Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ana Sanches Silva | -- | 3308 | 2023-02-28 13:48:19 | | | |
| 2 | Camila Xu | Meta information modification | 3308 | 2023-03-01 01:36:26 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Barros, S.C.; Silva, A.S.; Torres, D. Antibiotics in Animal Origin Food. Encyclopedia. Available online: https://encyclopedia.pub/entry/41741 (accessed on 13 January 2026).
Barros SC, Silva AS, Torres D. Antibiotics in Animal Origin Food. Encyclopedia. Available at: https://encyclopedia.pub/entry/41741. Accessed January 13, 2026.
Barros, Sílvia Cruz, Ana Sanches Silva, Duarte Torres. "Antibiotics in Animal Origin Food" Encyclopedia, https://encyclopedia.pub/entry/41741 (accessed January 13, 2026).
Barros, S.C., Silva, A.S., & Torres, D. (2023, February 28). Antibiotics in Animal Origin Food. In Encyclopedia. https://encyclopedia.pub/entry/41741
Barros, Sílvia Cruz, et al. "Antibiotics in Animal Origin Food." Encyclopedia. Web. 28 February, 2023.
Copy Citation
Antibiotics are antimicrobial substances formed by or obtained from microorganisms that kill or inhibit the growth of other microorganisms. Antibiotics were first approved for use in livestock by the US Food and Drug Administration (FDA) in 1951. The European Union has forbidden the use of antibiotics as growth promoters since 2006. Its abusive use leads to the presence of antibiotic residues (AR) in foods of animal origin which is associated with antibiotic resistance.
multiresidue method
LC-MS/MS
HRMS
immunoassay
food processing
1. Physico-Chemical Properties of Different Classes of Antibiotics
The different classes of veterinary drugs are directly related to different chemical structures that consequently give rise to different physico-chemical properties (Figure 1).
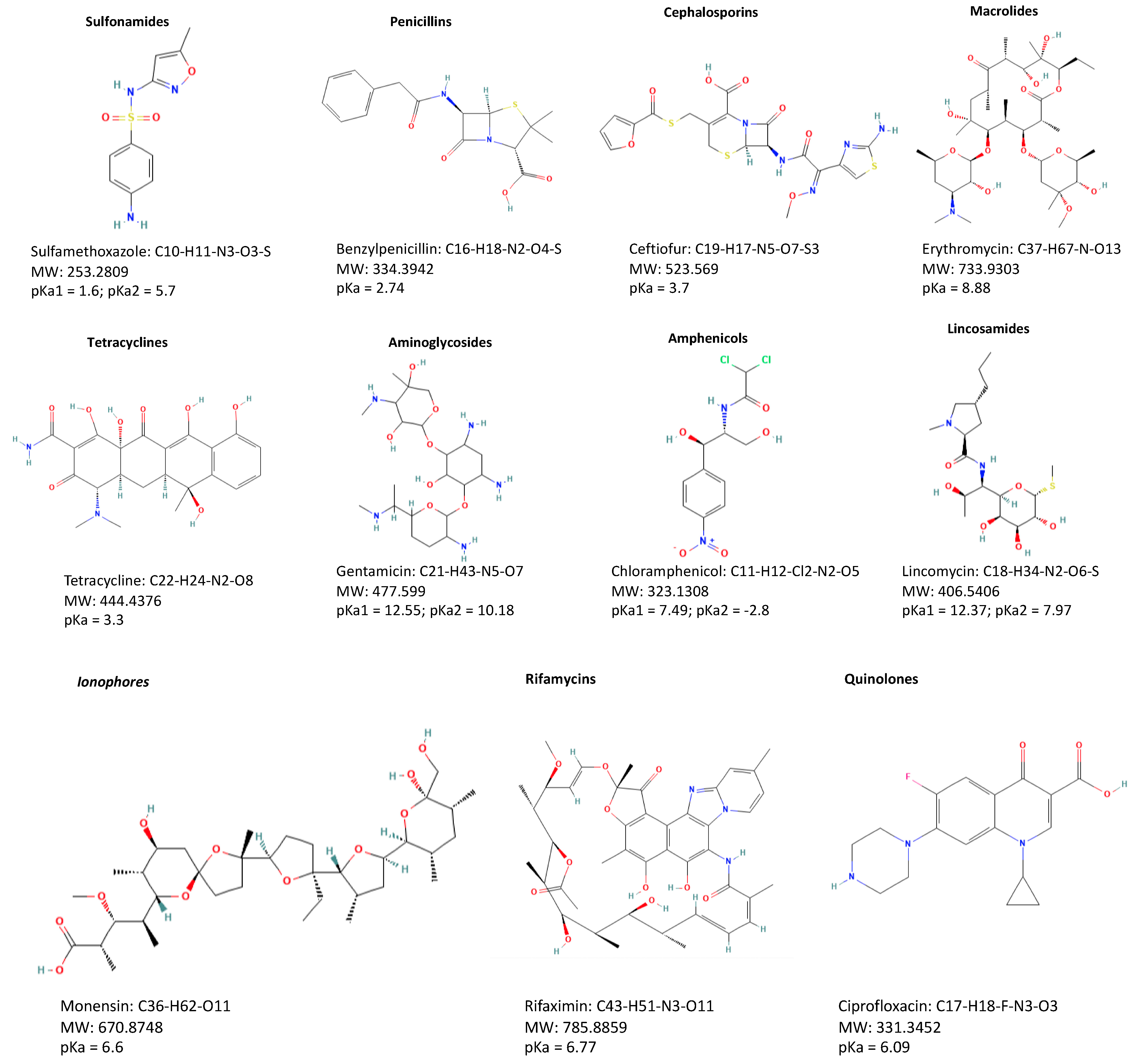
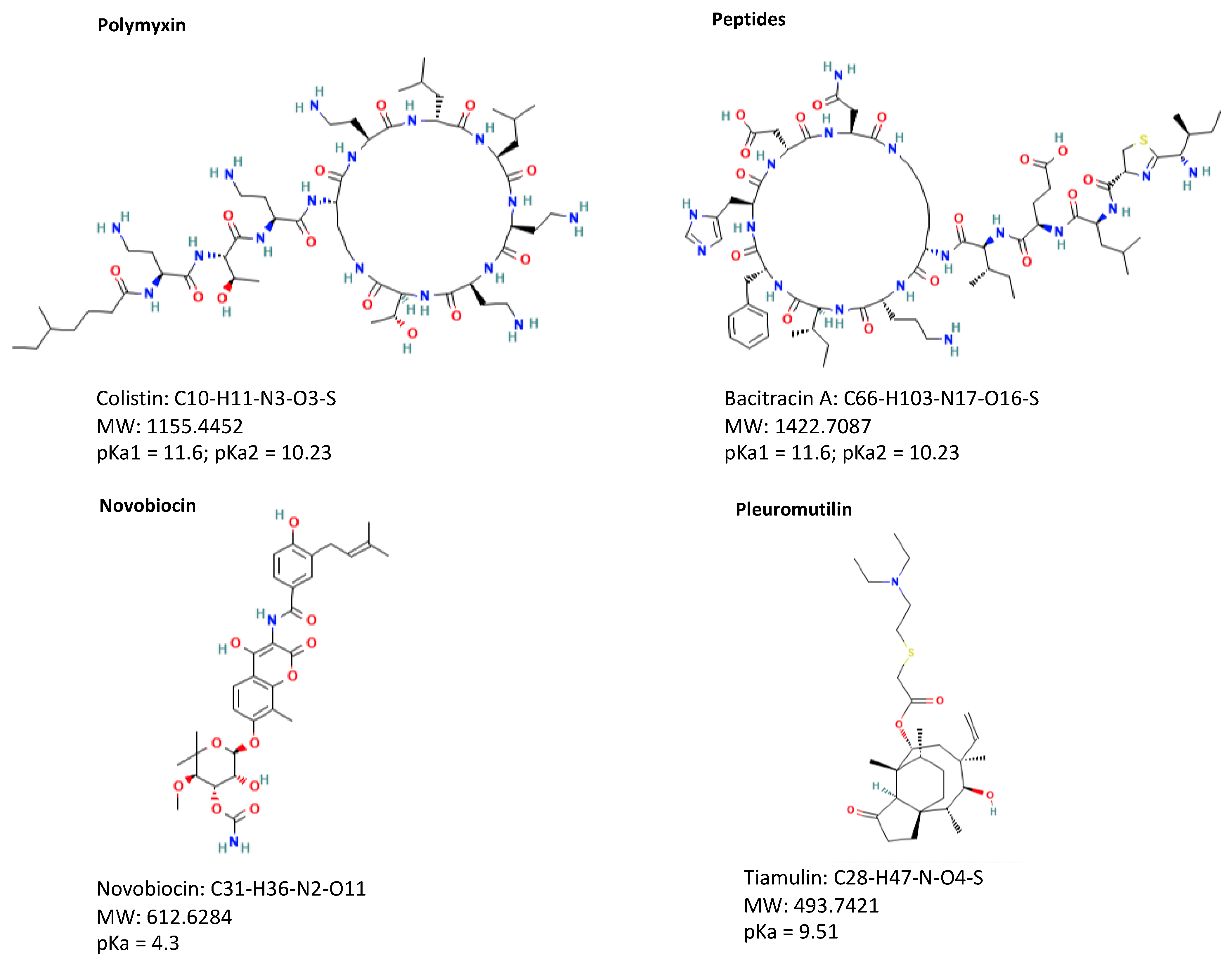
Figure 1. Structures of representative compounds from each class of antibiotics.
Table 1 compiles the classes of veterinary drugs most used in the EU according to the EFSA report on “monitoring of veterinary medicinal product residues and other substances in live animals and animal products” [1].
Table 1. Summary of the Appendix A (List of non-compliant results: targeted sampling) of the technical report of the monitoring data collected in 2018 on the presence of residues of veterinary medicinal products and certain substances in live animals and animal products in the European Union. European Food Safety Authority. Approved: 20 December 2019.
| Class | Substance | Aquaculture | Bovine | Eggs | Honey | Milk | Pig | Poultry | Rabbits | Sheep/goats | Eggs |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sulfonamides | Sulfadimethoxine | √ | √ | √ | √ | √ | √ | ||||
| Sulfadimidine | √ | √ | |||||||||
| Sulfamethoxypyridazine | √ | ||||||||||
| Sulfacetamide | √ | ||||||||||
| Sulfachlorpyrazine | √ | ||||||||||
| Sulfadiazine | √ | √ | √ | √ | √ | ||||||
| Sulfadimidine | √ | ||||||||||
| Sulfamethazin | √ | ||||||||||
| Sulfathiazole | √ | ||||||||||
| Tetracyclines | Oxytetracycline | √ | √ | √ | √ | √ | √ | √ | |||
| Epi-Oxytetracycline | √ | ||||||||||
| Chlortetracyclin | √ | ||||||||||
| Doxycycline | √ | √ | √ | √ | |||||||
| Penicilines | Amoxycillin | √ | √ | √ | |||||||
| Benzylpenicillin (Penicillin G) |
√ | √ | |||||||||
| Ampicillin | √ | ||||||||||
| Cloxacillin | √ | ||||||||||
| Macrolides | Gamithromycin | √ | |||||||||
| Spiramycine | √ | ||||||||||
| Neospiramycin | |||||||||||
| Tilmicosine | √ | √ | √ | √ | √ | ||||||
| Tulathromycin | √ | √ | |||||||||
| Erythromycin | √ | ||||||||||
| Quinolonas, including fluoroquinolones | Enrofloxacin | √ | √ | √ | √ | √ | √ | √ | |||
| Oxolinic Acid | √ | ||||||||||
| Ciprofloxacin | √ | √ | |||||||||
| Oxolinic Acid | √ | ||||||||||
| Danofloxacin | √ | √ | |||||||||
| Sarafloxacin | √ | ||||||||||
| Flumequine | √ | √ | |||||||||
| Aminoglycodides | Dihydrostreptomycin | √ | √ | √ | √ | ||||||
| Neomycin | √ | ||||||||||
| Streptomycin | √ | ||||||||||
| Aminosidin (Paromycin, Paromomycin) | √ | ||||||||||
| Phenicols | Florfenicol | √ | |||||||||
| Lincosamides | Lincomycine | √ | |||||||||
| Diaminopyrimidinas | Trimethoprim | √ | √ | √ | √ |
1.1. Sulfonamides
Sulfonamides (SA) are sulfanilamide derivatives that serve as the structural nucleus for many of the compounds in this class. They have varied pharmacological and bactericidal effects, as well as changing physico-chemical properties, depending on the attachment or replacement of different functional groups in the amido group or replacement in the amino group.
Despite their amphoteric nature, SA typically act like weak organic acids and are much more soluble in alkaline aqueous solutions than in acidic solutions.
The pKa value is 4.8–8.6 [2]. While a group of diaminopyrimidines (aditoprim, methoprim, pyrimethamine trimethoprim, ormetoprim) is commonly used alone, they are ineffective against bacteria, and resistance develops quickly. When separate SA are combined, however, a sequential blockade of microbial enzyme systems occurs, with bactericidal consequences. Trimethoprim/sulfamethoxazole (SMX) (co-trimoxazole), trimethoprim/sulfadiazine (SDZ) (co-trimazine), trimethoprim/sulfadoxine (co-trimoxine), and ormetoprim/sulfadimethoxine are examples of such potentiated SA preparations [3].
1.2. Tetracyclines
Tetracyclines (TC) occur naturally in three forms. Chlortetracycline (CTC), oxytetracycline (OTC), and desmethyltetracycline, some of which are semi-synthetic derived (tetracycline (TC), rolitetracycline, metalocycline, minocycline), doxycycline (DC), lymocycline, etc.). The elimination time can be further divided into short-acting (TC, OTC, CTC), medium-acting (desmethylchlorotetracycline and metcycline) and long-acting (DC and minocycline).
TCs are semi-synthetic products with a bulkier side chain than minocycline and are stable in dry powder form but not in aqueous solution, particularly at higher pH ranges (7–8.5) [2]. They are water-soluble, strongly polar compounds [4]. They are also poor bases, with pKa values ranging from 3.2 to 9.8 and a variety of chromophore groups. The class of tetracyclines has a good chelating capacity because positions C1 and C11 have two distinct ketone groups [5].
1.3. Penicillins
Penicillins, especially the β-lactam ring, are somewhat unstable and sensitive to light, heat, oxidizing and reducing agents, heavy metals and extreme pH. During sample preparation, they typically exhibit very low analyte stability [6].
Penicillins are acid and base sensitive, and their sensitivity varies depending on the nature of the side chain. Furthermore, the presence of nitrogen in the β-lactam induces a reaction with chemical substances, such as nucleophiles like methanol, which is increased by heating and acid catalysis [7]. The best application of β-lactam is in synergy with β-lactamase inhibitors. Cefoperazone with sulbactam or amoxicillin with clavulanic acid are good examples of combinations to increase the effectiveness of these classes of compounds. For instance, the most frequent β-lactamic antibiotics are ampicillin, cefapirin, cloxacillin, penicillin G and amoxicillin [8].
1.4. Cephalosporins
Cephalosporins have chemical and physical characteristics that are somewhat similar to penicillins however are more resistant to temperature and pH changes. Cephalosporins are a class of weak acids produced from 7-aminocephalosporanic acid.
The first-generation cephalosporins, including cephalothin (no longer sold in the United States), cephaloridine, cephradine, cefazolin and cephalexin, cephapirin and cefadroxil, are examples of molecules of this group. Cefoperazone, cefotaxime, ceftiofur, ceftriaxone, and many others, including cefovecin and cefpodoxime, are among the second-generation cephalosporins. Cefepime is a fourth-generation cephalosporin antibiotic [2].
1.5. Macrolides
Macrolides are a type of antibiotic that is commonly used in veterinary medicine to treat respiratory diseases or as a food additive to promote growth.
A macrolide is a complex mixture of antibiotics that varies in the chemical substitutions of the structure’s multiple carbon atoms, as well as the neutral amino sugars. For example, Erythromycin is predominantly erythromycin A, but types B, C, D, and E may also be present [9].
Macrolides are basic because they contain a dimethylamino group. While it is not soluble in water, it does dissolve in more polar organic solvents. In alkaline (pH 10) and acidic (pH for erythromycin) conditions, macrolides are often inactivated. Furthermore, because of their numerous functional groups, they can perform a wide range of chemical reactions [2].
1.6. Quinolones, including Fluoroquinolones
Quinolones share several similar functional groups that are important for their antimicrobial effect, despite the diversity of their ring structures. The carboxylic group at position three makes the compounds acidic. However, the 7-piperazinyl quinolones even have basic amino substituents. In solution, the 7-piperazinylquinolones are cationic, zwitterionic or anionic, whereas the opposite quinolones will solely be neutral or anionic. Thanks to the various form of substituents, quinolones have reciprocally rather totally different physical properties [10]. Some quinolones are eliminated unchanged (e.g., ofloxacin), some are partially metabolized (e.g., ciprofloxacin, enrofloxacin), and a couple are completely degraded [11]. Metabolites are typically active; enrofloxacin is de-ethylated to make ciprofloxacin.
Enrofloxacin is an antimicrobial drug that was developed specifically for application in veterinary medicine in the late 1980s. Enrofloxacin is a fluoroquinolone antibacterial agent of the third generation. This antibiotic is efficacious against a broad spectrum of infections in animals and is applied in the prevention or treatment of infectious diseases [12].
Administered orally in chickens, turkeys, pigs and cattle (through food, milk replacer and/or drinking water) or by injection to pigs or cattle parenterally [13]. Enrofloxacin is well absorbed, dispersed into tissues, and excreted in high amounts in the urine and feces after oral administration. One of the main metabolites of enrofloxacin is ciprofloxacin, which is metabolized in the liver [14].
1.7. Aminoglycosides
An aminocyclitol group characterizes aminoglycoside antibiotics, and a glycosidic bond connects the amino sugar to the aminocyclitol ring.
Gentamicin is a mixture of gentamicin C1 and C2, whereas neomycin is a combination of neomycin B and C as well as framycin [2].
Its solubility in water is improved by the presence of hydroxyl groups, whereas its solubility in fat is decreased by the presence of amine groups. These drugs’ pKas are usually between 8 and 10 [15].
1.8. Phenicols
Chloramphenicol is a straightforward neutral nitrobenzene derivative. It is extremely lipid soluble and can be employed as a free base or an ester [2].
The methylsulfonyl group of thiamphenicol and florfenicol replaces the nitrophenol group of chloramphenicol; florfenicol also includes fluorine molecules. These structural improvements can increase effectiveness and minimize toxicity, and fluorine molecules can reduce bacterial resistance in the case of florfenicol.
1.9. Lincosamides
Lincosamides (LCs) are a group of drugs very similar to macrolide drugs. The main chemical property that differentiates them from macrolides is an uncommon eight-carbon sugar. They are more soluble in salt forms of amino acid and sulphur-containing octose because they are monobasic (hydrochlorides and phosphates). They are capable of forming good salts with hydrochloric acid (HCl) [5]. The main members of the LS class are lincomycin and clindamycin [16].
1.10. Polymyxins
This group of polypeptide antibiotics includes polymyxin B and polymyxin E or colistin. Polymyxin has a synergistic effect when combined with enhanced SA, TC and other antibacterial agents. They also reduce the activity of endotoxins in body fluids and may be beneficial for endotoxemia [2]
1.11. Bacitracins
Bacitracin is a branched, cyclic decapeptide antibiotic. Bacitracin A is the most active and the main component of commercial bacitracin. Bacitracin has a broad range of actions, but it is most often used to treat Gram-positive bacteria. Since there is little resistance, bacitracin is typically used in conjunction with neomycin and polymyxin to broaden the antibacterial spectrum [17].
1.12. Novobiocin
Novobiocin is a narrow-spectrum antibiotic that, at higher concentrations, can have an antibacterial or bactericidal impact. It mostly inhibits Gram-positive bacteria but also a few Gram-negative bacteria. It works in tandem with tetracycline [2].
1.13. Tiamulin
One semisynthetic derivate of pleuromutilin is tiamulin. It consists of a tricyclic motilin core with a C-14 glycolic acid side chain, in which the C-21 keto group is essential for antibacterial activity. Studies have found that the side chain containing thioacetate in tiamulin has particularly strong antibacterial activity. It is possible to study the binding of pleuromutilins in more detail to examine compounds with a range of C-14 substituents. Although the tricyclic core has a hydrophobic interaction in the binding site, and the C-21 carbonyl group seems to be in the position of polar interaction, the rest of the C-14 side chain in the studied compound only forms a small contact. It does not appear to be involved in any major interaction [18].
1.14. Ionophores
Ionophores are fat-soluble molecules that carry ions through the membranes of lipid cells. They play an important role in improving the health and feed efficiency of livestock and poultry production. An ionophore that is usually utilized is Monensin. This compound is derived from Streptomyces and has the propriety of forming complexes with monovalent cations (including sodium and potassium). Monensin inhibits protein transport in cells, resulting in antibacterial and antimalarial impact. Monensin is commonly used in feed to avoid coccidiosis and increase feed production in the beef and dairy industries [2].
1.15. Rifamycins
Rifamycins belong to the family of antibiotics of ansamycin, whose name results from its basket-like structure containing aliphatic chains that connect the two ends of the naphthoquinone nucleus. The four structures of rifamycins presently authorized for use in the United States are rifampicin, rifabutin, rifapentin and rifaximin [19]
2. Effect of Food Processing on the Residues of Antibiotics Found in Animal Products
When veterinary medicines are administered to animals, residues can remain in meat, milk or eggs if proper precautions are not followed. The application of MRL in raw foods does not consider the changes that occur during the processing of these foods. Since the largest foods of animal provenience are usually eaten after preparation, it is critical to consider the effects of various heat treatments on residues when evaluating human exposure, determining MRL, and assessing toxicity[20][21]
Regarding research on the effect of heat procedures on antibiotic residues, usually, they show their results as a percentage of degradation after treatment. Based on the available literature, it can be inferred that the respective heat treatment reduces the concentration of antibiotic residues or bioactivity in the food product. As a result, the values published in the literature vary widely depending on the type of treatment utilized, pH, temperature and matrix [21]. Food cooking can be done in a variety of ways, including boiling, scalding, steaming, baking, roasting, frying, microwave cooking, grilling, barbecuing, smoking, sous vide and confit. These procedures involve the application of heat at different temperatures and times for food preparation. The percentage of β-lactams residues in food that degrade after cooking ranges from 0.1% to 100%.
It is reported that the stability of β-lactam under heating is very low, a consequence of the high ring strain of the small β-lactone ring, which contributes to their hydrolysis.
The molecules of cephalexin and cefuroxime are unstable in biological matrices, including at moderate temperatures of 60–80 °C; they are more susceptible to heat than other β-lactam antibiotics. In the case of meat, long-term roasting resulted in a high degree of ampicillin degradation. For milk and water, when a classic sterilization procedure is applied (120 °C for 15–20 min), β-lactams antibiotics are significantly reduced [21]. Canton et al. [22] investigated how cooking affects the stability of veterinary drug residues in chicken eggs.
For the study of the degradation of the AMX, eggs can be prepared at different times and in different ways (methods), namely, making omelets, microwaving, heating, and boiling. The major reduction in egg residues was proportional to the cooking time when measuring the stability of AMX residues by boiling. After microwaving or making an omelet, there was a substantial loss of water. The AMX residue in eggs was unstable and was significantly reduced during all cooking processes. The amount of AMX residue reduction is proportional to the time each cooking process takes to cook (microwaving, cooking, boiling and omelete making). During the microwaving and omelet-making processes, the most residue was removed. According to reports, penicillin G of AMX in milk is degraded by about 20%, cephalexin by about 27%, and cefuroxime by about 35%.
In the past, meat has been shown to degrade penicillin. According to O’Brien et al. [23], the degradation of ampicillin after roasting bovine tissue is highly variable and appears to be dependent on the temperature reached while cooking as well as the cooking time. It is not known to the author if, until now, there is a conclusive and effective study about the effect of degradation of this class of antibiotics in food.
Research studies demonstrate that the kind of food matrix and cooking process affect TC deterioration in chickens and pigs. The stability of TC, tetracycline, OTC, CTC, and DC in the chicken thigh and breast samples after boiling, roasting, and microwaving was studied using varied temperatures and periods. Tetracycline degradation percentages vary from 2% to 100% when subjected to heat treatments [21].
Cooking time increases TC loss, with DC being the most heat-stable and OTC being the least. For example, to ensure 90% TC destruction in chicken meat, microwave (2450 MHz), boil (100 °C), and roast (180 °C) it for 24, 53, and 102 min, respectively. This suggests that standard cooking methods will not be sufficient to destroy these antibiotics [24].
Time and temperature are the two most important elements in reducing antibiotic residue during cooking. Microwaved, roasted and cooked chicken resulted in 74%, 48% and 35% OTC losses, respectively. There was a decrease in the OTC in the frying process. This effect can be attributed to water loss in chicken meat [25]. Shaltout et al. [26] investigated the impact of microwave and boiling treatments on OTC residue reduction in chicken muscles. The percent of reduction waste was 81.48% and 77.93%, respectively. Grilling and roasting, according to research, are the most efficient and least effective cooking procedures for reducing OTC and DC concentrations in chicken, respectively. Only one investigation on the stability of OTC in shrimp samples was reported in the last decade in the case of fish. Kleechaya et al. [27] studied the degradation of OTC in black tiger prawns. The obtained results were a reduction of residual OTC by 30–60% by boiling, baking and frying, while in the shell, OTC was reduced by 20% in each cooking method. The study of the degradation of TC’s in their respective epimers, 4-epi-TC, 4-epi-OTC, 4-epi-CTC, 4-epi-DC and anhydro-TC’s is extremely relevant for risk assessment in the consumer. TCs degrade in various ways depending on the pH of the medium. Thus far, there has been little research into the toxicity of TC breakdown products. Anhydrotetracyclines are considered hazardous, causing reversible kidney damage. However, it is unknown if hazardous degradation products will be generated in considerable quantities during ordinary household cooking processes due to a paucity of studies on the characteristics of TC degradation products during diverse processing circumstances [21].
The thermal degradation percentages of macrolides ranged between 0% and 93%. [21]. Erythromycin is the most heat-sensitive compound of the macrolide family. Milk heat treatment studies for 20 min at 120 °C showed that the residual amount of erythromycin was reduced by more than 90%, while the residual value of other macrolides was much lower [21]. Reduction of tilmicosin of 37%, 46% and 41% in boiling, frying, and microwaving cooking methods, respectively, was related by Hussein et al. [25]. Salaramoli et al. [28] used HPLC to determine the amount of tylosin in raw and cooked samples. The study’s findings revealed that when cooked and uncooked chicken meatballs were compared, the cooked samples had a significant reduction in tylosin amounts, both by microwave heating and boiling.
Chloramphenicol, florfenicol, and thiamphenicol are examples of broad-spectrum antibiotics in the amphenicol group. It was demonstrated that the bioactivity of chloramphenicol in beef after roasting for 2 h decreased by 70%. Its degradation in beef is almost 5 times higher than in water [21]. These conclusions were reported by Franje et al. [29] and Clarke et al. [30]. Both reported that the greater degradation of chloramphenicol, which is lipophilic, could be due to the meat’s low water-binding ability after heating. [21]. The class of quinolones is not much affected by processing methods. Oxalinic acid and flumequine in salmon, enrofloxacin and ciprofloxacin in Latin fish were highly stable during heating [31]. Boiling, roasting, and frying, on the other hand, minimize the content of oxolinic acid in shrimp by 20% to 30% [32]. Heating methods such as frying, boiling, grilling, microwave cooking, and roasting all appear to affect quinolone residues in meat samples. Boiling and microwaving reduced enrofloxacin levels in the uncooked thigh and chicken breast muscles, whereas oven-roasting and grilling raised them [24].
Hasanen et al. [[33] studied ciprofloxacin residues in chicken and turkey carcasses. They concluded that ciprofloxacin residues are heat-stable and are not degraded by any cooking method, except microwaves (800 W) for 15–20 min in muscles and 3–5 min in the liver and kidney, also freezing for one month at −20 °C can degrade ciprofloxacin and its metabolites to levels below the permitted limits, but not below detectable levels. Roca et al. [34] related quinolones’ stability by heating milk. Quinolones are very resistant to heat treatments, with maximum concentration losses of 13 percent for ciprofloxacin and 12 percent for norfloxacin at 120 °C for 20 min. Quinolones’ high stability poses a risk to human health because antibiotic residues will remain in milk after heat treatment and thus enter the dairy industry and consumers [34]. A study by Ismail-Fitry et al. [35], in relation to the effect of deep-frying at different temperatures and times on sulfonamide (SA) residues in chicken meatballs, concluded that frying chicken meat-balls at 180 °C for 6 min results in better appearance and quality status of meat for consumption as well as a reduction of SA residues. Deep-frying could aid in the reduction of SA residues in chicken meatballs, with maximum reductions of 38, 28, 41, and 28% obtained for sulfadiazine (SDZ), sulfamethazine (SMZ), sulfamethoxazole (SMX), and sulfaquinoxaline (SQX) at the maximum frying time and temperature, respectively. Ismail-Fitry et al. [35] concluded that the increasing order of degradation of sulfonamides was deep-frying, boiling and microwaving, while the SDZ was the most heat-labile SA. Javadi et al. [36] used a microbial inhibition approach to reduce the concentration of sulfadiazine and trimethoprim residue in broiler edible tissues after various cooking processes. The microwave method is similar to the one that causes the greatest reduction in SDZ and trimethoprim residues in cooked muscle samples.
Zhao et al. [37] studied the degradation kinetics of six kinds of SA, SDZ, SMX, sulfasalazine, SMZ, SMX and sulfadimethoxine in eggs at simulated cooking temperatures. SDZ and sulfadimethoxine had the lower and biggest or ample half-life time, respectively. The LC-MS/MS methodology was used by Roca et al. [38] to investigate the kinetics degradation of eight types of SA, sulfadimethoxine, sultathiazole, sulfapyridine, sulfacloropiridazine, sulphaquinoxaline, SDZ, sulfamerazine and SMZ when skimmed milk is heated at 60, 70, 80, 80, 90 and 100 °C. The results obtained show that sulphonamides are very stable molecules that can resist even the most common heat treatments performed in the dairy industry without degrading significantly. The degradation of sulfamerazine, SMZ, SDZ, and SQX in milk was explained by the slow reaction rate at low temperatures and the quick increase at high temperatures. The high collision energy between molecules was sufficient to disrupt the pre-existing connection, resulting in a higher degree of degradation [38]. The collision level of sulfadimethoxine and sulfathiazole, on the other hand, was low, indicating that the reaction rate and degradation rate were both low [21].
References
- Report for 2019 on the results from the monitoring of veterinary medicinal product residues and other substances in live animals and animal products. EFSA Support. Publ. 2021, 18, 1–82.
- Veterinary Manual Available online: https://www.merckvetmanual.com/pharmacology/antibacterial-agents.
- Remko, M.; von der Lieth, C.-W. Theoretical study of gas-phase acidity, pKa, lipophilicity, and solubility of some biologically active sulfonamides. Bioorg. Med. Chem. 2004, 12, 5395–5403.
- Pérez-Rodríguez, M.; Pellerano, R.G.; Pezza, L.; Pezza, H.R. An overview of the main foodstuff sample preparation technologies for tetracycline residue determination. Talanta 2018, 182, 1–21.
- Manimekalai, M.; Rawson, A.; Sengar, A.S.; Kumar, K.S. Development, Optimization, and Validation of Methods for Quantification of Veterinary Drug Residues in Complex Food Matrices Using Liquid-Chromatography—A Review. Food Anal. Methods 2019, 12, 1823–1837.
- Lopes, R.P.; Reyes, R.C.; Romero-González, R.; Frenich, A.G.; Vidal, J.L.M. Development and validation of a multiclass method for the determination of veterinary drug residues in chicken by ultra high performance liquid chromatography–tandem mass spectrometry. Talanta 2012, 89, 201–208.
- Rossi, R.; Saluti, G.; Moretti, S.; Diamanti, I.; Giusepponi, D.; Galarini, R. Multiclass methods for the analysis of antibiotic residues in milk by liquid chromatography coupled to mass spectrometry: A review. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2018, 35, 241–257.
- Bessaire, T.; Mujahid, C.; Beck, A.; Tarres, A.; Savoy, M.C.; Woo, P.M.; Mottier, P.; Desmarchelier, A. Screening of 23 β-lactams in foodstuffs by LC–MS/MS using an alkaline QuEChERS-like extraction. Food Addit. Contam. - Part A Chem. Anal. Control. Expo. Risk Assess. 2018, 35, 661–673.
- Mazzei, T.; Mini, E.; Novelli, A.; Periti, P. Chemistry and mode of action of macrolides. J. Antimicrob. Chemother. 1993, 31, 1–9.
- Stolker, A.A.M.; Brinkman, U.A.T. Analytical strategies for residue analysis of veterinary drugs and growth-promoting agents in food-producing animals - A review. J. Chromatogr. A 2005, 1067, 15–53.
- Parshikov, I.A.; Sutherland, J.B. Microbial transformations of antimicrobial quinolones and related drugs. J. Ind. Microbiol. Biotechnol. 2012, 39, 1731–1740.
- Razzagh, M.; Reza, N. Determination of enrofloxacin residue in chicken eggs using FPT and ELISA methods. J. Res. Heal. 2015, 5, 159–164.
- Moghadam, N.R.; Arefhosseini, S.R.; Javadi, A.; Lotfipur, F.; Ansarin, M.; Tamizi, E.; Nemati, M. Determination of Enrofloxacin and Ciprofloxacin Residues in Five Different Kinds of Chicken Tissues by Dispersive Liquid-Liquid Microextraction Coupled with HPLC. Iran. J. Pharm. Res. 2018, 17, 1182–1190.
- Petrović, J.; Baltic, M.; Cupic, V.; Stefanović, S.; Stojanovic, D. Residues of enrofloxacin and its main metabolite ciprofloxacin in broiler chickens. Acta Vet. Brno. 2006, 56.
- Martínez-Villalba, A.; Moyano, E.; Galceran, M.T. Ultra-high performance liquid chromatography–atmospheric pressure chemical ionization–tandem mass spectrometry for the analysis of benzimidazole compounds in milk samples. J. Chromatogr. A 2013, 1313, 119–131.
- Salikin, J.; Abdullah, A. Determination of macrolide antibiotics in chicken tissues by liquid chromatography-electrospray mass spectrometry. AIP Conf. Proc. 2013, 1571, 702–709.
- Jank, L.; Martins, M.T.; Arsand, J.B.; Hoff, R.B.; Barreto, F.; Pizzolato, T.M.; Campos Motta, T.M. High-throughput method for macrolides and lincosamides antibiotics residues analysis in milk and muscle using a simple liquid-liquid extraction technique and liquid chromatography-electrospray-tandem mass spectrometry analysis (LC-MS/MS). Talanta 144, 686–695.
- Jank, L.; Martins, M.T.; Arsand, J.B.; Hoff, R.B.; Barreto, F.; Pizzolato, T.M. High-throughput method for the determination of residues of β-lactam antibiotics in bovine milk by LC-MS/MS. Food Addit. Contam. - Part A Chem. Anal. Control. Expo. Risk Assess. 32, 1992–2001.
- Dasenaki, M.E.; Thomaidis, N.S. Multi-residue determination of 115 veterinary drugs and pharmaceutical residues in milk powder, butter, fish tissue and eggs using liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 2015, 880, 103–121.
- Dasenaki, M.E.; Thomaidis, N.S. Multi-residue determination of 115 veterinary drugs and pharmaceutical residues in milk powder, butter, fish tissue and eggs using liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 2015, 880, 103–121.
- Tian, L.; Khalil, S.; Bayen, S. Effect of thermal treatments on the degradation of antibiotic residues in food. Crit. Rev. Food Sci. Nutr. 2017, 57, 3760–3770.
- Canton, L.; Alvarez, L.; Canton, C.; Ceballos, L.; Farias, C.; Lanusse, C.; Moreno, L. Effect of cooking on the stability of veterinary drug residues in chicken eggs. Food Addit. Contam. - Part A Chem. Anal. Control. Expo. Risk Assess. 2019, 36, 1055–1067.
- O’Brien, J.J.; Campbell, N.; Conaghan, T. Effect of cooking and cold storage on biologically active antibiotic residues in meat. J. Hyg. (Lond). 1981, 87, 511–523.
- Sobral, M.M.C.; Cunha, S.C.; Faria, M.A.; Ferreira, I.M.P.L.V.O. Domestic Cooking of Muscle Foods: Impact on Composition of Nutrients and Contaminants. Compr. Rev. Food Sci. Food Saf. 2018, 17, 309–333.
- Hussein, M.A.; Morshedy, A.M.; Ahmed, M.M. Effect of cooking methods on some antibiotic residues in chicken meat. Jpn. J. Vet. Res. 2016, 64, S225-S225–S231.
- Shaltout, F. Impacts Of Different Types Of Cooking And Freezing On Antibiotic Residues In Chicken Meat. Food Sci. Nutr. 2019, 5.
- Kleechaya, W. Pharmacokinetics of oxytetracycline in black tiger shrimp, Penaeus monodon, and the effect of cooking on the residues. Aquac. Res. 2005, 254, 24–31.
- Salaramoli, J.; Heshmati, A.; Kamkar, A.; Hassan, J. Effect of cooking procedures on tylosin residues in chicken meatball. J. für Verbraucherschutz und Leb. 2015, 11.
- Franje, C.A.; Chang, S.-K.; Shyu, C.-L.; Davis, J.L.; Lee, Y.-W.; Lee, R.-J.; Chang, C.-C.; Chou, C.-C. Differential heat stability of amphenicols characterized by structural degradation, mass spectrometry and antimicrobial activity. J. Pharm. Biomed. Anal. 2010, 53, 869–877.
- Clarke, A.; Means, W.; Schmidt, G. Effects of Storage Time, Sodium Chloride and Sodium Tripolyphosphate on Yield and Microstructure of Comminuted Beef. J. Food Sci. 2006, 52, 854–856.
- Botsoglou, N.A.; Fletouris, D.J. Drug residues in foods : pharmacology, food safety, and analysis; Marcel Dekker: New York, 2001; ISBN 0-367-39792-7.
- Uno, K.; Aoki, T.; Kleechaya, W.; Tanasomwang, V.; Ruangpan, L. Pharmacokinetics of oxolinic acid in black tiger shrimp, Penaeus monodon Fabricius, and the effect of cooking on residues. Aquac. Res. 2006, 37, 826–833.
- Hasanen, F.; Mohammed, M.; H, M.; Hassan, W.; Amro, F. Ciprofloxacin residues in chicken and turkey carcasses. Benha Vet. Med. J. 2016, 31, 136–143.
- Roca, M.; Castillo, M.; Marti, P.; Althaus, R.L.; Molina, M.P. Effect of Heating on the Stability of Quinolones in Milk. J. Agric. Food Chem. 2010, 58, 5427–5431.
- Ismail-Fitry, M.R.; Jinap, S.; Bakar, J.; Saleha, A. Effect of Deep-Frying at Different Temperature and Time on Sulfonamide Residues in Chicken Meat-Balls. J. Food Drug Anal. 2008, 16, 81–86.
- Javadi, A.; Mirzaie, H.; Khatibi, A. Effect of roasting, boiling and microwaving cooking method on sulfadiazine+ trimethoprim residues in edible tissues of broiler by microbial inhibition method. African J. Microbiol. Res. 2011, 5, 16–19.
- Zhao, X.H.; Wu, P.; Zhang, Y.H. Degradation kinetics of six sulfonamides in hen eggs under simulated cooking temperatures. J. Serbian Chem. Soc. 2011, 76, 1093–1101.
- Roca, M.; Althaus, R.; Molina, M.P. Thermodynamic analysis of the thermal stability of sulphonamides in milk using liquid chromatography tandem mass spectrometry detection. Food Chem. 2013, 136, 376–383.
- Roca, M.; Althaus, R.; Molina, M.P. Thermodynamic analysis of the thermal stability of sulphonamides in milk using liquid chromatography tandem mass spectrometry detection. Food Chem. 2013, 136, 376–383.
More
Information
Subjects:
Others
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
975
Revisions:
2 times
(View History)
Update Date:
01 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




