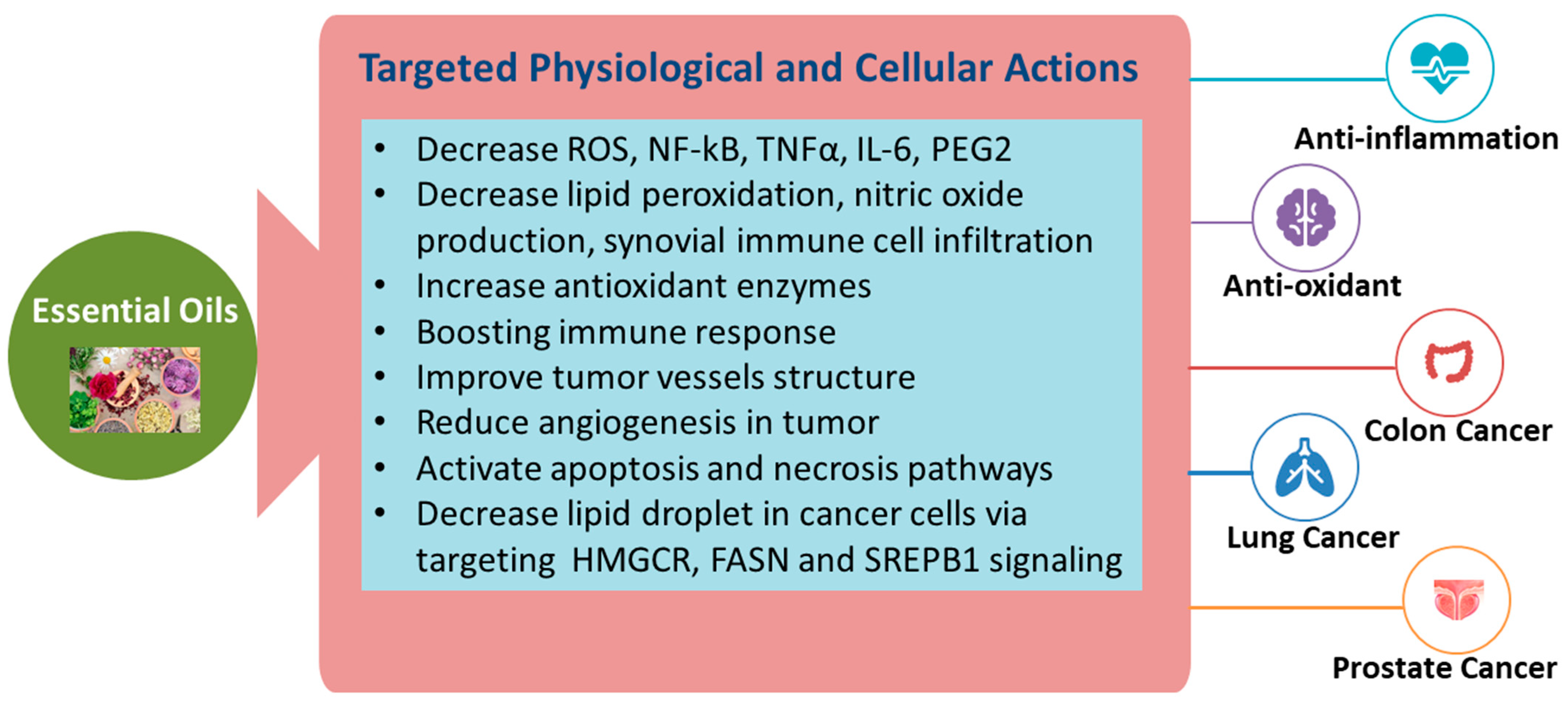
Figure 1. Targeted actions of EOs for the reduction of inflammation and cancer.
2. EOs: A Remedy for the Management of Metabolic Syndrome
Metabolic syndrome (MetS) is a group of risk factors that predispose an individual to cardiovascular disease and type II diabetes mellitus of metabolic origin. Typical MetS risk factors include obesity, high blood pressure, high blood sugar, and dyslipidemia (high triglycerides and/or low high-density lipoprotein cholesterol) [28]. The pathophysiology and the exact etiology of the development of MetS are not very clear [29]. However, MetS has been associated with increased oxidative stress [30] and is characterized by elevated inflammatory markers [31]. Furthermore, obesity and insulin resistance lead to an increase in proinflammatory cytokines such as TNF-α and IL-6.
2.1. EOs and Obesity
Obesity could be classified as the primary initiating factor of MetS, and attaining a healthy body weight is one of the first lines of treatment. Multiple plant-derived EOs, which are rich sources of volatile organic compounds, have long been used for the complementary treatment of obesity [32]. Several in vitro and in vivo studies suggest that the anti-obesity effects of EOs are achieved by the down-regulation of adipogenic transcription factors such as PPARγ and CEBPα at both protein and mRNA levels, the elevation of the plasma glycerol concentration (a marker of lipolysis), as well as the suppression of fat accumulation and intracellular triglycerides [33]. The EOs from the Citrus family have been widely investigated for their anti-obesity properties [33]. In an animal study, sweet orange EO was suggested as a potential dietary supplement for weight loss, since it decreased the expression of peroxisome proliferators-activated receptor-γ (PPARγ), upregulated uncoupling protein 2 (UCP-2), hormone-sensitive lipase (HSL) and carnitine palmitoyltransferase I (CPT-I), and inhibited the expression of acetyl-CoA carboxylase (AAC) [34]. The bioactive compound (+)-limonene, extracted from citrus peel EO, exhibited anti-obesity properties [35]. Moreover, grapefruit and citrus EOs are commonly used in aromatherapy for weight loss programs [36][37].
2.2. EOs and Diabetes
Diabetes is a common complication of MetS. It is important to note that EOs may help in the management of diabetes mellitus (DM), but they cannot be used as a cure. The EOs exhibit their antidiabetic action by inhibiting α-amylase and α-glucosidase enzymes, and by increasing insulin sensitivity or/and insulin secretion [38]. EOs can also be used to reduce common complications of diabetes, such as ulcers and loss of skin integrity, and can decrease the duration of the infection [39]. EOs from Asian ginseng (Panax quinquefolius), fenugreek (Trigonella foenum-graecum), and aloe (Aloe vera) have been observed to improve glucose tolerance [40]. Lavender EO (Lavandula stoechas L.) was protective against diabetes and decreased the associated renal and hepatic injuries in diabetic rats [41]. A mixture of cinnamon bark (Cinnamomum zeylanicum), cumin (Cuminum cyminum), fenugreek (Trigonella foenum-graecum), and oregano (Origanum vulgare) was observed to lower glucose levels by enhancing insulin sensitivity [42]. One animal study suggested that bioactive compounds in cinnamon (Cinnamomum veru) can regulate adipocyte gene expression to improve glucose transport and insulin signaling [43]. Under in vitro conditions, hydro-distilled EO from clove buds inhibited the activities of α-amylase and α -glucosidase [44]. However, a meta-analysis identified only one randomized controlled study on the impact of EO on diabetes and thereby, a conclusive statement that provides evidence-based health claims on this issue cannot be made [45].
2.3. EOs and Hypertension
Hypertension is another hallmark risk factor for the development of MetS. Antihypertensive medications and lifestyle modifications are the primary treatments for hypertension [46]. Preliminary studies indicate that EOs are effective in decreasing blood pressure and heart rate [47][48]. Ref. [47] blended four EOs, namely lavender (Lavandula officinalis), ylang-ylang (Cananga odorata), marjoram (Origanum majorana), and neroli (Citrus aurantium). The immediate and long-term effects of the EOs were evaluated by monitoring the 24-h ambulatory blood pressure and salivary cortisol levels, respectively. It was observed that the EO blend significantly decreased the daytime blood pressure and salivary cortisol concentration. However, there was an insignificant decrease in nighttime blood pressure [47]. Carvacrol, the major component of oregano EO, may help decrease blood pressure as it causes peripheral vasodilatation and inhibits the contraction elicited by intracellular Ca2+ influx through CAV (voltage-dependent calcium) channels and transient receptor potential (TRP) channels [49][50][51][52]. A systematic review indicated no significant effect of inhaled EOs on blood pressure reduction in patients with hypertension [53].
2.4. EO and Dyslipidemia
Dyslipidemia is one of the major contributors to MetS. Lemon balm EO (Melissa officinalis) decreased triglycerides and reduced the expression of genes involved in fatty acid synthesis [47]. The purple yam (Dioscorea alata L.) was reported to be effective in controlling adipose tissue mass and increasing high-density lipoprotein cholesterol (HDL-C), decreasing triglyceride (TG), total cholesterol (TC), and low-density lipoprotein cholesterol (LDL-C) concentrations associated with gut microbiota modulation [54]. The antihyperlipidemic effect of EOs could be due to their ability to activate lipoprotein lipase [38].
Interestingly, some studies investigated the impact of EOs on two or more risk factors for MetS rather than concentrating only on one risk factor. Cinnamon extracts (Cinnamomum veru) exhibited antidiabetic properties and had a beneficial impact on lipid profiles [55][56][57]. Hedge nettles from the Stachys species, commonly referred to as “mountain tea” are consumed in various parts of the world as a herbal tea to treat different medical conditions [58]. The bioactive compounds in this tea include germacrene D, β-pinene, α-pinene, hexahydrofarnesyl acetone, and valeranone. These compounds were observed to play a role in glucose homeostasis and prevent the absorption of fats by inhibiting enzymes such as cholinesterases, glucosidase, amylase, tyrosinase, and lipases respectively [59]. Similarly, lemon balm extract also prevented or concomitantly treated DM-associated dyslipidemia and hypercholesterolemia [60].
2.5. Dosage, Bioactive Metabolites, Therapeutic, and Adverse Effects of EOs
The researchers have reviewed the therapeutic effects, dosage, and possible side effects of EOs extracted from different sources such as peppermint, chamomile, fennel, rosemary, fenugreek, garlic, cumin, and lavender, among others, against various diseases, including microbial infections, obesity, diabetes, hypertension, and dyslipidemia (Table 1). Previous studies suggested that 225 mg/day of peppermint EOs may reduce the microbial dysbiosis and the total symptom score in patients with IBS [61][62][63]. Consumption of 100 mg/kg/b.w of chamomile EO prevents obesity and dyslipidemia, by reducing the weight gain and improve the lipid profile, and kidney and liver functions in animal models [64]. In addition, 3 g of chamomile EOs decreased the HOMA-IR index, serum HbA1C, TAG, TC, and LDL levels in patients with type 2 diabetes [65][66][67][68]. The use of 15 mg/kg/b.w and 7.5 mg/kg of fennel and rosemary EOs confer protection against hypertension and improve cardiac and renal function through anti-inflammatory and antioxidant activities [69][70]. Moreover, the consumption of 50 mg/kg/b.w of garlic EOs exerted anti-obesity and anti-hyperlipidemic effects by reducing HFD-induced body weight gain and adipose tissue weight [71][72]. The bioactive ingredients responsible for the therapeutic effects of EOs are polyphenols, flavonoids, tocopherols, menthone, tanins, luteolin, apigenin, transanethole, terpenoids, estragole, fenchone, limonene, diallyl disulfide, cuminaldehyde, α-pinene, γ-terpinene, linalool, and linalyl acetate [61][62][63][64][65][66][67][68][69][70][71][72][73][74][75]. The majority of these bioactive components have strong antioxidant activities, like phenolic compounds, terpenoids, luteolin, apigenin, estragole, fenchone, and limonene [64][65][66][67][68][74][75]. On the other hand, there are some side effects associated with the consumption of EOs. Therefore, it is important to know about the possible effects of EOs before using them. Previous studies demonstrated that EOs act as an endocrine disrupting chemical (EDC), an exogenous agent that interferes with hormonal release, action, storage, and metabolism in the body. However, EO may act as an agonist to the estrogen receptor alpha (ERα),antagonist to the androgen receptor (AR), and may cause endocrine disruption [76]. Regular exposure to lavender and tea tree oil is associated with abnormal breast growth and premature gynecomastia in adolescents [77][78]. Mild skin irritation was associated with consumption of chamomile tea in patients with type 2 DM [65].
Table 1. Dosage, bioactive metabolites, therapeutic, and adverse effects of EOs.
| Essential Oil | Experimental Condition | Usage/Dosage | Duration | Bioactive Compounds | Therapeutic Effects | Possible Side Effects/Toxicity | Reference |
|---|---|---|---|---|---|---|---|
| Peppermint | 57 patients with IBS | Capsule/225 mg per day | 4 weeks | Polyphenols, flavonoids, tocopherols, menthone, and tanins | Significant reduction in the total IBS symptoms score | Not detected | [61][62] |
| Chamomile | HFD-fed Wistar rats | Water extract/100 mg/kg b.w. | 6 weeks | Phenolic compounds and terpenoids | Prevention of body weight gain; decrease in levels of serum TAG, TC, LPL, urea, and creatinine, ALT, and AST; decrease in MDA levels, increase in antioxidant enzyme activity (SOD, catalase, GPx) and GSH levels in the liver and kidney | Not detected | [63][64] |
| 64 patients with T2DM | Chamomile tea (3 g/150 mL hot water) three times per day | 8 weeks | Luteolin and apigenin | Decrease in HOMA-IR index, serum HbA1C, insulin, TAG, TC, and LDL levels; no changes in HDL levels Decrease in serum MDA, increase in serum total antioxidant capacity and antioxidant enzyme activities (SOD, GPx, and catalase) |
Mild adverse event | [65][66][67][68] | |
| HFD-fed C57BL/6J mice | 10 mg/kg b.w. | 12 weeks | Apigenin | Decrease in body weight, visceral fat weight, plasma lipid levels (TAG, TC, LDL), postprandial glucose levels, and reduction of hepatic SREBP-1c and SREBP-2 expressions | No side effect | [69] | |
| Fennel and rosemary | 48 male albino rats | Volatile oils-15 and 7.5 mg/kg b.w. respectively | 4 weeks | Trans-anethole, terpenoids, estragole, fenchone, and limonene | Showed cardio and hepato- protective effect and safety towards kidney and blood sugar. Oxidative stress and inflammatory biomarkers were significantly improved | Not detected | [70] |
| Fenugreek | 60 diabetic male Wistar rats | 5% (w/w) | 8 weeks | Terpenenes | Glucose, triglyceride (TG), and total-cholesterol (TC) and LDL-cholesterol (LDL-C) levels decreased significantly in the plasma and liver of diabetic rats and increased the HDL-Cholesterol (HDL-Ch) level, modulated key enzyme related to hypertension | Not detected | [71] |
| Garlic | HFD-fed C57BL/6J mice | 50 mg/kg b.w. | 12 weeks | Diallyl disulfide, DADS | Exerted anti-obesity and anti-hyperlipidemic effects by reducing HFD-induced body weight gain and adipose tissue weight | Not detected | [72] |
| Cumin | Male rats with hepatotoxicity | 400 mg/kg | 2 weeks | Cuminaldehyde, α-pinene and γ-terpinene | Normalized acetaminophen-induced liver enzyme elevation and preserved liver structure | Not detected | [73] |
| Lavender | 75 diabetic neuropathic patients | Massage-2.5 cc of 3% lavender oil | 4 weeks | Linalool and linalyl acetate | Significantly increase the quality of life domain, reduce neuropathic pain | Not detected | [74] |
| 52 diabetic patients with insomnia | Inhalation | 4 weeks | Linalyl acetate and linalool | improve sleep quality and quantity, quality of life, and mood | Not detected | [75] |
3. Enhance Breast Milk Production and Childcare
Breastfeeding is beneficial for both mother and child from an immunological, physiological, psychological, and nutritional perspective [79][80]. However, poor milk production is considered one of the most common issues leading to early cessation of breastfeeding. Factors such as cesarean delivery, preterm birth, pain, fatigue, anxiety, return to work, emotional stress, and postpartum depression may affect milk production [81][82].
Medical and herbal galactagogues are often used to augment and increase milk production [83][84][85]. Nonetheless, aromatherapy accompanied by massage has been used historically as an alternative therapy [86][87]. Previous literature suggests that lavender oil has a comforting effect on the central nervous system, and a massage with lavender oil can increase milk production and prolactin levels in mothers [88][89][90]. A combination of aromatherapy and massage using EOs increased milk production to a larger extent, as compared to individual treatments [91]. Similarly, jasmine oil has been observed to increase milk secretion [92]. Furthermore, the application of menthol essence and peppermint is effective against nipple fissures common in lactating mothers [93][94].
EOs have also been observed to be beneficial during pregnancy and labor. Lemon EO (citrus lemon) effectively reduced nausea and vomiting during pregnancy [95]. Additionally, a randomized controlled trial observed that lavender aromatherapy massages helped reduce pain and the duration of labor [96]. Moreover, a triple-blind, randomized, placebo-controlled trial revealed that inhaling lavender essence may help decrease pain after a Caesarean section when used along with other medical and therapeutic approaches [97].
Literature reveals that EOs are also beneficial for infants and children. In one study, massaging with lavender oil was effective in relieving infants of the symptoms of colic [98]. During bathing, adding lavender oil reduced stress and crying and enhanced sleep in very young infants [99]. Using lavender aromatherapy during dental treatment decreased children’s anxiety and perception of pain [100]. Lavender oil was also effective in alleviating pain during blood sampling and vaccinations in children [101]. Similar results were shown in decreasing anxiety in children with diabetes using orange oil aromatherapy [102]. Aromatherapy using Rosa damascena EO showed improved sleeping quality in children with sleep disorders [103]. Moreover, EOs decreased chemotherapy-induced nausea and vomiting, decreased distress in burn patients, and the prevalence of rhinitis symptoms in children [104][105][106].
4. EOs: Natural Antibiotics
Foodborne pathogens are a common cause of foodborne illnesses that affect millions of people every year, sometimes with severe and lethal consequences [107]. As commonly used food preservatives are chemical in nature and may only be added up to a certain degree to prevent changes in taste/odor, there is a fair demand to identify naturally sourced compounds that would exert a similar effect without altering the organoleptic properties negatively [108]. Previously, extensive investigation into the use of EOs as food preservatives in the food industry has been conducted [25]. EOs have been observed to be effective against both pathogenic and non-pathogenic organisms [109][110][111][112][113][114]. They have been observed to be effective against gram-positive (Staphylococcus aureus and Listeria monocytogenes) and gram-negative bacteria (Escherichia coli and Salmonella enteritidis) [115]. Several studies conducted on food materials reported bactericidal or bacteriostatic activity of EOs against Salmonella enterica, Escherichia coli O157:H7, Staphylococcus aureus, Listeria monocytogenes, Lactobacillus plantarum, Saccharomyces cerevisiae, and Candida albicans strains [113][114][116][117]. Three citrus fruit EOs, mandarin (Citrus reticulata), wilking (Citrus reticulata cv. Wilking blanco), and clementine (Citrus clementina), were examined for their antimicrobial potential. Mandarin EO demonstrated the best bacterial growth inhibition, followed by clementine EOs [7]. The oils were significantly effective against Candida albicans, Escherichia coli, Listeria innocua, methicillin-resistant S. aureus, and Staphylococcus aureus [7]. Clove and melaleuca EO showed an inhibitory effect on S. aureus, E. coli, and C. albicans [118].
Phenolic EOs such as carvacrol, thymol, and others consist of hydrophobic ends that interact with different areas of microbial cells (e.g., cell wall and cytoplasmic membrane) and break the membrane structure, thereby causing a loss of cellular constituents and resulting in cell death [119].
5. Other Beneficial Effects of EOs
Mixtures containing the EOs of sage, oregano [120], J. oxycedrus subsp. oxycedrus, and J. phoenicea [121], have been reported to encourage wound healing. Similarly, a combination of sesame and lemon EOs accelerated the healing process of wounds in male Albino Wistar rats [122]. EOs have also been observed to cause subtle ST-segment elevation and decrease the levels of malondialdehyde and myeloperoxidase in rat models that had been induced with a myocardial infarction [123]. Malondialdehyde and myeloperoxidase are the markers that indicate necrosis of the myocardium; thus, the ability of EOs to reduce the levels of these enzymes points towards better heart health. Previous studies have also observed the positive effects of EOs on hepatic function. EOs of rosemary (Rosmarinus officinalis L.) and fennel (Foeniculum vulgare) have been associated with decreasing the levels of alanine aminotransferase (ALT) and alkaline phosphatase (ALP) [124][125]. These biomarkers indicate liver function insufficiency/damage. Acetominophen toxicity accounts for 50% of overdose-related acute liver failure and approximately 20% of liver transplant cases in the United States. EOs have also aided in treating such cases. EO extracted from Nepeta cataria L. increased the mRNA expression of uridine diphosphate glucuronosyltransferases and sulfotransferases [126]. These enzymes aid in metabolizing acetominophen into a nontoxic form that can be excreted through urine.
The beneficial effect of EOs on brain health has also been observed. Rosemary EO enhanced memory, concentration, alertness, and locomotor activity, besides stimulating the cerebral cortex and causing mood relaxation [127]. EOs from sage, rosemary, and Stachys inflata Benth inhibited acetylcholine esterase (AChE) even better than the drug donepezil [59][128]. This is a significant observation, especially for Alzheimer’s disease patients, as elevated activity of the enzyme (AChE) has been associated with the disease. The inhibitory potential of the AChE enzyme has been associated with the di- and triterpenes present in the EOs [128].
EOs have also been shown to exert beneficial effects on asthmatic patients; eucalyptol extracted from eucalyptus oil reduced the dependence on oral steroids by such patients [129]. Their usage has also been observed to reduce symptoms associated with primary dysmenorrhea [130] and labor pain [131]. Other benefits recorded include the ability to inhibit tyrosinase (anti-hyperpigmentation effect) [59], elastase (anti-wrinkle effect) [132], anti-viral [133], and anti-fungal activity [132][133]. Overall, EOs may positively change the course of many health issues, including obesity, dyslipidemia, hypertension, diabetes, and infections, along with improvements in brain, heart, and liver health (Figure 2).
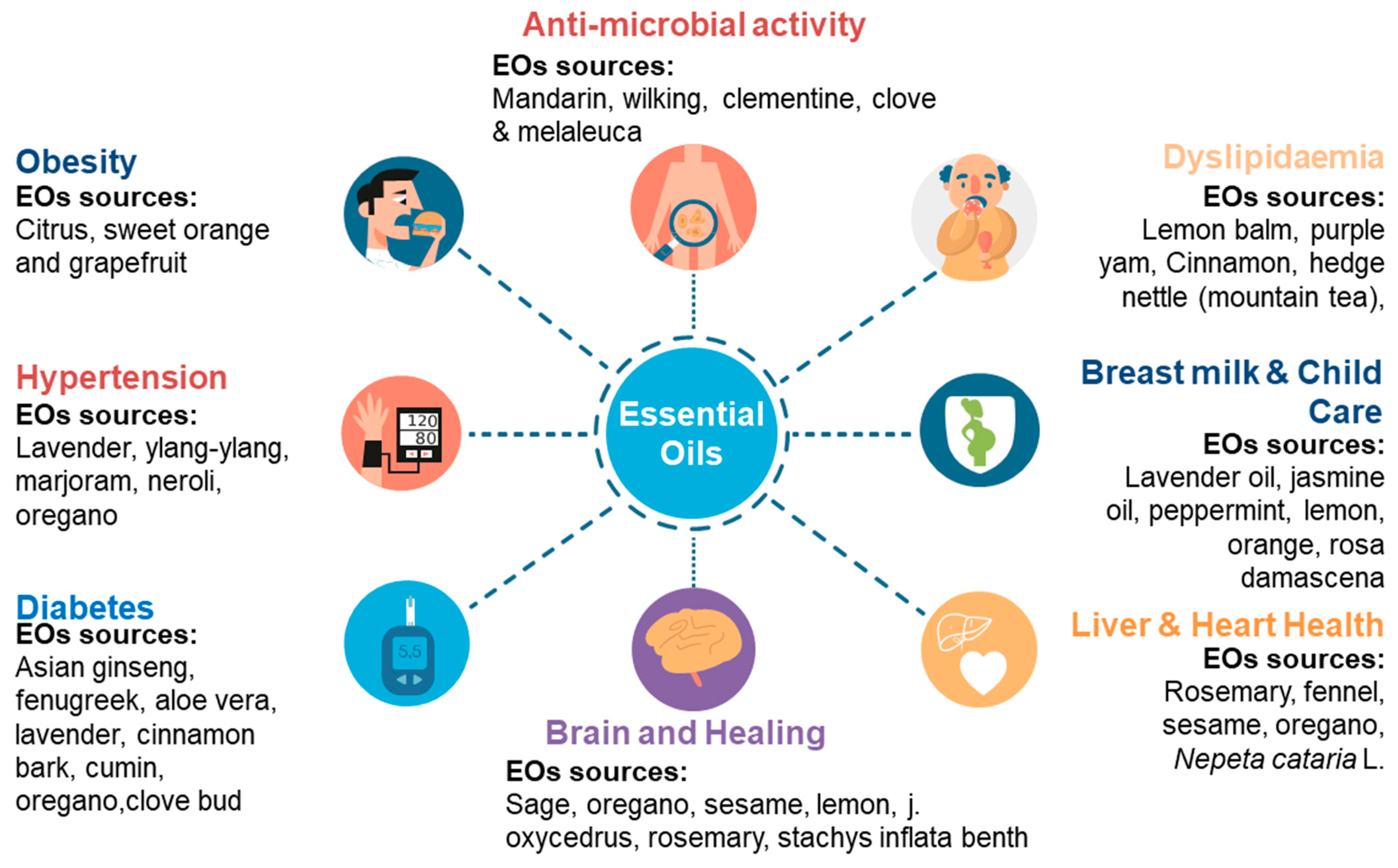
Figure 2. Sources of EOs and their respective health-promoting properties.
References
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules 2017, 22, 70.
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crops Prod. 2014, 62, 250–264.
- Spisni, E.; Petrocelli, G.; Imbesi, V.; Spigarelli, R.; Azzinnari, D.; Donati Sarti, M.; Campieri, M.; Valerii, M.C. Antioxidant, anti-inflammatory, and microbial-modulating activities of essential oils: Implications in colonic pathophysiology. Int. J. Mol. Sci. 2020, 21, 4152.
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175.
- De Lavor, É.M.; Fernandes, A.W.C.; de Andrade Teles, R.B.; Leal, A.E.B.P.; de Oliveira Júnior, R.G.; Gama e Silva, M.; De Oliveira, A.P.; Silva, J.C.; de Moura Fontes Araujo, M.T.; Coutinho, H.D.M. Essential oils and their major compounds in the treatment of chronic inflammation: A review of antioxidant potential in preclinical studies and molecular mechanisms. Oxidative Med. Cell. Longev. 2018, 2018, 6468593.
- Loizzo, M.R.; Tundis, R.; Bonesi, M.; Sanzo, G.D.; Verardi, A.; Lopresto, C.G.; Pugliese, A.; Menichini, F.; Balducchi, R.; Calabrò, V. Chemical profile and antioxidant properties of extracts and essential oils from Citrus× limon (L.) burm. Cv. Femminello comune. Chem. Biodivers. 2016, 13, 571–581.
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboody, M.S.; Vijayakumar, R.; Baskaran, D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci. Technol. 2019, 2, 49–55.
- Lombardo, G.E.; Cirmi, S.; Musumeci, L.; Pergolizzi, S.; Maugeri, A.; Russo, C.; Mannucci, C.; Calapai, G.; Navarra, M. Mechanisms underlying the anti-inflammatory activity of bergamot essential oil and its antinociceptive effects. Plants 2020, 9, 704.
- Afroz, M.; Zihad, S.N.K.; Uddin, S.J.; Rouf, R.; Rahman, M.S.; Islam, M.T.; Khan, I.N.; Ali, E.S.; Aziz, S.; Shilpi, J.A. A systematic review on antioxidant and antiinflammatory activity of Sesame (Sesamum indicum L.) oil and further confirmation of antiinflammatory activity by chemical profiling and molecular docking. Phytother. Res. 2019, 33, 2585–2608.
- Rosalina, R.; Weerapreeyakul, N. An insight into sesamolin: Physicochemical properties, pharmacological activities, and future research prospects. Molecules 2021, 26, 5849.
- Sankar, D.; Rao, M.R.; Sambandam, G.; Pugalendi, K. Effect of sesame oil on diuretics or ß-blockers in the modulation of blood pressure, anthropometry, lipid profile, and redox status. Yale J. Biol. Med. 2006, 79, 19.
- Jabbari, N.; Eftekhari, Z.; Roodbari, N.H.; Parivar, K. Evaluation of Encapsulated Eugenol by Chitosan Nanoparticles on the aggressive model of rheumatoid arthritis. Int. Immunopharmacol. 2020, 85, 106554.
- Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Vazquez-Olivo, G.; Heredia, J.B. Essential oils of oregano: Biological activity beyond their antimicrobial properties. Molecules 2017, 22, 989.
- Jan, S.; Rashid, M.; Abd_Allah, E.F.; Ahmad, P. Biological efficacy of essential oils and plant extracts of cultivated and wild ecotypes of Origanum vulgare L. BioMed Res. Int. 2020, 2020, 8751718.
- Boudries, H.; Loupassaki, S.; Ladjal Ettoumi, Y.; Souagui, S.; Bachir Bey, M.; Nabet, N.; Chikhoune, A.; Madani, K.; Chibane, M. Chemical profile, antimicrobial and antioxidant activities of Citrus reticulata and Citrus clementina (L.) essential oils. Int. Food Res. J. 2017, 24, 1782.
- Sharma, M.; Grewal, K.; Jandrotia, R.; Batish, D.R.; Singh, H.P.; Kohli, R.K. Essential oils as anticancer agents: Potential role in malignancies, drug delivery mechanisms, and immune system enhancement. Biomed. Pharmacother. 2022, 146, 112514.
- Yang, C.; Chen, H.; Chen, H.; Zhong, B.; Luo, X.; Chun, J. Antioxidant and anticancer activities of essential oil from Gannan navel orange peel. Molecules 2017, 22, 1391.
- Thomas, V.; Giles, D.; PM Basavarajaswamy, G.; Kumar Das, A.; Patel, A. Coumarin derivatives as anti-inflammatory and anticancer agents. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2017, 17, 415–423.
- Sylvestre, M.; Pichette, A.; Longtin, A.; Nagau, F.; Legault, J. Essential oil analysis and anticancer activity of leaf essential oil of Croton flavens L. from Guadeloupe. J. Ethnopharmacol. 2006, 103, 99–102.
- Feng, Y.; Deng, L.; Guo, H.; Zhao, Y.; Peng, F.; Wang, G.; Yu, C. The anti-colon cancer effects of essential oil of Curcuma phaeocaulis through tumour vessel normalisation. Front. Oncol. 2021, 4459, 728464.
- Perumalsamy, H.; Shanmugam, R.; Kim, J.-R.; Anandapadmanaban, G.; Huq, M.; Dua, K.; Chellappan, D.K.; Yoon, T.H.; Balusamy, S.R. Nanoemulsion and Encapsulation Strategy of Hydrophobic Oregano Essential Oil Increased Human Prostate Cancer Cell Death via Apoptosis by Attenuating Lipid Metabolism. Bioinorg. Chem. Appl. 2022, 2022, 9569226.
- Iriti, M.; Colnaghi, G.; Chemat, F.; Smadja, J.; Faoro, F.; Visinoni, F.A. Histo-cytochemistry and scanning electron microscopy of lavender glandular trichomes following conventional and microwave-assisted hydrodistillation of essential oils: A comparative study. Flavour Fragr. J. 2006, 21, 704–712.
- Fornari, T.; Vicente, G.; Vázquez, E.; García-Risco, M.R.; Reglero, G. Isolation of essential oil from different plants and herbs by supercritical fluid extraction. J. Chromatogr. A 2012, 1250, 34–48.
- Oliveira, F.d.A.; Andrade, L.N.; De Sousa, É.B.V.; De Sousa, D.P. Anti-ulcer activity of essential oil constituents. Molecules 2014, 19, 5717–5747.
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Medicines 2016, 3, 25.
- Gandhi, G.R.; Vasconcelos, A.B.S.; Haran, G.H.; da Silva Calisto, V.K.; Jothi, G.; Quintans, J.d.S.S.; Cuevas, L.E.; Narain, N.; Júnior, L.J.Q.; Cipolotti, R. Essential oils and its bioactive compounds modulating cytokines: A systematic review on anti-asthmatic and immunomodulatory properties. Phytomedicine 2020, 73, 152854.
- Sharma, P.R.; Mondhe, D.M.; Muthiah, S.; Pal, H.C.; Shahi, A.K.; Saxena, A.K.; Qazi, G.N. Anticancer activity of an essential oil from Cymbopogon flexuosus. Chem. -Biol. Interact. 2009, 179, 160–168.
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A consensus statement from the international diabetes federation. Diabet. Med. 2006, 23, 469–480.
- Cornier, M.-A.; Dabelea, D.; Hernandez, T.L.; Lindstrom, R.C.; Steig, A.J.; Stob, N.R.; Van Pelt, R.E.; Wang, H.; Eckel, R.H. The metabolic syndrome. Endocr. Rev. 2008, 29, 777–822.
- Sharma, P. Inflammation and the metabolic syndrome. Indian J. Clin. Biochem. 2011, 26, 317–318.
- Awadallah, S.; Hasan, H.; Attlee, A.; Raigangar, V.; Unnikannan, H.; Madkour, M.; Abraham, M.S.; Rashid, L.M. Waist circumference is a major determinant of oxidative stress in subjects with and without metabolic syndrome. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 2541–2547.
- Pandey, A.; Tripathi, P.; Pandey, R.; Srivatava, R.; Goswami, S. Alternative therapies useful in the management of diabetes: A systematic review. J. Pharm. Bioallied Sci. 2011, 3, 504.
- Rashed, A.A.; Mohd Nawi, M.N.; Sulaiman, K. Assessment of essential oil as a potential anti-obesity agent: A narrative review. J. EssEntial Oil Res. 2017, 29, 1–10.
- Li, D.; Wu, H.; Dou, H. Weight loss effect of sweet orange essential oil microcapsules on obese SD rats induced by high-fat diet. Biosci. Biotechnol. Biochem. 2019, 83, 923–932.
- Suryawanshi, J.A.S. An overview of Citrus aurantium used in treatment of various diseases. Afr. J. Plant Sci. 2011, 5, 390–395.
- Niijima, A.; Nagai, K. Effect of olfactory stimulation with flavor of grapefruit oil and lemon oil on the activity of sympathetic branch in the white adipose tissue of the epididymis. Exp. Biol. Med. 2003, 228, 1190–1192.
- Rochlani, Y.; Pothineni, N.V.; Kovelamudi, S.; Mehta, J.L. Metabolic syndrome: Pathophysiology, management, and modulation by natural compounds. Ther. Adv. Cardiovasc. Dis. 2017, 11, 215–225.
- Heghes, S.C.; Filip, L.; Vostinaru, O.; Mogosan, C.; Miere, D.; Iuga, C.A.; Moldovan, M. Essential oil-bearing plants from Balkan Peninsula: Promising sources for new drug candidates for the prevention and treatment of diabetes mellitus and dyslipidemia. Front. Pharmacol. 2020, 11, 989.
- Mowat, A.; Macsween, R.; Percy-Hobbs, L.; Foulis, A. Liver, biliary tract and pancreas. In Muir’s Textbook of Pathology, 13th ed.; MacSween, R., Whaley, K., Eds.; Arnold: London, UK, 1993; pp. 674–741.
- Rountree, R. Herb, drugs and blood sugar. Herbs Health 2001, 26.
- Sebai, H.; Selmi, S.; Rtibi, K.; Souli, A.; Gharbi, N.; Sakly, M. Lavender (Lavandula stoechas L.) essential oils attenuate hyperglycemia and protect against oxidative stress in alloxan-induced diabetic rats. Lipids Health Dis. 2013, 12, 1–9.
- Talpur, N.; Echard, B.; Ingram, C.; Bagchi, D.; Preuss, H. Effects of a novel formulation of essential oils on glucose–insulin metabolism in diabetic and hypertensive rats: A pilot study. Diabetes Obes. Metab. 2005, 7, 193–199.
- Cao, H.; Graves, D.J.; Anderson, R.A. Cinnamon extract regulates glucose transporter and insulin-signaling gene expression in mouse adipocytes. Phytomedicine 2010, 17, 1027–1032.
- Oboh, G.; Akinbola, I.A.; Ademosun, A.O.; Sanni, D.M.; Odubanjo, O.V.; Olasehinde, T.A.; Oyeleye, S.I. Essential oil from clove bud (Eugenia aromatica Kuntze) inhibit key enzymes relevant to the management of type-2 diabetes and some pro-oxidant induced lipid peroxidation in rats pancreas in vitro. J. Oleo Sci. 2015, 64, 775–782.
- Schirmer, M.A.; Phinney, S.D. γ-Linolenate reduces weight regain in formerly obese humans. J. Nutr. 2007, 137, 1430–1435.
- Gupta, R.; Guptha, S. Strategies for initial management of hypertension. Indian J. Med. Res. 2010, 132, 531.
- Kim, I.-H.; Kim, C.; Seong, K.; Hur, M.-H.; Lim, H.M.; Lee, M.S. Essential oil inhalation on blood pressure and salivary cortisol levels in prehypertensive and hypertensive subjects. Evid. -Based Complement. Altern. Med. 2012, 2012, 984203.
- Hur, M.-H.; Oh, H.; Lee, M.S.; Kim, C.; Choi, A.-N.; Shin, G.-R. Effects of aromatherapy massage on blood pressure and lipid profile in Korean climacteric women. Int. J. Neurosci. 2007, 117, 1281–1287.
- Aydin, Y.; Kutlay, Ö.; Ari, S.; Duman, S.; Uzuner, K.; Aydin, S. Hypotensive effects of carvacrol on the blood pressure of normotensive rats. Planta Med. 2007, 73, 1365–1371.
- Earley, S.; Gonzales, A.L.; Garcia, Z.I. A dietary agonist of transient receptor potential cation channel V3 elicits endothelium-dependent vasodilation. Mol. Pharmacol. 2010, 77, 612–620.
- Dantas, B.P.V.; Alves, Q.L.; de Assis, K.S.; Ribeiro, T.P.; de Almeida, M.M.; de Vasconcelos, A.P.; de Araújo, D.A.M.; de Andrade Braga, V.; de Medeiros, I.A.; Alencar, J.L. Participation of the TRP channel in the cardiovascular effects induced by carvacrol in normotensive rat. Vasc. Pharmacol. 2015, 67, 48–58.
- Almanaitytė, M.; Jurevičius, J.; Mačianskienė, R. Effect of carvacrol, TRP channels modulator, on cardiac electrical activity. BioMed Res. Int. 2020, 2020, 6456805.
- Freeman, M.; Ayers, C.; Peterson, C.; Kansagara, D. Aromatherapy and Essential Oils: A Map of the Evidence; Department of Veterans Affairs (US): Washington, DC, USA, 2019. Available online: https://pubmed.ncbi.nlm.nih.gov/31851445/ (accessed on 14 November 2020).
- Li, T.; Teng, H.; An, F.; Huang, Q.; Chen, L.; Song, H. The beneficial effects of purple yam (Dioscorea alata L.) resistant starch on hyperlipidemia in high-fat-fed hamsters. Food Funct. 2019, 10, 2642–2650.
- Ziegenfuss, T.N.; Hofheins, J.E.; Mendel, R.W.; Landis, J.; Anderson, R.A. Effects of a water-soluble cinnamon extract on body composition and features of the metabolic syndrome in pre-diabetic men and women. J. Int. Soc. Sport. Nutr. 2006, 3, 1–9.
- Couturier, K.; Batandier, C.; Awada, M.; Hininger-Favier, I.; Canini, F.; Anderson, R.; Leverve, X.; Roussel, A.-M. Cinnamon improves insulin sensitivity and alters the body composition in an animal model of the metabolic syndrome. Arch. Biochem. Biophys. 2010, 501, 158–161.
- Shen, Y.; Jia, L.-N.; Honma, N.; Hosono, T.; Ariga, T.; Seki, T. Beneficial effects of cinnamon on the metabolic syndrome, inflammation, and pain, and mechanisms underlying these effects—A review. J. Tradit. Complement. Med. 2012, 2, 27–32.
- Goren, A.C. Use of Stachys species (Mountain Tea) as herbal tea and food. Rec. Nat. Prod. 2014, 8, 71.
- Bahadori, M.B.; Maggi, F.; Zengin, G.; Asghari, B.; Eskandani, M. Essential oils of hedgenettles (Stachys inflata, S. lavandulifolia, and S. byzantina) have antioxidant, anti-Alzheimer, antidiabetic, and anti-obesity potential: A comparative study. Ind. Crops Prod. 2020, 145, 112089.
- Weidner, C.; Wowro, S.J.; Freiwald, A.; Kodelja, V.; Abdel-Aziz, H.; Kelber, O.; Sauer, S. Lemon balm extract causes potent antihyperglycemic and antihyperlipidemic effects in insulin-resistant obese mice. Mol. Nutr. Food Res. 2014, 58, 903–907.
- Cappello, G.; Spezzaferro, M.; Grossi, L.; Manzoli, L.; Marzio, L. Peppermint oil (Mintoil) in the treatment of irritable bowel syndrome: A prospective double blind placebo-controlled randomized trial. Dig. Liver Dis. 2007, 39, 530–536.
- Singh, R.; Shushni, M.A.M.; Belkheir, A. Antibacterial and Antioxidant Activities of Mentha Piperita L. Arab. J. Chem. 2015, 8, 322–328.
- Jabri, M.A.; Sakly, M.; Marzouki, L.; Sebai, H. Chamomile (Matricaria recutita L.) decoction extract inhibits in vitro intestinal glucose absorption and attenuates high fat diet-induced lipotoxicity and oxidative stress. Biomed. Pharmacother. 2017, 87, 153–159.
- Fotakis, C.; Tsigrimani, D.; Tsiaka, T.; Lantzouraki, D.Z.; Strati, I.F.; Makris, C.; Tagkouli, D.; Proestos, C.; Sinanoglou, V.J.; Zoumpoulakis, P. Metabolic and antioxidant profiles of herbal infusions and decoctions. Food Chem. 2016, 211, 963–971.
- Rafraf, M.; Zemestani, M.; Asghari-Jafarabadi, M. Effectiveness of chamomile tea on glycemic control and serum lipid profile in patients with type 2 diabetes. J. Endocrinol. Investig. 2015, 38, 163–170.
- Bayliak, M.M.; Dmytriv, T.R.; Melnychuk, A.V.; Strilets, N.V.; Storey, K.B.; Lushchak, V.I. Chamomile as a potential remedy for obesity and metabolic syndrome. EXCLI J. 2021, 20, 1261–1286.
- Hieu, T.H.; Dibas, M.; Surya, D.K.A.; Sherif, N.A.; Hashmi, M.U.; Mahmoud, M.; Trang, N.T.T.; Abdullah, L.; Nghia, T.L.B.; Hirayama, K.; et al. Therapeutic efficacy and safety of chamomile for state anxiety, generalized anxiety disorder, insomnia, and sleep quality: A systematic review and meta-analysis of randomized trials and quasi-randomized trials. Phytother. Res. 2019, 33, 1604–1615.
- Zemestani, M.; Rafraf, M.; Asghari-Jafarabadi, M. Chamomile tea improves glycemic indices and antioxidants status in patients with type 2 diabetes mellitus. Nutrition 2016, 32, 66–72.
- Wu, L.; Guo, T.; Deng, R.; Liu, L.; Yu, Y. Apigenin ameliorates insulin resistance and lipid accumulation by endoplasmic reticulum stress and SREBP-1c/SREBP-2 pathway in palmitate-induced HepG2 Cells and highfat diet-fed mice. J. Pharmacol. Exp. Ther. 2021, 377, 146–156.
- Al-Okbi, S.Y.; Hussein, A.M.S.; Elbakry, H.F.H.; Fouda, K.A.; Mahmoud, K.F.; Hassan, M.E. Health Benefits of Fennel, Rosemary Volatile Oils and their Nano-Forms in Dyslipidemic Rat Model. Pak. J. Biol. Sci. 2018, 21, 348–358.
- Hamden, K.; Keskes, H.; Belhaj, S.; Mnafgui, K.; Feki, A.; Allouche, N. Inhibitory potential of omega-3 fatty and fenugreek essential oil on key enzymes of carbohydrate-digestion and hypertension in diabetes rats. Lipids Health Dis. 2011, 10, 226.
- Lai, Y.S.; Chen, W.C.; Ho, C.T.; Lu, K.H.; Lin, S.H.; Tseng, H.C.; Lin, S.Y.; Sheen, L.Y. Garlic essential oil protects against obesity-triggered nonalcoholic fatty liver disease through modulation of lipid metabolism and oxidative stress. J. Agric. Food Chem. 2014, 62, 5897–5906.
- Ebada, M.E. Essential oils of green cumin and chamomile partially protect against acute acetaminophen hepatotoxicity in rats. An. Da Acad. Bras. De Ciências 2018, 90, 2347–2358.
- Rivaz, M.; Rahpeima, M.; Khademian, Z.; Dabbaghmanesh, M.H. The effects of aromatherapy massage with lavender essential oil on neuropathic pain and quality of life in diabetic patients: A randomized clinical trial. Complement. Ther. Clin. Pract. 2021, 44, 101430.
- Nasiri, L.Z.; Hajimonfarednejad, M.; Riasatian, M.; Abolhassanzadeh, Z.; Iraji, A.; Vojoud, M.; Heydari, M.; Shams, M. Efficacy of inhaled Lavandula angustifolia Mill. Essential oil on sleep quality, quality of life and metabolic control in patients with diabetes mellitus type II and insomnia. J. Ethnopharmacol. 2020, 251, 112560.
- Diaz, A.; Luque, L.; Badar, Z.; Kornic, S.; Danon, M. Prepubertal gynecomastia and chronic lavender exposure: Report of three cases. J. Pediatr. Endocrinol. Metab. 2016, 29, 103–107.
- Henley, D.V.; Lipson, N.; Korach, K.S.; Bloch, C.A. Prepubertal Gynecomastia Linked to Lavender and Tea Tree Oils. N. Engl. J. Med. 2007, 356, 479–485.
- Ramsey, J.T.; Li, Y.; Arao, Y.; Naidu, A.; Coons, L.A.; Diaz, A.; Korach, K.S. Lavender Products Associated with Premature Thelarche and Prepubertal Gynecomastia: Case Reports and Endocrine-Disrupting Chemical Activities. J. Clin. Endocrinol. Metab. 2019, 104, 5393–5405.
- Kramer, M.S.; Chalmers, B.; Hodnett, E.D.; Sevkovskaya, Z.; Dzikovich, I.; Shapiro, S.; Collet, J.-P.; Vanilovich, I.; Mezen, I.; Ducruet, T. Promotion of Breastfeeding Intervention Trial (PROBIT): A randomized trial in the Republic of Belarus. JAMA 2001, 285, 413–420.
- Gartner, L.M.; Morton, J.; Lawrence, R.A.; Naylor, A.J.; O’Hare, D.; Schanler, R.J.; Eidelman, A.I. Breastfeeding and the use of human milk. Pediatrics 2005, 115, 496–506.
- Zanardo, V.; Savona, V.; Cavallin, F.; D’Antona, D.; Giustardi, A.; Trevisanuto, D. Impaired lactation performance following elective delivery at term: Role of maternal levels of cortisol and prolactin. J. Matern. -Fetal Neonatal Med. 2012, 25, 1595–1598.
- Hector, D.; King, L.; Webb, K.; Heywood, P. Factors affecting breastfeeding practices. Applying a conceptual framework. New South Wales Public Health Bull. 2005, 16, 52–55.
- Zuppa, A.A.; Sindico, P.; Orchi, C.; Carducci, C.; Cardiello, V.; Catenazzi, P.; Romagnoli, C. Safety and efficacy of galactogogues: Substances that induce, maintain and increase breast milk production. J. Pharm. Pharm. Sci. 2010, 13, 162–174.
- Nice, F.J. Common herbs and foods used as galactogogues. ICAN: Infant Child Adolesc. Nutr. 2011, 3, 129–132.
- Goksugur, S.B.; Karatas, Z. Breastfeeding and galactogogues agents. Acta Med. Anatolia 2014, 2, 113–118.
- Matsumoto, T.; Asakura, H.; Hayashi, T. Does lavender aromatherapy alleviate premenstrual emotional symptoms? A randomized crossover trial. BioPsychoSocial Med. 2013, 7, 1–8.
- Hossein Koulivand, P.; Khaleghi Ghadiri, M.; Gorji, A. Lavender and the Nervous System. Evid Based Complement. Alternat. Med. 2013, 2013, 681304.
- Agustie, P.R.; Hadisaputro, S.; Runjati, R.; Soejoenoes, A.; Mashudi, I.D.; Widyawati, M.N. Effect of oxytocin massage using lavender essential oil on prolactin level and breast milk production in primiparous mothers after caesarean delivery. Belitung Nurs. J. 2017, 3, 337–344.
- Asazawa, K.; Kato, Y.; Yamaguchi, A.; Inoue, A. The effect of aromatherapy treatment on fatigue and relaxation for mothers during the early puerperal period in Japan: A pilot study. Int. J. Community Based Nurs. Midwifery 2017, 5, 365.
- Susanti, K.D.M.B.E.; Politeknik, J.K.; Politeknik, J.K. The Effect of Oxytocin Massage Method Using Lavender Essential Oils on The Smooth Production of Breast Milk at Mother Postpartum in Rejang Lebong Regency. In 1st International Conference on Inter-Professional Health Collaboration (ICIHC 2018); Atlantis Press: Amsterdam, The Netherlands, 2019; pp. 91–94. Available online: https://www.atlantis-press.com/proceedings/icihc-18/55916774 (accessed on 14 November 2020).
- Widyawati, M.N.; Hadisaputro, S.; Anies, A.; Soejoenoes, A. Effect of massage and aromatherapy on stress and prolactin level among primiparous puerperal mothers in Semarang, Central Java, Indonesia. Belitung Nurs. J. 2016, 2, 48–57.
- Sabharwal, S.; Sudan, S.; Ranjan, V. Jasminum sambac linn (motia): A review. Int. J. Pharm. Res. Bio-Sci. 2013, 2, 108–130.
- Akbari, S.A.A.; Alamolhoda, S.H.; Baghban, A.A.; Mirabi, P. Effects of menthol essence and breast milk on the improvement of nipple fissures in breastfeeding women. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2014, 19, 629.
- Shuo-Shin, T.; Hsiu-Hung, W.; Fan-Hao, C. The effects of aromatherapy on postpartum women: A systematic review. J. Nurs. Res. 2020, 28, e96.
- Safajou, F.; Shahnazi, M.; Nazemiyeh, H. The effect of lemon inhalation aromatherapy on nausea and vomiting of pregnancy: A double-blinded, randomized, controlled clinical trial. Iran. Red Crescent Med. J. 2014, 16, e14360.
- Zahra, A.; Leila, M.S. Lavender aromatherapy massages in reducing labor pain and duration of labor: A randomized controlled trial. Afr. J. Pharm. Pharmacol. 2013, 7, 426–430.
- Olapour, A.; Behaeen, K.; Akhondzadeh, R.; Soltani, F.; al Sadat Razavi, F.; Bekhradi, R. The effect of inhalation of aromatherapy blend containing lavender essential oil on cesarean postoperative pain. Anesthesiol. Pain Med. 2013, 3, 203.
- Çetinkaya, B.; Başbakkal, Z. The effectiveness of aromatherapy massage using lavender oil as a treatment for infantile colic. Int. J. Nurs. Pract. 2012, 18, 164–169.
- Field, T.; Field, T.; Cullen, C.; Largie, S.; Diego, M.; Schanberg, S.; Kuhn, C. Lavender bath oil reduces stress and crying and enhances sleep in very young infants. Early Hum. Dev. 2008, 84, 399–401.
- Ghaderi, F.; Solhjou, N. The effects of lavender aromatherapy on stress and pain perception in children during dental treatment: A randomized clinical trial. Complement. Ther. Clin. Pract. 2020, 40, 101182.
- Sezavar, M.; Ahmadi, R.; Shojaei, H.; Jafari, M.; Hashemi, I.; Attaei Nakhaie, A.R.; Nasibeh, R.; Zolala, S.; Ashrafinia, F.; Khojastehfard, Z. The Effect of Lavender Oil for Relief Painful Producer in Children and Infants: A Systematic Review. Int. J. Pediatr. 2020, 8, 11177–11185.
- Motaghi, M.; Borji, M.; Moradi, M. The effect of orange essence aromatherapy on anxiety in school-age children with diabetes. Biomed. Pharmacol. J. 2017, 10, 159–164.
- Keyhanmehr, A.S.; Movahhed, M.; Sahranavard, S.; Gachkar, L.; Hamdieh, M.; Afsharpaiman, S.; Nikfarjad, H. The effect of aromatherapy with Rosa damascena essential oil on sleep quality in children. Res. J. Pharmacogn. 2018, 5, 41–46.
- O’Flaherty, L.-A.; van Dijk, M.; Albertyn, R.; Millar, A.; Rode, H. Aromatherapy massage seems to enhance relaxation in children with burns: An observational pilot study. Burns 2012, 38, 840–845.
- Zorba, P.; Ozdemir, L. The preliminary effects of massage and inhalation aromatherapy on chemotherapy-induced acute nausea and vomiting: A quasi-randomized controlled pilot trial. Cancer Nurs. 2018, 41, 359–366.
- Kilina, A.; Kolesnikova, M. The efficacy of the application of essential oils for the prevention of acute respiratory diseases in organized groups of children. Vestn. Otorinolaringol. 2011, 5, 51–54.
- World Health Organization. The Future of Food Safety: Transforming Knowledge into Action for People, Economies and the Environment: Technical Summary by FAO and WHO. 2020. Available online: https://apps.who.int/iris/handle/10665/333621 (accessed on 14 November 2020).
- Falleh, H.; Jemaa, M.B.; Saada, M.; Ksouri, R. Essential oils: A promising eco-friendly food preservative. Food Chem. 2020, 330, 127268.
- Kalemba, D.; Kunicka, A. Antibacterial and antifungal properties of essential oils. Curr. Med. Chem. 2003, 10, 813–829.
- Oussalah, M.; Caillet, S.; Saucier, L.; Lacroix, M. Inhibitory effects of selected plant essential oils on the growth of four pathogenic bacteria: E. coli O157: H7, Salmonella typhimurium, Staphylococcus aureus and Listeria monocytogenes. Food Control 2007, 18, 414–420.
- Silva, F.; Ferreira, S.; Duarte, A.; Mendonca, D.I.; Domingues, F.C. Antifungal activity of Coriandrum sativum essential oil, its mode of action against Candida species and potential synergism with amphotericin B. Phytomedicine 2011, 19, 42–47.
- Fitzgerald, D.; Stratford, M.; Gasson, M.; Ueckert, J.; Bos, A.; Narbad, A. Mode of antimicrobial action of vanillin against Escherichia coli, Lactobacillus plantarum and Listeria innocua. J. Appl. Microbiol. 2004, 97, 104–113.
- Osaili, T.M.; Hasan, F.; Dhanasekaran, D.K.; Obaid, R.S.; Al-Nabulsi, A.A.; Ayyash, M.; Karam, L.; Savvaidis, I.N.; Holley, R. Effect of active essential oils added to chicken tawook on the behaviour of Listeria monocytogenes, Salmonella spp. and Escherichia coli O157: H7 during storage. Int. J. Food Microbiol. 2021, 337, 108947.
- Osaili, T.M.; Hasan, F.; Dhanasekaran, D.K.; Obaid, R.S.; Al-Nabulsi, A.A.; Karam, L.; Savvaidis, I.N.; Olaimat, A.N.; Ayyash, M.; Al-Holy, M. Effect of yogurt-based marinade combined with essential oils on the behavior of Listeria monocytogenes, Escherichia coli O157: H7 and Salmonella spp. in camel meat chunks during storage. Int. J. Food Microbiol. 2021, 343, 109106.
- Barbosa, L.N.; Rall, V.L.M.; Fernandes, A.A.H.; Ushimaru, P.I.; da Silva Probst, I.; Fernandes Jr, A. Essential oils against foodborne pathogens and spoilage bacteria in minced meat. Foodborne Pathog. Dis. 2009, 6, 725–728.
- Chorianopoulos, N.; Kalpoutzakis, E.; Aligiannis, N.; Mitaku, S.; Nychas, G.-J.; Haroutounian, S.A. Essential oils of Satureja, Origanum, and Thymus species: Chemical composition and antibacterial activities against foodborne pathogens. J. Agric. Food Chem. 2004, 52, 8261–8267.
- Friedman, M.; Henika, P.R.; Levin, C.E.; Mandrell, R.E. Antibacterial activities of plant essential oils and their components against Escherichia coli O157: H7 and Salmonella enterica in apple juice. J. Agric. Food Chem. 2004, 52, 6042–6048.
- Pereira dos Santos, E.; Nicácio, P.H.M.; Coêlho Barbosa, F.; Nunes da Silva, H.; Andrade, A.L.S.; Lia Fook, M.V.; de Lima Silva, S.M.; Farias Leite, I. Chitosan/essential oils formulations for potential use as wound dressing: Physical and antimicrobial properties. Materials 2019, 12, 2223.
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253.
- Süntar, I.; Akkol, E.K.; Keleş, H.; Oktem, A.; Başer, K.H.C.; Yeşilada, E. A novel wound healing ointment: A formulation of Hypericum perforatum oil and sage and oregano essential oils based on traditional Turkish knowledge. J. Ethnopharmacol. 2011, 134, 89–96.
- Tumen, I.; Süntar, I.; Keleş, H.; Küpeli Akkol, E. A therapeutic approach for wound healing by using essential oils of Cupressus and Juniperus species growing in Turkey. Evid.-Based Complement. Altern. Med. 2012, 2012.
- Valizadeh, A.; Shirzad, M.; Pourmand, M.R.; Farahmandfar, M.; Sereshti, H.; Amani, A. Preparation and comparison of effects of different herbal oil ointments as wound-healing agents. Cells Tissues Organs 2019, 207, 177–186.
- Ziaee, M.; Khorrami, A.; Ebrahimi, M.; Nourafcan, H.; Amiraslanzadeh, M.; Rameshrad, M.; Garjani, M.; Garjani, A. Cardioprotective effects of essential oil of Lavandula angustifolia on isoproterenol-induced acute myocardial infarction in rat. Iran. J. Pharm. Res. IJPR 2015, 14, 279.
- Rašković, A.; Milanović, I.; Pavlović, N.; Ćebović, T.; Vukmirović, S.; Mikov, M. Antioxidant activity of rosemary (Rosmarinus officinalis L.) essential oil and its hepatoprotective potential. BMC Complement. Altern. Med. 2014, 14, 1–9.
- Özbek, H.; Uğraş, S.; Dülger, H.; Bayram, I.; Tuncer, I.; Öztürk, G.; Öztürk, A. Hepatoprotective effect of Foeniculum vulgare essential oil. Fitoterapia 2003, 74, 317–319.
- Tan, J.; Li, J.; Ma, J.; Qiao, F. Hepatoprotective effect of essential oils of Nepeta cataria L. on acetaminophen-induced liver dysfunction. Biosci. Rep. 2019, 39, BSR20190697.
- Ayaz, M.; Sadiq, A.; Junaid, M.; Ullah, F.; Subhan, F.; Ahmed, J. Neuroprotective and anti-aging potentials of essential oils from aromatic and medicinal plants. Front. Aging Neurosci. 2017, 9, 168.
- Rocamora, C.R.; Ramasamy, K.; Lim, S.M.; Majeed, A.B.A.; Agatonovic-Kustrin, S. HPTLC based approach for bioassay-guided evaluation of antidiabetic and neuroprotective effects of eight essential oils of the Lamiaceae family plants. J. Pharm. Biomed. Anal. 2020, 178, 112909.
- Juergens, U.R.; Dethlefsen, U.; Steinkamp, G.; Gillissen, A.; Repges, R.; Vetter, H. Anti-inflammatory activity of 1.8-cineol (eucalyptol) in bronchial asthma: A double-blind placebo-controlled trial. Respir. Med. 2003, 97, 250–256.
- Iravani, M. Clinical effects of Zataria multiflora essential oil on primary dysmenorrhea. J. Med. Plants 2009, 8, 54–60.
- Tabatabaeichehr, M.; Mortazavi, H. The effectiveness of aromatherapy in the management of labor pain and anxiety: A systematic review. Ethiop. J. Health Sci. 2020, 30, 728281.
- Bouyahya, A.; Lagrouh, F.; El Omari, N.; Bourais, I.; El Jemli, M.; Marmouzi, I.; Salhi, N.; Faouzi, M.E.A.; Belmehdi, O.; Dakka, N. Essential oils of Mentha viridis rich phenolic compounds show important antioxidant, antidiabetic, dermatoprotective, antidermatophyte and antibacterial properties. Biocatal. Agric. Biotechnol. 2020, 23, 101471.
- Brochot, A.; Guilbot, A.; Haddioui, L.; Roques, C. Antibacterial, antifungal, and antiviral effects of three essential oil blends. Microbiologyopen 2017, 6, e00459.
More
Information
Subjects:
Others
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
917
Revisions:
2 times
(View History)
Update Date:
07 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





